 Journal of Materials Science and Chemical Engineering, 2014, 2, 16-25 Published Online October 2014 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2014.210003 How to cite this paper: Szczerba, J., Jastrzębska, I., Pędzich, Z. and Bućko, M.M. (2014) Corrosion of Basic Refractories in Contact with Cement Clinker and Kiln Hot Meal. Journal of Materials Science and Chemical Engineering, 2, 16-25. http://dx.doi.org/10.4236/msce.2014.210003 Corrosion of Basic Refractories in Contact with Cement Clinker and Kiln Hot Meal Jacek Szczerba, Ilona Jastrzębska, Zbigniew Pędzich, Mirosław M. Bućko Department of Materials Science and Ceramics, AGH University of Science and Technology, Kraków, Poland Email: jszczerb@agh.edu.pl Received July 2014 Abstract This paper investigates the new phases formation in the magnesia-spinel, magnesia-zirconia-cal- cia and magnesia-hercynite refractory products as a result of their reaction with kiln hot meal and cement clinker (both rich in SO3 and Cl ions) at the temperature range 1200°C - 1450˚C. The labor - atory corrosion tests were conducted by means of pulverous method and by special coating test. The phase identity of the products as well as the mixes after corrosion tests was established by powder X-ray diffraction method. The microstructure development of the contact zones between coating and the products were studied using scanning electron microscopy SEM with EDS spec- troscopy. The differences in the reactions proceeding between the phases of the test bricks and the phases of kiln hot meal and cement clinker in dependence on the temperatures have been discussed. Keywords Corrosion, Refractories, Clinker, CaZrO3, MgO, FeAl2O4 1. Introduction During work of basic refractory lining in the sintering zone of rotary kiln devoted for sintering of Portland clinker, the components of the products undergo an intensive interaction in the first place. This is associated with the influence of the highest temperatures in the sintering zone of the kiln. The length of it is usually identi- fied with the range of temperatures of the shifting furnace charge, where chemical processes of phase composi- tion formation of Portland clinker occur in the presence of a liquid phase [1] [2]. The liquid phase in the system of basic oxide components CaO-SiO2-Al2O3-Fe2O3 of cement clinker occurs in zero-point of C3S-C2S-C3A- C4AF subsystem at the temperature of 1338˚C [3]. The liquid phase may occur earlier at the temperature about 1280˚C, what is related with eutectic in the system C2S-C12A7-C2(A,F). Moreover, presence of MgO and R2O oxides (R = K, Na) lowers the temperature of liquid phase occurrence to about 1260˚C [4]. The earlier appear- ance of the liquid phase is associated with eutectic in CF-CF2 system occurring at approximately 1200˚C. Nowadays, the magnesia-spinel products have the biggest share among many types of the basic products that are devoted for the application as linings in the sintering zone of cement kilns [5]-[7]. The corrosion process, besides wear by erosion and abrasion, is one of the crucial importances limiting the lifetime of the refractory lining. Therefore, this work aims to investigate the high temperature behaviour (from 1200˚C to 1450˚C) of a  J. Szczerba et al. few important, in the cement industry, basic refractories—magnesia-spinel MSp, magnesia-zirconia-calcia MCZ and magnesia-hercynite MH products. The present work was undertaken in order to get a better recognize and understanding of reactions between the components of the bricks and the furnace charge, leading to formation of the new phases. 2. Experimental Met hods The materials taken to investigation were industrial magnesia-spinel (MSp), magnesia-zirconia-calcia (MCZ) and magnesia-hercynite (MH) products (Table 1). Hot kiln meal enriched with alkali oxides, SO3 and chlorine and Portland clinker produced out of it (Table 1) were applied as the corrosive media in the present study. The meal and clinker came from the cement rotary kiln working with precalciner system and using alternative fuels. The corrosion test was carried out in the temperature range from 1200˚C to 1450˚C, corresponding to the tem- peratures of the shifting furnace charge in the segment of the sintering zone of cement rotary kiln, considering the changes in its phase composition during the sintering process [1] [2]. The examined materials from cement kiln came from often encountered system with an excess of SOx and with the high amount of Cl- ions, over alkali oxides level occurring in the kiln atmosphere. That is the reason for the low molar alkali sulphate module, designated as ASR (Table 1). Both for kiln hot meal and Portland clinker the ASR module took a value below 1 [2]. Table 1. The chemical and phase composition of the materials taken to examination. Chemical composition, wt.% Specification Product1) Corrosive media MSp MCZ MH Kiln hot meal (without loss of ignition) Portland clinker (without loss of ignition) SiO2 0.3 0.30 1.0 19.78 20.67 CaO 0.9 5.59 2.0 63.14 64.32 Al2O3 5.6 0.15 2.9 6.11 6.22 Fe2O3 0.4 0.42 8.1 2.46 3.07 MgO 92.8 82.74 85.3 1.75 2.03 ZrO2 - 10.63 - - - C/Swt. 3.0 18.6 2.0 - - K2O - - - 1.73 1.44 Na2O - - - 0.12 0.13 SO3 - - - 4.92 2.46 Cl- - - - 3.54 0.00 Modules (refer to corrosive media only)/dimensionless unit Lime saturation factor, LSF2) - - - 95.2 92.9 Silica ratio, SR3) - - - 2.3 2.2 Alumina ratio, AR4) - - - 2.5 2.0 Liquid phase, LP14505) - - - 27.5 28.8 Alkali sulphate module, ASR6) - - - −0.48 0.45 Theoretical phase composition7) of MSp.:M, MA, C2S,C3A; MCZ: M, CZ; MH:M, F2+A, M(A,F),C2S, Portland clinker according to Bogue [8],wt. %7): C3S-58,6; C2S-15,1; C3A-11,3; C4AF-9,4; CaSO4-4,2. 1)Symbols according to the standard PN-EN [9]; 2)LSFA/R>0.6 38 = 100·CaO/(2,8·SiO2 + 1.65·Al2O3 + 0.35·Fe2O3); 3) SR = SiO2/(Al2O3 + Fe2O3); 4)AR = Al 2O3/Fe2O3; 5)LP1450 = 3.0·Al2O3 + 2.25·Fe2O3 + MgO + Na2O + K2O; 6)ASR = (K2O/94 + Na2O/62 – Cl2/71)/(SO3/80); 7)M-MgO; A-Al2O3; C-CaO; S-SiO2; F-Fe2O3; f/F2+-FeO; Z-ZrO2.  J. Szczerba et al. The X-ray powder analysis of Portland clinker showed, besides the main phase of alite C3S, presence of the phases such as: belite β -C2S, tri-calcium aluminate C3A and calcium aluminoferrite phase corresponding to the structure of C4AF type. The determined phase composition of Portland clinker by XRD is agreeable with the theoretical one calculated according to Bogue [8] (Table 1). The XRD analysis of the kiln hot meal sample showed the presence of belite β -C2S, mayenite C12A7, anhy- drite CaSO4, sylvine KCl, quartz SiO2 and remains of calcium carbonate CaCO3. On the other hand, after cal- cining of kiln hot meal at 1200˚C/2 h, it exhibited the presence of belite β -C2S, calcium sulphate aluminate-yee- limite C3A3·CaSO4, aluminoferrite phase corresponding to structure C4AF, mayenite C12A7 and traces of potas- sium chloride KCl. These are the typical intermediate phases appearing at this stage of sintering of Portland clinker. The corrosion test of the products was carried out by means of the pulverous and special coating method [10]. The mixtures for the corrosion test by pulverous method consisted of the product/kiln hot meal or Portland clinker in mass ratio (3:1). Before the materials to be examined were prepared, the subsequently, the mixtures were moulded into pellets of mixtures were milled to obtain granulation <0.063 mm. Subsequently, the mixtures were moulded into pellets of 20 mm in diameter and 10 mm in thickness under the pressure of 100 MPa. Then, the pellets were heated up to 1200˚C (with kiln hot meal), 1300˚C and 1400˚C (with Portland clinker) with 2 hours soaking time at each temperature and then cooled down together with the laboratory electric furnace. Such samples were then milled to granulation <0.063 mm and their phase composition was determined by XRD. For the corrosion test by coating method the disks of 30 mm in diameter and 20 mm in height were bored from the products. The surface of the discs was ground and then they were pressed with kiln hot meal or Port- land clinker, of granulation <0.063 mm, in a mould of 50 mm in diameter, under the pressure of 50 MPa. Thus, the disks were covered both on the side rim as well as the upper surface with the layer of kiln hot meal and ce- ment clinker. The samples with Portland clinker for the coating corrosion test were heated up to 1450˚C with 5 hours soak- ing time at each temperature and cooled down together with the laboratory electric furnace. The metallographic specimens were prepared from the after-coating corrosion samples for the microstructure observations under Scanning Electron Microscope (SEM) equipped with EDS detector for chemical analysis in microareas. The starting products were also subjected to SEM/EDS analysis. 3. Results and Discussion 3.1. Products of the Reactions in the Powder Mixtures Examined by Pulverous Corrosion Method Table 2 and Table 3 contains results of the phase composition analysis of the mixtures product/kiln hot meal or Portland clinker after heating up to 1200˚C (kiln hot meal), and 1300˚C, 1400˚C (Portland clinker). As it can be observed from Table 2, in reaction of the mixture of MSp product with kiln hot meal at the temperature of 1200˚C, spinel MA almost totally disappeared from the product and calcium aluminate C12A7 was produced. Moreover, the presence of C3A3·CaSO4 phase as well as merwinite and brownmillerite was detected in the ma- terial. In the case of MCZ product MgO, CaZrO3 and β -Ca2SiO4 were identified as the major phases, while C3A3·CaSO4, C4AF, C3A were present in the minor amounts. This shows that the main constituents of the MCZ Table 2. Phase composition of the mixtures (3:1) (product/kiln hot meal) after heating up to 1200˚C. Sample Phases identified in the test material by XRD (in order of lower intensity) Kiln hot meal β -Ca2SiO4, C12A7, CaSO4, KCl, SiO2, remains of CaCO3 MSp/khma MgO, β -Ca2SiO4, MgAl2O4↓, C12A7, C3A3·CaSO4, C3MS2, C4AF MCZ/khma MgO, CaZrO3, β -Ca2SiO4, C3A3·CaSO4, C4AF, C3A MH/khma MgO, β -Ca2SiO4, C4AF, Fe3O4 akiln hot meal.  J. Szczerba et al. Table 3. Phase composition of the mixtures with (3:1) ratio (product/Portland clinker) after heating up to 1300˚C and 1400˚C. Sample Phases identified in the test material by XRD (in order of lower intensity) Portland clinker C3S, β -Ca2SiO4, C3A, C4AF, CaSO4 Corrosion test at 1300˚C MSp/Pcb MgO, β -Ca2SiO4, Q-C20A13M3S3, C3A3·CaSO4, MgAl2O4↓ MCZ/Pcb MgO, CaZrO3, Ca3SiO5, C4AF, C3A MH/Pcb MgO, C4AF, β -Ca2SiO4 Corrosion test at 1400°C MSp/Pcb MgO, β -Ca2SiO4, C2AS, Q - C20A13M3S3 MCZ/Pcb MgO, CaZrO3, Ca3SiO5, C4AF, C3A MH/Pcb MgO, C4AF, β -Ca2SiO4 bPortland clinker. product did not react during the corrosion test. The new phases, formed after the test, were a consequence of the reactions only between the components of hot kiln meal, and they constituted typical transitional phases appear- ing at 1200˚C during sintering of cement clinker. Regarding magnesia-hercynite sample (MH), presence of pe- riclase, alite and brownmillerite was detected after the corrosion test. Furthermore, a small amount of magnetite Fe3O4 was found in the discussed sample, probably as a consequence of hercynite decomposition and subsequent partial oxidation of FeO [11]. Table 3 shows phase composition of the mixtures in the mass ratio (3:1) (product/Portland clinker) after heating up to 1300˚C and 1400˚C. The mixture of MSp product with Portland clinker, heated up to 1300˚C, ex- hibited almost total disappearance of spinel MA from the product, decomposition of silicate C3S and aluminate phases, and calcium sulphate from Portland clinker (Table 1 and Table 3). As a result of the reaction new phas- es were formed, coexisting with MgO and C2S. They involve quaternary aluminate, so-called Q phase, which can be expressed by the formula C20A13M3S3 [12] and calcium sulphate aluminate C3A3·CaSO4, stable to 1350˚C [13]. However, in the mixture of MSp product with Portland clinker, heated up to 1400°C, apart from MgO and C2S, new phases were found, which coexisted with them in sub-solidus. These were gehlenite C2AS and Q phase [12]. Since in the mixtures with MSp the C4AF phase disappeared, it may be expected that iron came within a solid solution (henceforth designated as “ss.”) with Q phase. Therefore the formula for this phase, ear- lier suggested by Parker as C6A4MS (with incongruent melting point at 1380˚C), can not be excluded here - in this case with obvious substitutions of magnesium ions by ions of bivalent iron [8] [14]. The mixtures of MCZ product with Portland clinker, heated up to 1300˚C and 1400˚C, exhibit the same phas- es occurrence at both temperatures of the test. In these mixtures XRD analysis enabled to identify the main components of the products—CaZ rO3 and MgO as well as the main constituents of cement clinker-C3S, C3A and C4AF. The attained results imply that no reaction underwent between components of the MCZ product and the corrosive media. As it comes to the MH product mixed with the cement clinker and subsequently sintered, at both 1300˚C and 1400˚C the same phases were identified. These were MgO, C4AF and β -Ca2SiO4, where brownmillerite com- prised the product of the reaction between hercynite decomposition products and CaO. The lime was provided from the C3S and C3A decomposition, present in the Portland clinker. It is also proved by the disappearance of these phases in the after-corrosion sample. 3.2. Phase Evolution and Microstructure Development of the Products after Coating Corrosion with Portla nd Cli nker 3.2.1. Magnesia-Spinel Product (MSp) Base components of MSp product are periclase and spinel MA. This product consists of coarse-grained fraction of magnesia clinker, with low content of Fe2O3 oxide (Table 1) and fused spinel MA, surrounded by magnesia  J. Szczerba et al. matrix (Figure 1). The additive phases of coarser grains of magnesia clinker are calcium silicate C2S and tri- calcium aluminate C3A, or rather their solid solutions. Those phases are dispersed among spinel and periclase grains. In the grains of fused spinel MA and in the magnesia matrix, the aluminate phases exist in the proximity of the latter as a solid solution CA-C6A4MS. In SEM image of the MSp product, after the reaction with the components of Portland clinker at 1450˚C (Figure 2), changes were observed both in the range of matrix as well as in the area of bigger grains of magne- sia clinker and fused spinel MA. In the product MSp, between the periclase crystals, prismatic crystals of Park- er’s phase of C6A4(M,f)S type, crystallized out of liquid phase, which “moistened” them well. The elongated crystals of the C6A4(M,f)S phase were integrated with periclase crystals and spinel MA, creating constant enve- lopes also around them. The determined chemical composition of the Parker’s phase [8], carried out by method of chemical analysis in microarea (EDS), corresponds to the solid solution of the C6A4MS-C6A4fS series. In this phase, oxides of TiO2, K2O and Na2O were also dissolved in small amounts. Between crystals of C6A4(M,f)S, an aluminate-ferrite phase appeared, as a solid solution C2(A,F), enriched with SiO2. In the chemical composition of this phase, oxides of MgO and TiO2 were also detected. This oxides may, together with oxide SiO2 and phase C2(A,F), form the solid solutions [15] [16]. Figure 1. SEM image of the MSp product: spinel MA (1); aluminate phase ss. CA-C6A4MS (2); grain of magnesia clinker (3); black areas are pores. Figure 2. SEM image of the MSp product after corrosion by Portland clinker. Elongated crystals of Parker’s phase C6A4(M,f)S (4) and C2(A,F) phase enriched with MgO and SiO2 (2), filling the areas between MgO crystals (3) and spinel MA (1).  J. Szczerba et al. 3.2.2. Magnesia-Zirconia-Calcia Product (MCZ) The microstructure of MCZ product was characterized by the presence of coarse grains of magnesia clinker, visible in Figure 3 as dark grey areas, and the finer ones of calcium zirconate (exhibited in the SEM image as the light grey areas). The black areas stand for pores. The deposition of Portland clinker formed on the MCZ product (Figure 4) was mainly composed of the hex- agonal crystals of tri-calcium silicate C3S [1] with the admixtures of MgO and Al2O3 oxides that both stabilize the alpha and beta belite phase. The amount of admixtures was detected as being at the maximum level, com- pared to occurring in the industrial clinkers [17]. Additionally, the inclusions of C2S phase in C3S were found in the formed coating. Figure 3. SEM image of the MCZ product, where light grains—CZ, dark grey areas—MgO, light grey areas—C2S, black areas are pores. Figure 4. SEM image of deposition composed of mainly of clinker formed on the MCZ product. The areas between C3S (3), with in- clusions of C2S, are filled up by C3A (1,4) and ss. C2(A,F) (2). 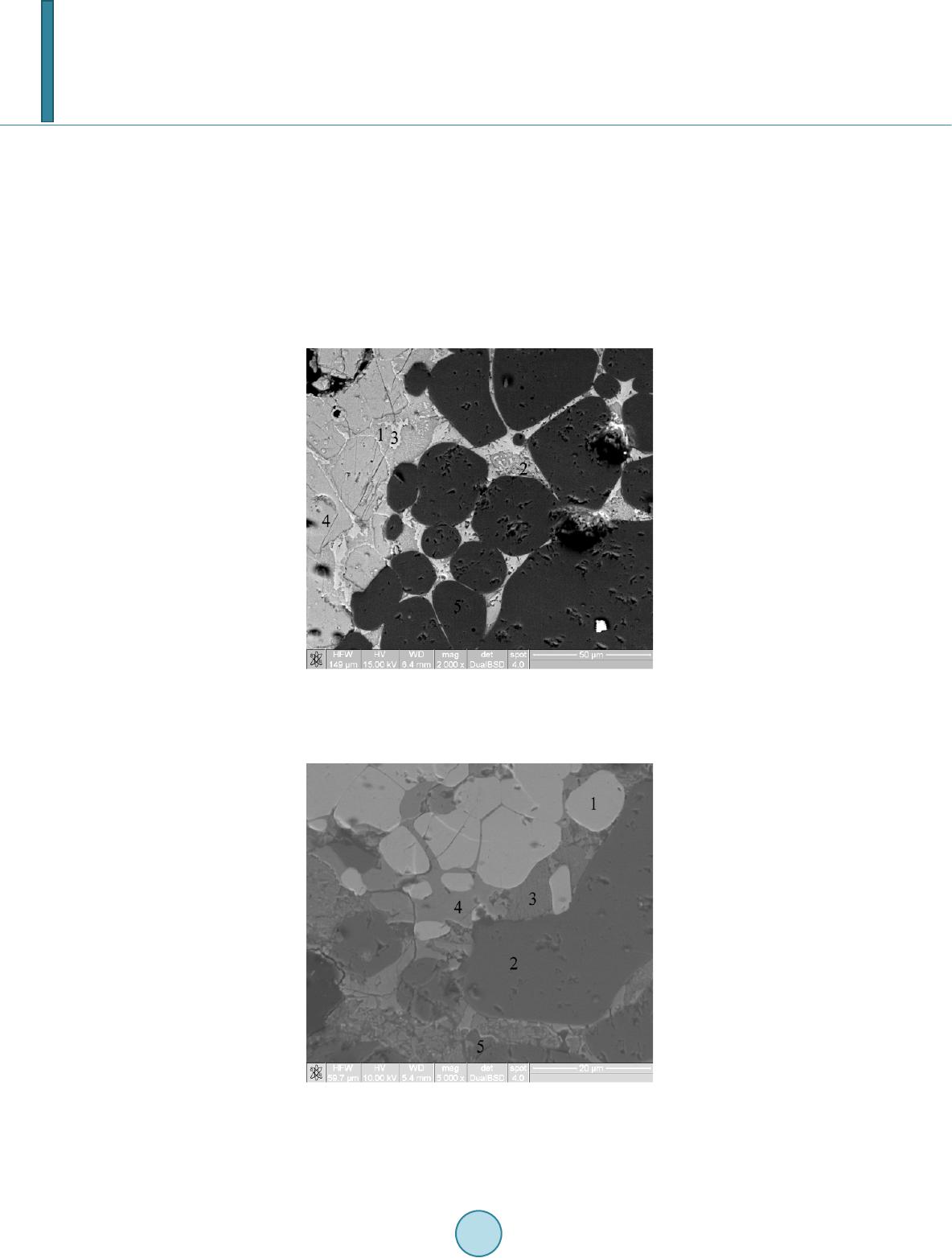 J. Szczerba et al. The areas between alite crystals in the contact zone were filled up by the solid solution of aluminate ferrite phase C2(A,F) (Figure 5, point 3) with the observed inclusions of C3A (Figure 5, points 1 and 2). The admix- tures of other oxides, typical for the industrial clinkers, were also detected in the above mentioned solid solu- tions. These were MgO in C3A, in higher amount when compared to the industrial clinker, as well as oxides SiO2 and Fe2O3, which reach the maximum level of their amount, present in the regular industrial clinker [17] [18]. Additionally, MgO, SiO2 and TiO2 present in C2(A,F) phase occurred in the high amount in comparison to industrial clinker. It was examined that the MCZ product was saturated in the area between MgO and CZ crystals by C3A (Fig- ure 6, point 3) and solid solution C2(A,F) (Figure 6, point 4) with rarely occurring C2S inclusions (Figure 6, point 5). The phases C3A and C2(A,F) contained in their chemical composition the same admixtures as were Figure 5. SEM image of the after-corrosion contact zone of the formed deposition composed of clinker on the MCZ product. Between C3S crystals (4) in the deposition and MgO crystals (5) in the product, the ss. C3A (1,2) as well as C2(A,F) (3) occur. The holes corresponds to pores. Figure 6. SEM image of the MCZ product after corrosion test with cement clinker. MgO (2) and CaZrO3 crystals (1) surrounded by the ss. of C3A (3) and ss. of C2(A,F) (4) enriched with magnesium, silicon and zirconium with the inclusions of belite β -Ca2SiO4 (5).  J. Szczerba et al. present in the area of the deposition, but with double amount of Fe2O3 and low amount of Na2O. On the other hand, C2(A,F) phase, besides the dissolved MgO and SiO2 oxides, possessed also ZrO2. The chemical composi- tion measured by EDS spectroscopy in point 4, indicated in Figure 6, was as follows: MgO—3.44%, Al2O3— 22.53%, SiO2—3.48%, CaO—44.26%, Fe2O3—20.52%, ZrO2—5.77%. In the chemical composition of C2S phase admixtures of oxides like MgO, Al2O3, Na2O and K2O were stated. These oxides possess ability to stabil- ize α, α’ and β -Ca2SiO4 [19] and protect the material against deteriorated phase transition to γ-belite. Moreover, in the belite phase ZrO2 oxide was found, which also enable the stabilization of the α, α’ and β variants of the alite phase [1] [4]. 3.2.3. Magnesia-Hercynite Product (MH) As it can be observed from the cross section of the MH product, depicted in Figure 7, it is mainly composed of the coarse grains of magnesia clinker enriched with ferric oxide. The second component are fused hercynite grains, with their average size in the range of 0.2 mm - 1 mm, which are surrounded by the magnesia matrix. The periclase crystals reach 60 µm in their average size and possess magnesium ferrite inclusions which are visible in the SEM image as small light dots. In addition, the Mg-Fe spinel was found as the phase that sur- rounds the MgO crystals together with di-calcium silicate. The crystals of fused hercynite are also not totally pure in their chemical composition. Besides FeAl2O4 phase, also the solid solutions of (Mg,Fe)(Al, Fe)2O4 and CA2 were detected. Furthermore, solid solution of (Mg,Fe) (Al,Fe)2O4 was found as the phase surrounding the magnesia and hercynite grains along with the silicates as C2S and C3MS2. This (Mg,Fe) (Al,Fe)2O4 solid solution arises from dissolution and mutual partial diffusion of Fe2+/3+ and Mg2+ ions [11]. Hercynite grains (Figure 7, point 1), in MH product, composed of the solid solutions of FeAl2O4 and MAF dispersed in the matrix, underwent dissolution by the cement clinker components. Similarly, magnesioferrite surrounding periclase crystals was also dissolved by clinker. In the image of the after-corrosion MH product (Figure 8) the solid solution C2(A,F) can be observed as light- grey areas between MgO crystals (dark grey areas). This intermediate phase is additionally enriched with mag- nesium and silica oxides. Moreover, crystals of alite C2S can be found, which are enriched with the admixtures such as MgO and Al2O3, that constitute components able to stabilize β - and α ’-C2S [17]. In the larger distance from the contact zone of the MH product the areas between the isometric crystals of MgO are filled up by alu- minate-ferrite phase of C4AF type, that was enriched with SiO2. This phase contains admixtures of MAF solid solution with small amounts of SiO2, CaO as well as the crystals of belite C2S stabilized by Fe2O3, Al2O3 and Na2O. Figure 7. SEM image of the MH product, coarse grain of magne- sia clinker (2) enriched with ferric oxide, grain of fused hercynite (1) located in the matrix of magnesia clinker.  J. Szczerba et al. Figure 8. SEM image of the MH product after corrosion test with Portland clinker in the area close to the formed deposition. 4. Summary and Conclusions Depending on the temperature, following the most important processes occur as a result of reaction between the components of the investigated products and the components of cement furnace charge: -the amount of spinel MA decreases in the MSp product and calcium silicate of C3S and calcium aluminate C3A disappear in Portland clinker; after reaction at 1200˚C calcium aluminate—mayenit C12A7 (with melting point 1413˚C) is formed; after reaction at 1300˚C—the phase C6A4(M, f)S (incongruently melting at 1380˚C) is formed (identified by X-ray analysis as so-called Q phase, expressed by the formula C20A13M3S3), and still ex- ists at 1400˚C, -the magnesia-zirconia-calcia product (MCZ) exhibits the excellent resistance to corrosion by both kiln hot meal and Portland clinker, in the range of temperature from 1200˚C to 1400˚C, what is evidenced by the practi- cally unchanged phase composition after the corrosion test under the above mentioned conditions. The con- ducted investigation showed that alternative materials for magnesia-spinel refractory are magnesia-zirconia- calcia materials, -after coating corrosion test of the magnesia-hercynite product (MH) dissolution of hercynite grains in the magnesia matrix was observed. Hercynite decomposed and products of its decomposition subsequently reacted with calcia, leading to formation of C2(A,F) or C4AF solid solutions, locating in the areas between magnesia grains and filling them very tightly. Taking into consideration kind of the reaction products, the magnesia-hercynite product will demonstrate an excellent ability to formation of the dense and stable protective coating. Acknowledgements The work was financially supported by the Polish State National Centre for Research and Development under Programme INNOTECH-K2/IN2/16/181920/NCBR/13. References [1] Kurdowski, W. (1991) “Chemia cementu”—“Chemistry of Cement” (in Polish), Scientific Publishing PWN, Warszawa, p. 27. [2] Szczerba, J. (2005) “Ceramika”—Ceramics” (in Polish), 88, p. 110. [3] Kühl, H. (1951) “Zement Chemie”—“Chemistry of Cement”. Verlag Technik Gmbh Berlin. [4] Lea, F.M. (1972) The Chemistry of Cement and Concrete. Chemical Publ. Comp. Inc., New York. [5] Bartha, P. (2004) Refractories Manual—Special Edition. InterCeram, 53, 14.  J. Szczerba et al. [6] Szczerba, J. (1997) “Materiały Ogniotrwałe”—“Refractory Materials” (in Polish). 49 [2], p. 59. [7] Ohno, M., Tokunaga, K., Tsuchiya, Y., Mizuno, Y. and Kozuka, H. (2003) Proceedings UNITECR’03, p. 27. [8] Bogue, R.H. (1955) The Chemistry of Portland Cement. Rinhold Publ. Corp., New York. [9] Szczerba, J. (2006) “Materiały Ceramiczne”—“Ceramic Materials” (in Polish), 58 [1], p. 6. [10] Szczerba, J. (2010) Chemical Corrosion of Basic Refractories by Cement Kiln Materials. Ceramics International, 36, 1877-1885. http://dx.doi.org/10.1016/j.ceramint.2010.03.019 [11] Liu, G., Li, N., Yan, W., Tao, G. and Li, Y. (2012) Composition and Structure of a Composite Spinel Made from Magnesia and Hercynite. Journal of Ceramic Processing Research, 13, 480-485. [12] Kapralik, I. and Hanic, F. (1964) Transaction of British Ceramic Society, 79, 128. [13] Turriziani, R. and Massazza, F. (1966) Annali di Chimica, 56, 1180. [14] Polesnig, W. and Zednicek, W. (1984) InterCeram, 33, 49. [15] Woerman, E., Hahn, T. and Ey sel, W. (1965) American Ceramic Society Bulletin, 44, 299. [16] Guinier, A. and Regourd, M. (1968) Proceedings of the 5th ICCC, Tokyo, I, p. 1. [17] Regourd, M. and Guinier, A. (1974) Proceedings of the 6th ICCC, Moscow, I, p. 25. [18] Ghosh, S.N. (1980) Proceedings of the 7th ICCP, Paris, II, II-18. [19] Woerman, E., Hahn, T. and Eysel, W. (1965) American Ceramic Society Bulletin, 44, p. 299.
|