 American Journal of Analytical Chemistry, 2011, 2, 276-283 doi:10.4236/ajac.2011.22034 Published Online May 2011 (http://www. SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Rapid Determination of Calcium in Milk and Water Samples by Reflectance Spectroscopy Hayati Filik, Duygu Aksu, Reşat Apak Department of Chemistry, Faculty of Engineering, Istanbul University, Istanbul, Turkey E-mail: filik@istanbul.edu .tr Received October 23, 2010; revised March 7, 2011; accepted May 16, 2011 Abstract Reflectance sp ectrosco py (RS) can be used as a rapi d and sen sitive metho d for the quantit ative determinati on of low amounts of calcium. In this analytical technique, the analyte in complex samples is extracted onto a solid sorbent matrix loaded with glyoxal bis (2-hydroxyanil (GBHA) and then quantified directly on the sor- bent surface. The measurements were carried out at a wavelength of 566.1 nm yielding the largest diver- gence of reflectance spectra before and after reaction with the analyte element. The optimum response was obtained in 0.2 mol⋅L–1 NaOH solution, and the response time of the sensor was about 5 min, depending on the concentrat ion of Ca(II). The calibration cu rve of Ca(II) was found to be linear on semi-logarithmic scale within the concentration range of 0.3 - 40 mg ⋅L–1, with a LOD o f 0.15 mg⋅L–1 in the low concentration range. The sensor response from different sensors (n = 5) gave an R.S.D. of 1.4% at 10 mg⋅L–1 Ca(II). The response characteristics of the sensor including dynamic range, reversibility, reproducibility, response time and life- time are discu ssed in detai l. The main advan tages of th is prototyp e device ar e sensi tivity an d higher select iv- ity over Mg(II). The proposed method has been successfully applied to the determination of Ca(II) in milk and drinking water samples. Keywords: Calcium, Reflectance, Optical Senso r , Glyoxal Bis (2-Hydroxyanil) 1. Introduction Calcium is an essential element for humans, and is also responsible for ‘water hardness’, a water qualit y parameter frequently tested in industrial plants and municip al water treatment facilities. Calcium is biologically required for numerous functions, such as building and maintaining the bones and teeth, blood clotting, transmitting of the nerve impulses and regulating heart's rhythm [1]. Milk and dairy products are convenient sources of calcium for many people [2]. Drinking water is an important source of calcium in the elderly, particularly because of in- creased needs and decreased consumption of dairy prod- ucts [3]. Therefore, low-cost, sensitive and selec tive me- thods are necessary to determine this ion. A traditional method for the quality control of cal- cium-containing products and hard water is complexo- metric titration with EDTA using suitable indicators and masking agents. Lower concentrations of Ca2+ in aqueous samples require more sensitive instrumental analytical techniques such as flame atomic absorption spectrometry (FAAS), inductively coupled plasma (ICP) emission spectrometry, capillary electrophoresis, ion chromatography, and spectrophotometry, though the most widely used one is AAS [1,4]. The use of different concentrations of ionization buffers (e.g., containing La(III)) has been recommended in the FAAS analysis of different classes of substrates [5-11]. Lanthanum salts enhance the atomic absorption signals of Ca, especially in phosphate- containing matrices. The addition of ioni- zation suppressants to both samples and calibration standards can reduce interference effects. Analyses of milk and milk products are routinely performed by a number of dairy laboratories to implement quality con- trol with regard to nutritional components. FAAS analy- sis of milk products require sample preparation such as dry a shing or wet digestion (for decomposition of a sam- ple into a homogeneous liquid phase). The complexity of Ca matrices (complexed with protein) and its low con- centration levels in aqueous solutions, together with possible effects of various matrix constituents, make direct measurement of this metal ion difficult [11]. Therefore, it is of great importance to develop a simple and rapid detection technique for th e routine quality con- 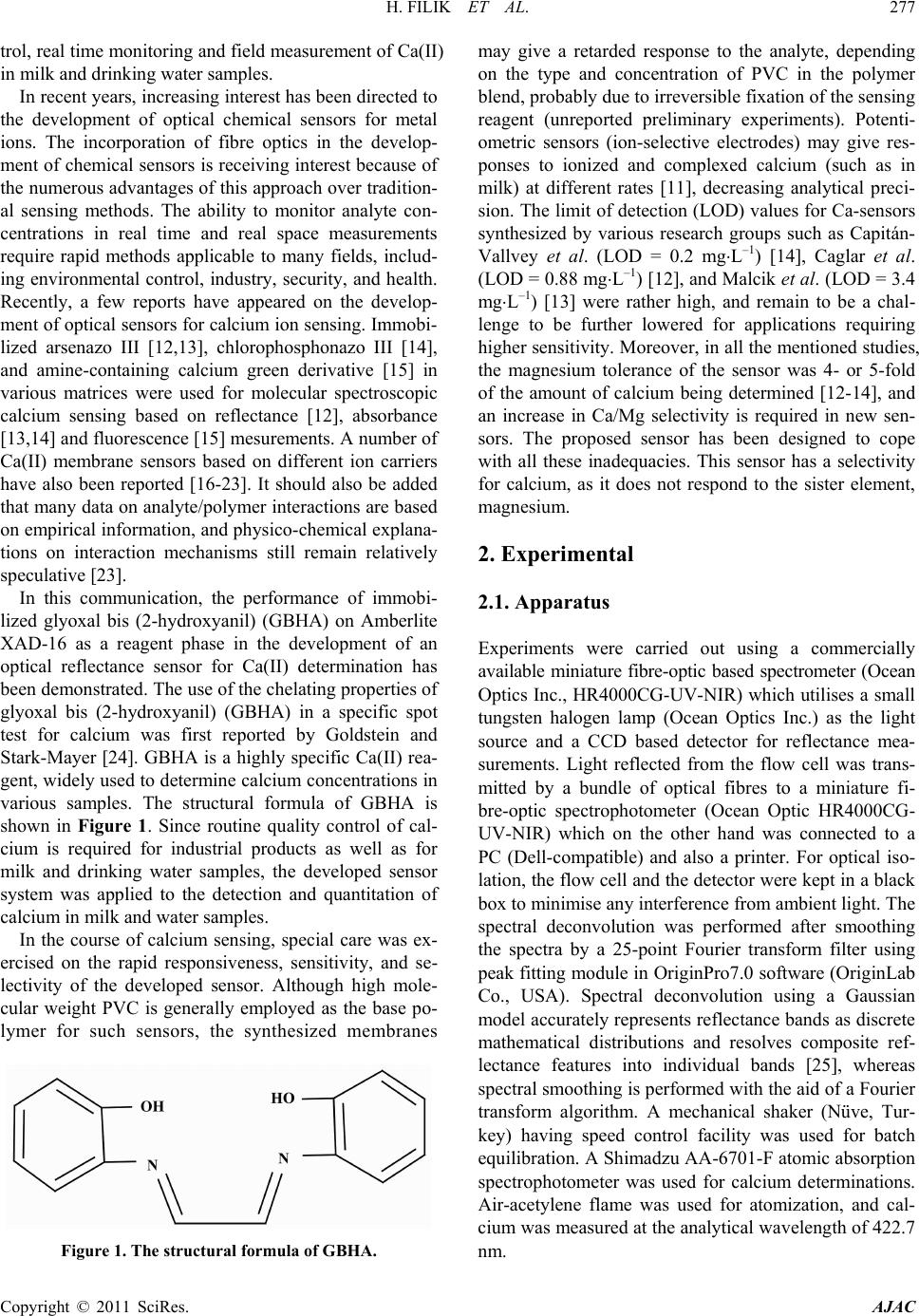 H. FILIK ET AL. Copyright © 2011 SciRes. AJAC trol, real time monitoring and field measurement of Ca(II) in milk and drinking water sample s. In recent years, increasing interest has been directed to the development of optical chemical sensors for metal ions. The incorporation of fibre optics in the develop- ment of chemical sensors is receiving interest because of the numerous advantages of this approach over tradition- al sensing methods. The ability to monitor analyte con- centrations in real time and real space measurements require rapid methods applicable to many fields, includ- ing environmental control, industry, security, and health. Recently, a few reports have appeared on the develop- ment of optical sensors for calcium ion sensing. Immobi- lized arsenazo III [12,13], chlorophosphonazo III [14], and amine-containing calcium green derivative [15] in various matrices were used for molecular spectroscopic calcium sensing based on reflectance [12], absorbance [13,14] and fluorescence [15] mesurements. A number of Ca(II) membrane sensors based on different ion carriers have also been reported [16-23]. It should also be added that many data on analyte/polymer interactions are based on empirical information, and physico-chemical explana- tions on interaction mechanisms still remain relatively speculative [23]. In this communication, the performance of immobi- lized glyoxal bis (2-hydroxyanil) (GBHA) on Amberlite XAD-16 as a reagent phase in the development of an optical reflectance sensor for Ca(II) determination has been demonstrated. The use of the chelating properties of glyoxal bis (2-hydroxyanil) (GBHA) in a specific spot test for calcium was first reported by Goldstein and Stark-Mayer [24]. GBHA is a highly specific Ca(II) rea- gent, widely used to determine calcium concentrations in various samples. The structural formula of GBHA is shown in Figure 1. Since routine quality control of cal- cium is required for industrial products as well as for milk and drinking water samples, the developed sensor system was applied to the detection and quantitation of calcium in milk and water samples. In the course of calcium sensing, special care was ex- ercised on the rapid responsiveness, sensitivity, and se- lectivity of the developed sensor. Although high mole- cular weight PVC is generally employed as the base po- lymer for such sensors, the synthesized membranes Figure 1. The structural formula of GBHA. may give a retarded response to the analyte, depending on the type and concentration of PVC in the polymer blend, probably due to irreversible fixation of the sensing reagent (unreported preliminary experiments). Potenti- ometric sensors (ion-selective electrodes) may give res- ponses to ionized and complexed calcium (such as in milk) at different rates [11], decreasing analytical preci- sion. The limit of detection (LOD) values for Ca-sensors synthesized by various research groups such as Capitán- Vallvey et al. (LOD = 0.2 mg⋅L–1) [14], Caglar et al. (LOD = 0 .88 mg⋅L–1) [12], and Malcik et al. (LOD = 3.4 mg⋅L–1) [13] were rather high, and remain to be a chal- lenge to be further lowered for applications requiring higher sensitivity. Moreover, in all the mentioned studies, the magnesium tolerance of the sensor was 4- or 5-fold of the amount of calcium being determined [12-14], and an increase in Ca/Mg selectivity is required in new sen- sors. The proposed sensor has been designed to cope with all these inadequacies. This sensor has a selectivity for calcium, as it does not respond to the sister element, magnesium. 2. Experimental 2.1. Apparatus Experiments were carried out using a commercially available miniature fibre-optic based spectrometer (Ocean Optics Inc., HR4000CG-UV-N I R) which utilises a small tungsten halogen lamp (Ocean Optics Inc.) as the light source and a CCD based detector for reflectance mea- surements. Light reflected from the flow cell was trans- mitted by a bundle of optical fibres to a miniature fi- bre-optic spectrophotometer (Ocean Optic HR4000CG- UV-NIR) which on the other hand was connected to a PC (Dell-compatible) and also a printer. For optical iso- lation, the flow cell and the detector were kept in a black box to minimise any interference from ambient light. The spectral deconvolution was performed after smoothing the spectra by a 25-point Fourier transform filter using peak fitting module in OriginPro7.0 software (OriginLab Co., USA). Spectral deconvolution using a Gaussian model accurately represents reflectance bands as discrete mathematical distributions and resolves composite ref- lectance features into individual bands [25], whereas spectral smoothing is performed with the aid of a Fourier transform algorithm. A mechanical shaker (Nüve, Tur- key) having speed control facility was used for batch equilibration. A Shimadzu AA-6701-F atomic absorption spectrophotometer was used for calcium determinations. Air-acetylene flame was used for atomization, and cal- cium was measured at the analytical wavelength of 422.7 nm.  H. FILIK ET AL. Copyright © 2011 SciRes. AJAC 2.2. Chemicals and Reagen t s All chemicals used were of analytical-reagent grade, ex- cept where otherwise stated; calcium-free distilled water was used throughout for the preparation of all solutions. All metal salts were used without further purification . Polystyrene–divinylbenzene (Amberlite XAD-16) resin was purchased from Sigma (Madrid, Spain). Glyoxal bis (2-hydroxyanil) (GBHA) was obtained from Riedel de-Haën (Seelze, Germany), and used without further purification. Accurately weighed amounts of calcium carbonate, dried at 110˚C for 1 h, were dissolved in di- lute hydrochloric acid, then diluted to the appropriate volume of stock solution. Working solutions were pre- pared by dilution as required. The stock calcium solution was standardized against ethylenediamine tetraacetate (EDTA) using murexide as metallochromic indicator for compleximetric titration. The stock color reagent was GBHA solution, prepared by dissolving 0.5 g of the rea- gent in 100 mL of ethanol (2 × 10–2 mol⋅L–1). 2.3. Sample Preparation Two UHT milk samples and mineral water samples of different commercial brands were supplied at random from the local market in Istanbul, Turkey. All the drink- ing water bottles were stored under refrigeration at 4˚C, and opened on the day of analysis. The samples were analyzed directly without pretreatment. Trichloroacetic acid method: A portion (5 mL) of milk sample was mixed with 20% trichloroacetic acid (TCA) in the volume ratio 1:1. After waiting for 30 min, the solution was centrifuged. The volume of the centrifugate was made up to 100 mL with water and an aliquot was used for the determinati on of c a l c ium. Ashing method: Milk samples (4 mL) were weighed into a porcelain crucible and dried on a hot plate. After charring, samples were incinerated in a muffle furnace at 500˚C. If necessary, the ash was bleached by treatment with HNO3 and heating in the muffle furnace for 1 h. Finally, the ashes were diluted with concentrated nitric acid (1 mL) and the mixture was heated to dryness. Then the residue was dissolved in water and transferred into a 50 mL calibrated flask, and made up to the mark with distilled water. 2.4. Impregnation Procedure Amberlite XAD-16 resin as obtained from the supplier contained organic and inorganic impurities. To remove these contaminants, it was washed successively with ethanol, water, 1.0 mol⋅L–1 NaOH, and 1.0 mol⋅L–1 HCl in this order. The XAD-16 beads were then washed with distilled water until neutral. The sensing material (GBHA) was physically immobilized by adsorption onto Amberlite XAD-16 polymer. The resin beads were dried in an oven at 105˚C for 4 h. A mass of 0.5 g dry resin (Amberlite XAD-16) beads was treated with 5 ml of 2.0 × 10–2 mol⋅L–1 GBHA and 5 ml ethanol, and the mixture was shaken at room temperature for 30 min. The result- ing white resin beads (loaded with ligand) were filtered off from the supernatant solution, and washed with dis- tilled water to remove the excess of GBHA. Then the resin beads were transfered onto a dry filter paper and pressed for easy drying. Finally, the white resin beads were kept under nitro ge n atmosphere when not in use. 2.5. Sensor Fabrication The sensor design was similar to that described by Ahmad and Narayanaswamy and Filik et al. [26-29]. The probe was built using disposable syringe tubes (Tyco Health- care Group LP, Büyükçekmece, Istanbul, Turkey). A sy- ringe column (5 milliliter plastic syringe, with graduations in milliliters and fifths of milliliters), with a nylon me m- brane and a stopcock, has been used for preconcentration of Ca(II). A syringe tube was filled to the 0.5 ml (i.e. 5 mm) mark lin e with the sensing layer (XAD-16-GBHA). The filling mass of the Amberlite XAD-16- G BHA resin was 299.3 ± 0.5 mg (n = 5). During measurements, the arbitrary unit and the detector were kept in a black box to minimise any interference from ambient light. 2.6. Recommended Procedure A 3 mL aliquot of the sample solution containing cal- cium (within the working range of 0.3 - 40 mg⋅L–1) was placed in a sensor system. Then, 1.0 mL of 1.0 mol⋅L–1 NaOH was added, and mixed well. The reflectance mea- surement was carried out by recording the optical signal 5 min after placement of the bifurcated optical fibre in the analyte solution. Reflectance spectra were recorded after 5 min (for full colour development). The measurements were expressed in the units of r elative reflectance, which is defined as the difference between the reflectance of the Ca-GBHA/XAD complex (Rc) and that of the immobi- lized reagent (GBHA/XAD) alone (Rf), both recorded at the same wavelength (566.1 nm). The blank value was obtained by the same procedure with the exception of Ca addition. 3. Results and Discussion 3.1. Reflectance Spectra Figure 2 shows the reflectance spectra of immobilized 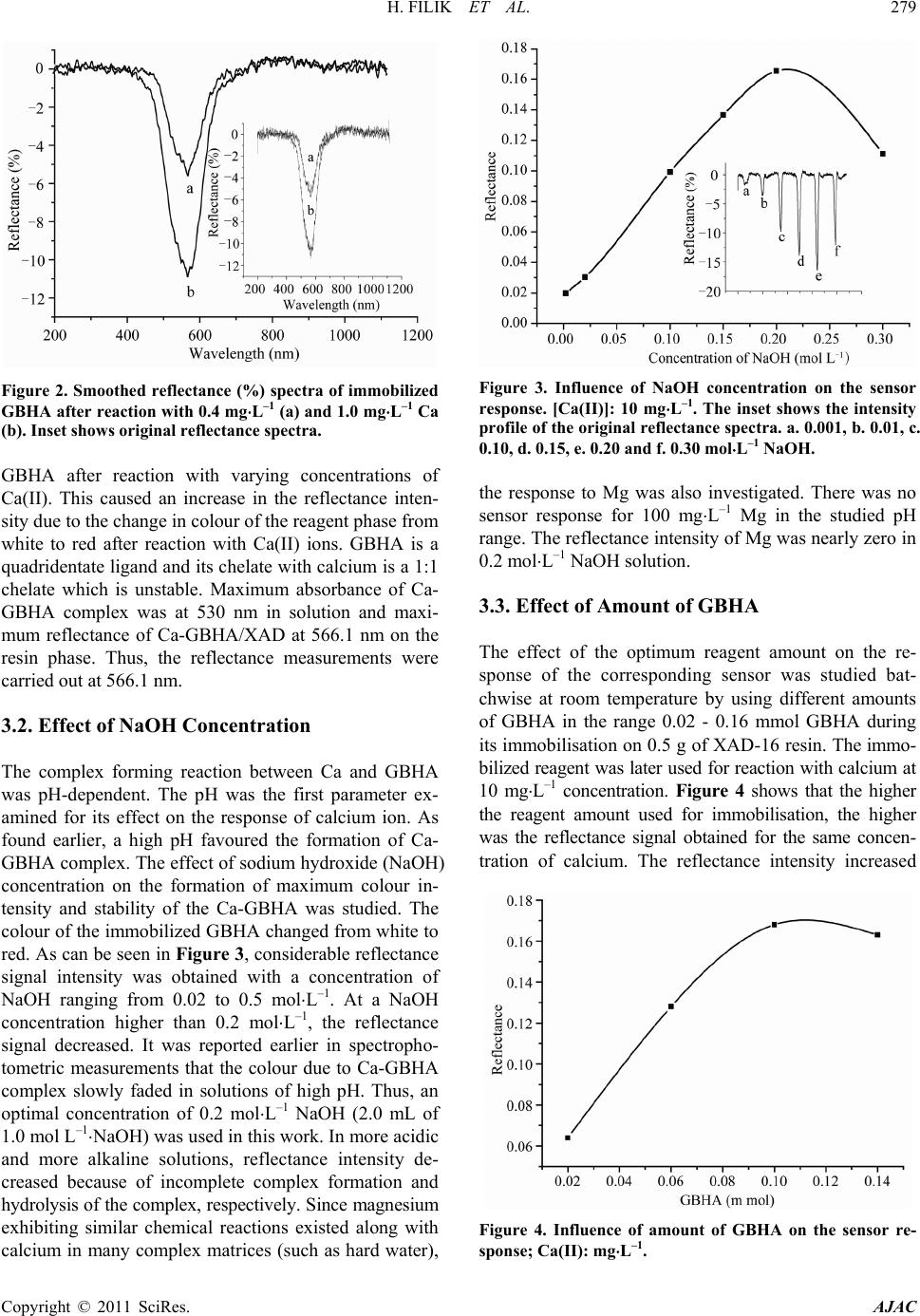 H. FILIK ET AL. Copyright © 2011 SciRes. AJAC Figure 2. Smoothed reflectance (%) spectra of immobilized GBHA after reaction with 0.4 mg⋅L–1 (a) and 1.0 mg⋅L–1 Ca (b). Inset shows original reflectance spectra. GBHA after reaction with varying concentrations of Ca(II). This caused an increase in the reflectance inten- sity due to the change in colour of the reagent phase from white to red after reaction with Ca(II) ions. GBHA is a quadridentate ligand and its chelate with calcium is a 1:1 chelate which is unstable. Maximum absorbance of Ca- GBHA complex was at 530 nm in solution and maxi- mum reflectance of Ca-GBHA/XAD at 566.1 nm on the resin phase. Thus, the reflectance measurements were carried out at 566. 1 nm. 3.2. Effect of NaOH Concentration The complex forming reaction between Ca and GBHA was pH-dependent. The pH was the first parameter ex- amined for its effect on the response of calcium ion. As found earlier, a high pH favoured the formation of Ca- GBHA complex. The effect of sodium hydroxide (NaOH) concentration on the formation of maximum colour in- tensity and stability of the Ca-GBHA was studied. The colour of the immobilized GBHA changed from white to red. As can be seen in Figure 3, considerable reflectance signal intensity was obtained with a concentration of NaOH ranging from 0.02 to 0.5 mol⋅L–1. At a NaOH concentration higher than 0.2 mol⋅L–1, the reflectance signal decreased. It was reported earlier in spectropho- tometric measurements that the colour due to Ca-GBHA complex slowly faded in solutions of high pH. Thus, an optimal concentration of 0.2 mol⋅L–1 NaOH (2.0 mL of 1.0 mol L–1⋅NaOH) was used in this work. In more acidic and more alkaline solutions, reflectance intensity de- creased because of incomplete complex formation and hydrolysis of the complex, re spectively. Sin ce magnesium exhibiting similar chemical reactions existed along with calcium in many complex matrices (such as hard water), Figure 3. Influence of NaOH concentration on the sensor response. [Ca(II)]: 10 mg⋅L–1. The inset shows the intensity profile of the original reflectance spectra. a. 0.001, b. 0.01, c. 0.10, d. 0.15, e. 0.20 and f. 0.30 mol⋅L–1 NaOH. the response to Mg was also investigated. There was no sensor response for 100 mg⋅L–1 Mg in the studied pH range. The reflectance intensity of Mg was nearly zero in 0.2 mol⋅L–1 NaOH solution. 3.3. Effect of Amount of GBHA The effect of the optimum reagent amount on the re- sponse of the corresponding sensor was studied bat- chwise at room temperature by using different amounts of GBHA in the range 0.02 - 0.16 mmol GBHA during its immobilisation on 0.5 g of XAD-16 resin. The i mmo- bilized reagent was later used fo r reaction with calcium at 10 mg⋅L–1 concentration. Fig ure 4 shows that the higher the reagent amount used for immobilisation, the higher was the reflectance signal obtained for the same concen- tration of calcium. The reflectance intensity increased Figure 4. Influence of amount of GBHA on the sensor re- sponse; Ca(II): mg⋅L–1. 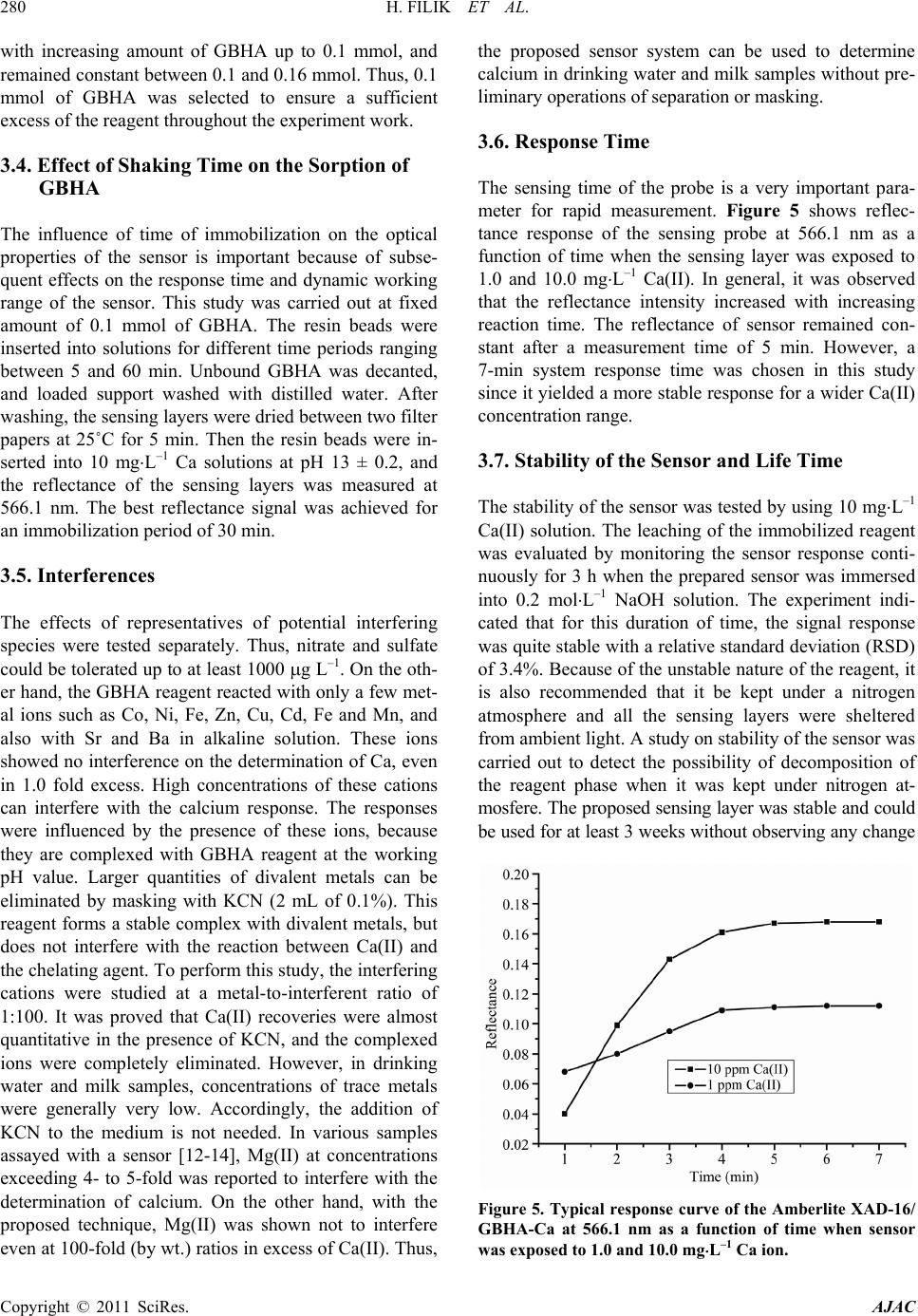 H. FILIK ET AL. Copyright © 2011 SciRes. AJAC with increasing amount of GBHA up to 0.1 mmol, and remained constan t between 0.1 and 0.16 mmol. Thus , 0.1 mmol of GBHA was selected to ensure a sufficient excess of the reagent throughout the experiment work. 3.4. Effect of Shaking Time on the Sorption of GBHA The influence of time of immobilization on the optical properties of the sensor is important because of subse- quent effects on the response time and dynamic working range of the sensor. This study was carried out at fixed amount of 0.1 mmol of GBHA. The resin beads were inserted into solutions for different time periods ranging between 5 and 60 min. Unbound GBHA was decanted, and loaded support washed with distilled water. After washing, the sensing layers were dried between two filter papers at 25˚C for 5 min. Then the resin beads were in- serted into 10 mg⋅L–1 Ca solutions at pH 13 ± 0.2, and the reflectance of the sensing layers was measured at 566.1 nm. The best reflectance signal was achieved for an immobi l ization peri od of 30 min. 3.5. Interferences The effects of representatives of potential interfering species were tested separately. Thus, nitrate and sulfate could be tolerated up to at least 1000 µg L–1. On the oth- er hand, the GBHA reagent reacted with only a few met- al ions such as Co, Ni, Fe, Zn, Cu, Cd, Fe and Mn, and also with Sr and Ba in alkaline solution. These ions showed no interference on the determination of Ca, even in 1.0 fold excess. High concentrations of these cations can interfere with the calcium response. The responses were influenced by the presence of these ions, because they are complexed with GBHA reagent at the working pH value. Larger quantities of divalent metals can be eliminated by masking with KCN (2 mL of 0.1%). This reagent forms a stable complex with divalent metals, but does not interfere with the reaction between Ca(II) and the chelating agent. To perform this study, the in terfering cations were studied at a metal-to-interferent ratio of 1:100. It was proved that Ca(II) recoveries were almost quantitative in the presence of KCN, and the complexed ions were completely eliminated. However, in drinking water and milk samples, concentrations of trace metals were generally very low. Accordingly, the addition of KCN to the medium is not needed. In various samples assayed with a sensor [12-14], Mg(II) at concentrations exceeding 4- to 5-fold was reported to interfere with the determination of calcium. On the other hand, with the proposed technique, Mg(II) was shown not to interfere even at 100-fold (by wt.) ratios in excess of Ca(II). Thus, the proposed sensor system can be used to determine calcium in drinking water and milk samples without pre- liminary operations of separation or masking. 3.6. Response Time The sensing time of the probe is a very important para- meter for rapid measurement. Figure 5 shows reflec- tance response of the sensing probe at 566.1 nm as a function of time when the sensing layer was exposed to 1.0 and 10.0 mg⋅L–1 Ca(II). In general, it was observed that the reflectance intensity increased with increasing reaction time. The reflectance of sensor remained con- stant after a measurement time of 5 min. However, a 7-min system response time was chosen in this study since it yielded a more stable response for a wider Ca(II) concentr a t ion range. 3.7. Stability of the Sensor and Life Time The stability of the sensor was tested by using 10 mg⋅L–1 Ca(II) solution. The leaching of the immobilized reagent was evaluated by monitoring the sensor response conti- nuously for 3 h when the prepared sensor was immersed into 0.2 mol⋅L–1 NaOH solution. The experiment indi- cated that for this duration of time, the signal response was quite stable with a r elative standard deviation (RSD) of 3.4%. Because of the unstable nature of the reagent, it is also recommended that it be kept under a nitrogen atmosphere and all the sensing layers were sheltered from ambient light. A study on stability of the sensor was carried out to detect the possibility of decomposition of the reagent phase when it was kept under nitrogen at- mosfere. The proposed sensing layer was stable and could be used for at least 3 weeks without observing any change Figure 5. Typical response curve of the Amberlite XAD-16/ GBHA-Ca at 566.1 nm as a function of time when sensor was exposed to 1.0 and 10.0 mg⋅L–1 Ca ion. 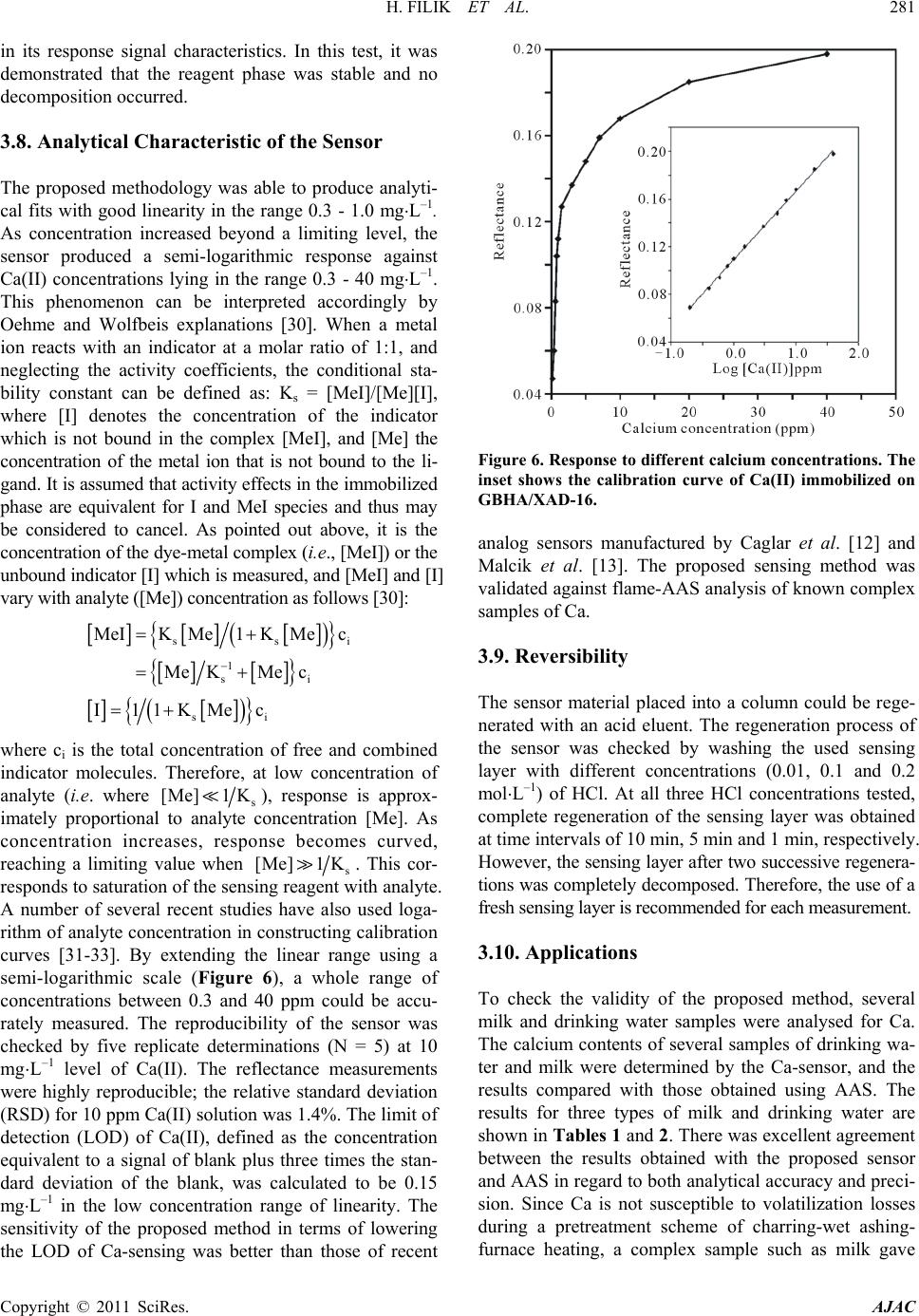 H. FILIK ET AL. Copyright © 2011 SciRes. AJAC in its response signal characteristics. In this test, it was demonstrated that the reagent phase was stable and no decompos i tion occur red. 3.8. Analytical Characteristic of the Sensor The proposed methodology was able to produce analyti- cal fits with good linearity in the range 0.3 - 1.0 mg⋅L–1. As concentration increased beyond a limiting level, the sensor produced a semi-logarithmic response against Ca(II) concentrations lying in the range 0.3 - 40 mg⋅L–1. This phenomenon can be interpreted accordingly by Oehme and Wolfbeis explanations [30]. When a metal ion reacts with an indicator at a molar ratio of 1:1, and neglecting the activity coefficients, the conditional sta- bility constant can be defined as: Ks = [MeI]/[Me][I], where [I] denotes the concentration of the indicator which is not bound in the complex [MeI], and [Me] the concentration of the metal ion that is not bound to the li- gand. It is ass umed that ac tivity ef fects in the immo bilized phase are equivalent for I and MeI species and thus may be considered to cancel. As pointed out above, it is the concentration of the dye-metal complex (i. e., [MeI]) or the unbound indicator [I] which is measured, and [MeI] and [I] vary with analyte ([Me] ) conc entrati on as follows [30] : [][ ][ ] ( ) { } [ ][ ] { } [ ][] ( ) { } s si 1 si si MeIK Me1K Mec MeKMec I1 1KMec − = + = + = + where ci is the total concentration of free and combined indicator molecules. Therefore, at low concentration of analyte (i.e. where s [Me]1 K), response is approx- imately proportional to analyte concentration [Me]. As concentration increases, response becomes curved, reaching a limiting value when s [Me]1 K. This cor- responds to saturation of the sensing reagent with analyte. A number of several recent studies have also used loga- rithm of analyte concentration in constructing calibration curves [31-33]. By extending the linear range using a semi-logarithmic scale (Figu re 6), a whole range of concentrations between 0.3 and 40 ppm could be accu- rately measured. The reproducibility of the sensor was checked by five replicate determinations (N = 5) at 10 mg⋅L–1 level of Ca(II). The reflectance measurements were highly reproducible; the relative standard deviation (RSD) fo r 10 ppm Ca(II) solution was 1.4%. The limit of detection (LOD) of Ca(II), defined as the concentration equivalent to a signal of blank plus three times the stan- dard deviation of the blank, was calculated to be 0.15 mg⋅L–1 in the low concentration range of linearity. The sensitivity of the proposed method in terms of lowering the LOD of Ca-sensing was better than those of recent Figure 6. Response to different calcium concentrations. The inset shows the calibration curve of Ca(II) immobilized on GBHA/XAD-16. analog sensors manufactured by Caglar et al. [12] and Malcik et al. [13]. The proposed sensing method was validated against flame-AAS analysis of know n complex samples of Ca. 3.9. Reversibility The sensor material placed into a column could be rege- nerated with an acid eluent. The regeneration process of the sensor was checked by washing the used sensing layer with different concentrations (0.01, 0.1 and 0.2 mol⋅L–1) of HCl. At all three HCl concentrations tested, complete regeneration of the sensing layer was obtained at time intervals of 10 min, 5 min and 1 min, respectively. However, the sen sing layer after two succes sive regenera- tions was comp letely decomposed. Theref ore, the use of a fresh sensing layer is rec ommended for e ach measurement. 3.10. Applications To check the validity of the proposed method, several milk and drinking water samples were analysed for Ca. The calcium contents of several samples of drinking wa- ter and milk were determined by the Ca-sensor, and the results compared with those obtained using AAS. The results for three types of milk and drinking water are shown in Tab les 1 and 2. There was excellent agreement between the results obtained with the proposed sensor and AAS in regard to both analytical accuracy and preci- sion. Since Ca is not susceptible to volatilization losses during a pretreatment scheme of charring-wet ashing- furnace heating, a complex sample such as milk gave  H. FILIK ET AL. Copyright © 2011 SciRes. AJAC Table 1. Analysis of three drinking water samples by using FAAS and the prop os ed sensor ( n = 3). Water Samples Declared (mg⋅L–1) FAAS (mg⋅L–1) Founda (mg⋅L–1) Pınar 6.0 6.1 ± 0.3 5.94 ± 0.6 Hayat 19.7 19.7 ± 0.2 19.9 ± 0.2 Erikli 4.0 4.0 ± 0.1 3.96 ± 0.3 aThe propose d method Table 2. Analysis of three milk samples by using FAAS and the propose d sensor (n = 3). Milk Samples Declared (mg⋅L–1) FAAS (mg⋅L–1) Founda (mg⋅L–1) Danone 1008 1010 ± 1 1015 ± 3 İçim 1120 1125 ± 2 1130 ± 4 Pınar 1200 1202 ± 2 1208 ± 5 aThe propose d method. correct Ca findings with the proposed method. 4. Conclusions The advantage of employing a GBHA-based sensor over the conventional methods for Ca(I I) determination lies in the ease of its detection, use of less amount of chemicals, and less time consumption. In the standard colorimetric determination, the precipitated Ca(II)-GBHA complex must be extracted into an organic solvent, and this intro- duces complexity in laboratory procedures as well as in difficult disposition of used solvents. The results of this study have shown that the proposed sensor is applicable to the determination of calcium in drinking water and milk samples. The application of the procedure can be extended to the determination of calcium in different samples having very low metal contents. There is defi- nite selectivity for Ca over Mg. The method for drinking water and milk is simple, fast and highly reproducible. 5. Acknowledgements The authors gratefully acknowledge the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Grant no: 109T856) for financial support. 6. References [1] V. Vičkačkaitė, S. Tautkus and R. Kazlauskas, “Deter- mination of Calcium in Mineral Wate rs by Flame Atomic Absorption Spectrometry,” Chemija, Vol. 18, No. 4, 2007, pp. 34-37. [2] A. J. Lanou, S. E. Berkow and N. D. Barnard, “Calcium, Dairy Products, and Bone Health in Children and Young Adults: A R eevaluation of the Evidence,” Pediatrics, Vol. 115, No. 3, 2005, pp. 736-743. doi:10.1542/peds.2004-0548 [3] S. Marque, H. Jacqmin-Gadda, J. -F. Dartigues and D. Commenges, “Cardiovascular Mortality and Calcium and Magnesium in Drinking Water: An Ecological Study in Elderly Peopl e, ” European J ournal of Epidemiology, Vol . 18, No. 4, 2003, pp. 305-309. doi:10.1023/A:1023618728056 [4] K. Grudpan, J. Jakmunee, Y. Vaneesorn, S. Watanesk, U Aye Maung and P. Sooksamiti, “Flow Injection Spectro- photometric Determination of Calcium Using Murexide as a Color Agent,” Talanta, Vol. 46, No. 6, 1998, pp. 1245-1247. doi:10.1016/S0039-9140(97)00410-4 [5] A. Moreno-Cid, M. C. Yebra, “Continuous Ultrasound- Assisted Extraction Coupled to a Flow Injection-Flame Atomic Absorption Spectrometric System for Calcium Determination in Seafood Samples,” Analytical and Bio- analytical Chemistry, Vol. 379, No. 1, 2005, pp. 77-82. doi:10.1007/s00216-003-2452-6 [6] B. Welz, “Atomic Absorption Spectrometry,” Vancouver Coastal Health, Weinheim, 1985. [7] R. Kataky, D. Parker and A. Teasdale, “Comparative Study of Trip odal Oxa-Amides and Oxaesters as Iono- phores in Potentiometric Ion-Selective Electrodes for Al- kali and Alkaline Earth Cations,” Analytica Chimica Acta, Vol. 276, No. 2, 1993, pp. 353-360. doi:10.1016/0003-2670(93)80404-9 [8] M. W. Welch, D. W. Hamar, M. J. Fettman, “Method Comparison for Calcium Det erminati on by Flame At omic Absorption Spectrophotometry in the Presence of Phos- phate,” Clinical Chemistry, Vol. 36, No. 2, 1990, pp. 351-354. [9] Z. Arslan, J. F. Tyson, “Determination of Calcium, Mag- nesium and Strontium in Soils by Flow Injection Flame Atomic Absorption Spectrometry,” Talanta, Vol. 50, No. 5, 1999, pp. 929-937. doi:10.1016/S0039-9140(99)00187-3 [10] A. P. Udoh, “Atomic Absorption Spectrometric Deter- mination of Calcium and other Metallic Elements in Some Animal Protein Sources,” Talanta, Vol. 52, No. 6, 2000, pp. 749-754. doi:10.1016/S0039-9140(00)00368-4 [11] T. J. Cardwell, R. W. Cattrall, G. J. Cross, R. I. Mrzljak and G. R. Scoll ary, “Determination of Calcium in Waters, Milk and Wine by Discontinuous-Flow Analysis,” Ana- lyst, Vol. 115, No. 10, 1990, pp. 1235-1237. doi:10.1039/an9901501235 [12] P. Caglar, S. A. Tuncel, N. Malcik and J. P. Landers, “A Microchip Sensor for Calcium Determination,” Analyti- cal and Bioanalytical Chemistry, Vol. 386, No. 5, 2006, pp. 1303-1312. [13] N. Malcik, J. P. Ferrance, J. P. Landers and P. Caglar, “The Performance of a Microchipbased Fiber Optic De- tection Technique for the Determination of Ca2+ ions in Urine,” Sensors Actuators B, Vol. 107, No. 1, 2005, pp. 24-31. doi:10.1007/s00216-006-0776-8 [14] L. F. Capitán-Vallvey, P. A. de Cienfuegos-Gálvez, M. D.  H. FILIK ET AL. Copyright © 2011 SciRes. AJAC F. Ramos and R. Avidad-Castañeda, “Determination of Calcium by a Single-Use Optical Sensor,” Sensors Actu- ators B, Vol. 71, No. 1-2, 2000, pp. 140-146. doi:10.1016/S0925-4005(00)00602-X [15] M. Shortreed, R. Kopelman, M. Kuhn, B. Hoyland, “Flu- orescent Fiber-Optic Calcium Sensor for Physiological Measurements,” Analytical Chemistry, Vol. 68, No. 8, 1996, pp. 1414-1418. doi:10.1021/ac950944k [16] U. Schaller, E. Bakker and E. Pretsch, “Carrier Mechan- ism of Acidic Ionophores in Solvent Polymeric Mem- brane Ion-Selective Electrodes,” Analytical Chemistry, Vol. 67, No. 18, 1995, pp. 3123-3132. doi:10.1021/ac00114a005 [17] U. Schaller, E. Bakker, U. E. Spichiger and E. Pretsch, “Ionic Additives for Ion-Selective Electrodes Based on Electr ically Charge d Carriers,” Analytical Chemistry, Vol. 66, 1994, pp. 391-398. doi:10.1021/ac00075a013 [18] A. Kumar, S. K. Mittal, “PVC Based Dibenzo-18- Crown-6 Electrode for Ca(II) Ions,” Sensors Actuators B, Vol. 99, No. 2-3, 2004, pp. 340-346. [19] A. El-Jammal, A. A. Bouklouze, G. J. Patriarche and G. D. Christian, “Use of Ethylene -Vinylacetate as a New Membrane Matrix for Calcium Ion-Selective Electrode Preparation,” Talanta, Vol. 38, No. 8, 1991, pp. 929-935. doi:10.1016/0039-9140(91)80274-4 [20] T. Dimitrakopoulos, J. R. Farrell and P. J. Iles, “Photo- Cured Calcium Ion-Selective Electrode for Use in Flow Injection Potentiometry that Tolerates High Perchlorate Levels,” Electroanalysis, Vol. 8, No. 4, 1996, pp. 391-395. doi:10.1002/elan.1140080417 [21] K. Suzuki, K. Watanabe, Y. Matsumoto, M. Kobayashi, S. Sato, D. Siswanta and H. Hisamoto, “Design and Synthe- sis of Calcium and Magnesium Ionophores Based on Double-Armed Diazacrown Ether Compounds and Their Application to an Ion Sensing Component for an Ion-Se- lective Electrode,” Analytical Chemistry, Vol. 67, No. 2, 1995, 324-334. doi:10.1021/ac00098a016 [22] W. E. Morf, K. Seiler, B. Rusterholz, W. Simon, “Design of a Novel Calcium-Selective Optode Membrane Based on Neutral Ionophores,” Analytical Chemistry, Vol. 62, No. 7, 1990, pp. 738-742. doi:10.1021/ac00206a018 [23] U. Lange, N. V. Roznyatovskaya and V. M. Mirsky, “Conducting Poly mers in Chemical Sensors and Arrays,” Analytica Chimica Acta, Vol. 614, No. 1, 2008, pp. 1-26. doi:10.1016/j.aca.2008.02.068 [24] D. Goldstein and R. Stark-Mayer, “New Specific Te st for Calcium”, Analytica Chimica Acta, Vol. 19, 1958, pp. 437-439. doi:10.1016/S0003-2670(00)88191-X [25] J. M. Sunshine, C. M. Pieters and S. F. Pratt, “Deconvo- lution of Mineral Absorption Bands: An Improved Ap- proach,” Journal of Geophysical Research, Vol. 95, No. B5, 1990, pp. 6955-6966. doi:10.1029/JB095iB05p06955 [26] M. Ahmad and R. Narayanaswamy, “Fibre Optic Reflec- tance Sensor for the Determination of Aluminium (III) in Aqueous Environment,” Analytica Chimica Acta, Vol. 291, No. 3, 1994, pp. 255-260. doi:10.1016/0003-2670(94)80020-0 [27] S. H. Alabbas, D. C. Ashworth, B. Bezzaa, S. A. Momin and R. Narayanaswamy, “Factors Affecting the Response Time of an Optical-Fibre Reflectance pH Sensor,” Sen- sors Actuators A, Vol. 51, No. 2, 1996, 129-134. doi:10.1016/0924-4247(95)01212-5 [28] H. Filik, M. M. Hayvali, E. Kiliç, R. Apak, D. Aksu, Z. Yanaz and T. Cengel, “Development of an Optical Fibre Reflectance Sensor for P-Aminophenol Detection Based on Immobilised Bis-8-hydroxyquinoline,” Talanta, Vol. 77, No. 1, 2008, pp. 103-109. doi:10.1016/j.talanta.2008.05.045 [29] H. Filik, D. Aksu, R. Apak, I. Sener and E. Kılıc, “An Optical Fibre Reflectance Sensor for P-aminophenol De- termination Based on Tetrahydroxycalix[4]arene as Sensing Reagent,” Sensors Actuators B, Vol. 136, No. 1-2, 2009, pp. 105-112. doi:10.1016/j.snb.2008.11.011 [30] I. Oehme and O. S. Wolfbeis, “Optical Sensors for De- termination of Heavy Metal Ions,” Microchimica Acta, Vol. 126, No. 3, 1997, pp. 177-192. doi:10.1007/BF01242319 [31] N. A. Yusof and M. Ahmad, “A Flow Cell Optosensor for Lead Based on Immobilized Gallocynin in Chitosan Membrane,” Talanta, Vol. 58, No. 3, 2002, pp. 459-466. doi:10.1016/S0039-9140(02)00308-9 [32] N. Mahendra, P. Gangaiya, S. Sotheeswaran and R. Na- rayanaswamy, “Investigation of a Fibre Optic Copper Sensor Based on Immobilised [alpha]-Benzoinoxime (cu- pron),” Sensors Actuators B, Vol. 90, No. 1-3, 2003, pp. 118-123. doi:10.1016/S0925-4005(03)00044-3 [33] A. A. Ensafi and M. Fouladgar, “Development of a Sp ec- trophotometric Optode for the Determination of Hg (II) ,” IEEE Sensors Journal, Vol. 8, No. 4, 2008, pp. 347-353. doi:10.1109/JSEN.2008.917482
|