W. DU ET AL.
Copyright © 2011 SciRes. AJAC
Table 3. Concentration of quinolones in the two brands of
honey.
Concentrati on (ng/g)
Sample 1 Sample 2
norfloxacin - 23.1/21.9*
*Guideline of Japan MHLW.
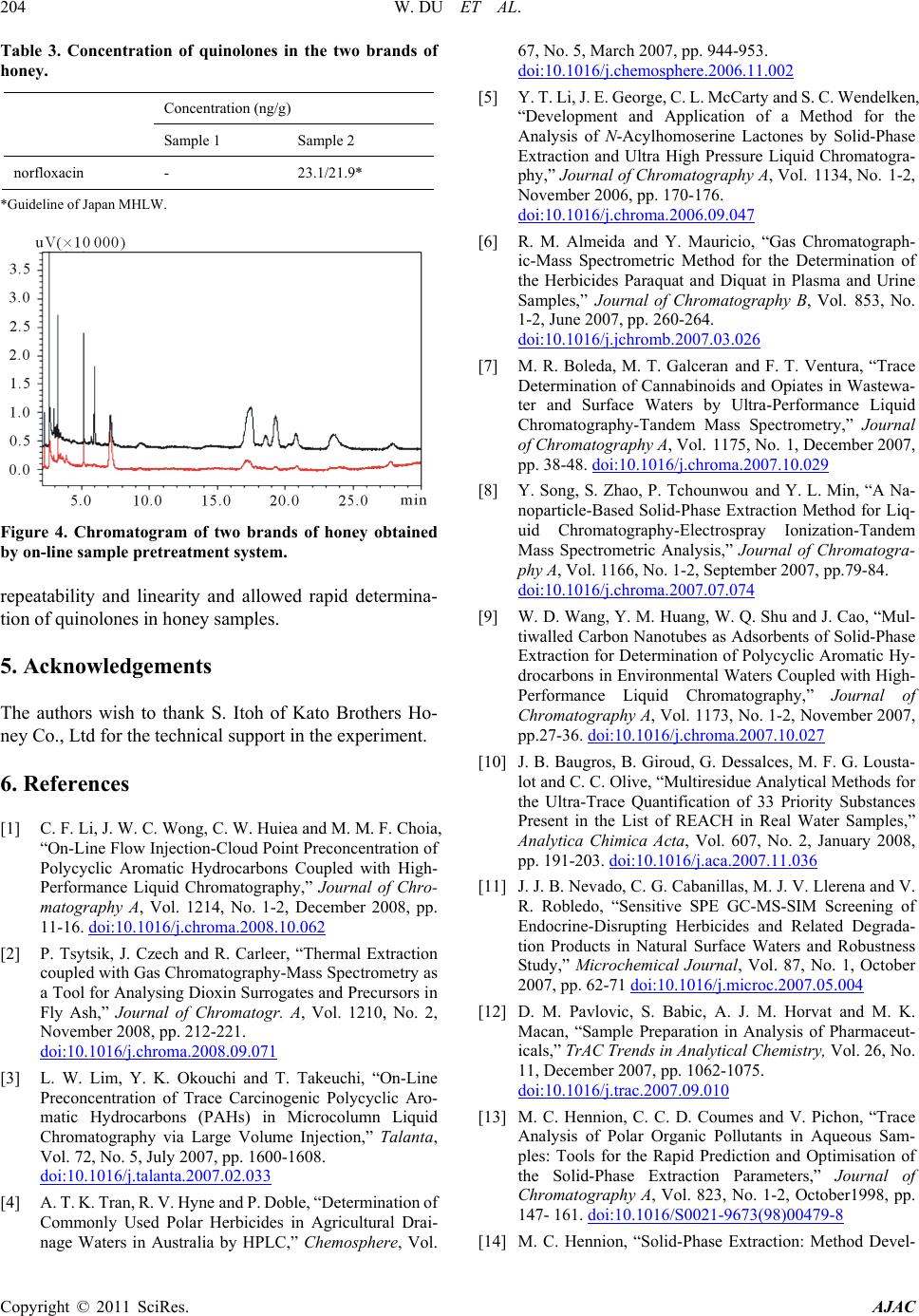
Figure 4. Chromatogram of two brands of honey obtained
by on-line sample pret reatment system.
repeatability and linearity and allowed rapid determina-
tion of quinolones in honey samples.
5. Acknowledgements
The authors wish to thank S. Itoh of Kato Brothers Ho-
ney Co., Ltd for the technical support in the experiment.
6. References
[1] C. F. Li, J. W. C. Wong, C. W. Huiea and M. M. F. Choia,
“On-Line Flow Injection-Cloud Point Preconcentration of
Polycyclic Aromatic Hydrocarbons Coupled with High-
Performance Liquid Chromatography,” Journal of Chro-
matography A, Vol. 1214, No. 1-2, December 2008, pp.
11-16. doi:10.1016/j.chroma.2008.10.062
[2] P. Tsytsik, J. Czech and R. Carleer, “Thermal Extraction
coupled with Gas Chromatography-Mass Spectrometry as
a Tool for Analysing Dioxin Surrogates and Precursors in
Fly Ash,” Journal of Chromatogr. A, Vol. 1210, No. 2,
November 2008, pp. 212-221.
doi:10.1016/j.chroma.2008.09.071
[3] L. W. Lim, Y. K. Okouchi and T. Takeuchi, “On-Line
Preconcentration of Trace Carcinogenic Polycyclic Aro-
matic Hydrocarbons (PAHs) in Microcolumn Liquid
Chromatography via Large Volume Injection,” Talanta,
Vol. 72, No. 5, July 2007, pp. 1600-1608.
doi:10.1016/j.talanta.2007.02.033
[4] A. T. K. Tran, R. V. Hyne and P. D oble, “Determination of
Commonly Used Polar Herbicides in Agricultural Drai-
nage Waters in Australia by HPLC,” Chemosphere, Vol.
67, No. 5, March 2007, pp. 944-953.
doi:10.1016/j.chemosphere.2006.11.002
[5] Y. T. Li, J. E. G eorge, C. L . McCarty and S . C. Wendelk en,
“Development and Application of a Method for the
Analysis of N-Acylhomoserine Lactones by Solid-Phase
Extraction and Ultra High Pressure Liquid Chromatogra-
phy,” Journal of Chromatography A, Vol. 1134, No. 1-2,
November 2006, pp. 170-176.
doi:10.1016/j.chroma.2006.09.047
[6] R. M. Almeida and Y. Mauricio, “Gas Chromatograph-
ic-Mass Spectrometric Method for the Determination of
the Herbicides Paraquat and Diquat in Plasma and Urine
Samples,” Journal of Chromatography B, Vol. 853, No.
1-2, June 2007, pp. 260-264.
doi:10.1016/j.jchromb.2007.03.026
[7] M. R. Boleda, M. T. Galceran and F. T. Ventura, “Trace
Determination of Cannabinoids and Opiates in Wastewa-
ter and Surface Waters by Ultra-Performance Liquid
Chromatography-Tandem Mass Spectrometry,” Journal
of Chromatography A, Vol. 1175, No. 1, December 2007,
pp. 38-48. doi:10.1016/j.chroma.2007.10.029
[8] Y. Song, S. Zhao, P. Tchounwou and Y. L. Min, “A Na-
noparticle-Based Solid-Phase Extraction Method for Liq-
uid Chromatography-Electrospray Ionization-Tandem
Mass Spectrometric Analysis,” Journal of Chromatogra-
phy A, Vol. 1166, No. 1-2, September 2007, pp.79-84.
doi:10.1016/j.chroma.2007.07.074
[9] W. D. Wang, Y. M. Huang, W. Q. Shu and J. Cao, “Mul-
tiwalled Carbon Nanotubes as Adsorbents of Sol i d -Phase
Extraction for Determination of Polycy clic Aromatic Hy-
drocarbons in Environmental Waters Coupled with High-
Performance Liquid Chromatography,” Journal of
Chromatography A, Vol. 1173, No. 1-2, November 2007,
pp.27-36. doi:10.1016/j.chroma.2007.10.027
[10] J. B. Baugros, B. Giroud, G. Dessalces, M. F. G. Lousta-
lot and C. C. Olive, “Multiresidue Analytical Methods for
the Ultra-Trace Quantification of 33 Priority Substances
Present in the List of REACH in Real Water Samples,”
Analytica Chimica Acta, Vol. 607, No. 2, January 2008,
pp. 191-203. doi:10.1016/j.aca.2007.11.036
[11] J. J. B. Nevado, C. G. Cabanillas, M. J. V. Llerena and V.
R. Robledo, “Sensitive SPE GC-MS-SIM Screening of
Endocrine-Disrupting Herbicides and Related Degrada-
tion Products in Natural Surface Waters and Robustness
Study,” Microchemical Journal, Vol. 87, No. 1, October
2007, pp. 62-71 doi:10.1016/j.microc.2007.05.004
[12] D. M. Pavlovic, S. Babic, A. J. M. Horvat and M. K.
Macan, “Sample Preparation in Analysis of Pharmaceut-
icals,” TrAC Trends in Analytical Chemistry, Vol. 26, No.
11, December 2007, pp. 1062-1075.
doi:10.1016/j.trac.2007.09.010
[13] M. C. Hennion, C. C. D. Coumes and V. Pichon, “Trace
Analysis of Polar Organic Pollutants in Aqueous Sam-
ples: Tools for the Rapid Prediction and Optimisation of
the Solid-Phase Extraction Parameters,” Journal of
Chromatography A, Vol. 823, No. 1-2, October1998, pp.
147- 161. doi:10.1016/S0021-9673(98)00479-8
[14] M. C. Hennion, “Solid-Phase Extraction: Method Devel-