Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 476-480 doi:10.4236/msa.2011.25064 Published Online May 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Sol-Gel Preparation of Nanoscale TiO2/SiO2 Composite for Eliminating of Con Red Azo Dye Abdolreza Nilchi, Simin Janitabar-Darzi, Somayeh Rasouli-Garmarodi Nuclear Science and Technology Research Institute, Tehran, Iran. Email: anilchi@aeoi.org.ir Received May 1st, 2010; revised June 21st, 2010; accepted May 11th, 2011. ABSTRACT A new anatase/SiO2 nanocomposite was synthesized by sol-gel method at room temperature using titanium tetrachlo- ride and tetraethylorthosilicate as raw materials. Characterization of the product was carried out by means of X-ray diffraction (XRD), X-ray fluorescence spectroscopy (XRF), transmission electron microscopy (TEM), Brunauer-Em- mett-Teller (BET) specific surface areas, Thermogravimetry analysis (TGA), Fourier transform infrared (FT-IR), and UV-vis absorption spectroscopy. Thermal phase transformation studies of composite were carried out up to 1100˚C which showed the establishment of anatase TiO2 phase. The presence of some tetrahedral coordination of TiO2 species in SiO2 matrix was confirmed by UV-Vis study. The produced TiO2/SiO2 nanocomposite has good photocatalytic pro- perties due to its anatase phase, existence of tetrahedral coordination of TiO2 in the SiO2 matrix and very large surface area. Furthermore, the synthesized anatase/SiO2 shows significant adsorption ability towards Congo Red (CR) azo dye in comparison with the pure commercial TiO2 which is known as Degussa, P25. Keywords: TiO2/SiO2, Nanocomposites, Sol-Gel Preparation, Photocatalyst, Congo Red 1. Introduction In the area of advanced oxidation technology, titanium dioxide semiconductor photocatalysis has been widely studied because of its potential application in air clean-up and water purification. TiO2 is largely used as photo- catalyst due to its beneficial characteristics: high photo- catalytic efficiency, physical and chemical stability, low cost and low toxicity [1-9]. TiO2/SiO2 composites are very promising in field of heterogeneous photocatalysis, since they could provide simultaneously enhanced photocatalytic and thermal pro- perties compared to pure TiO2 photocatalyst [10-13]. It has been reported that photocatalytic reactivity of TiO2/ SiO2 nanocomposites is highly dependent on the Ti/Si ratios [14-17]. The photocatalytic activity and mechani- cal stability was reported to improve by the addition of about 50% SiO2 [18]. In the present study, anatase/SiO2 nanocomposite was synthesized via sol-gel method at room temperature. The effect of calcination temperature on particles size, BET surface area and phase transformation of anatase to rutile TiO2 were investigated. Moreover, characterization of the coordination sphere of Ti ions incorporated into silica matrix of the composite was studied. These investiga- tions could provide vital information for the design of highly efficient photocatalytic systems in the degradation of toxic compounds diluted in a liquid phase. 2. Experimental 2.1. Chemicals The chemicals used in this study were titanium tetrachlo- ride (TiCl4, 99.9%), Fluka, as a titanium precursor, tetrae- thylorthosilicate (TEOS, 98%), as silica source, Congo Red (C32H22N6Na2O6S2), HNO3 (70 wt%, d = 1.42 g·cm−1), NH4OH (25 wt%), and anhydrous ethanol (C2H5OH) from Merck. 2.2. Preparation of TiO2/SiO2 Composite Titanium tetrachloride was added to distilled water under vigorous stirring in an ice water bath. The produced dis- persion was treated by NH4OH and pH adjusted to 7. The resulting solid was collected by filtration and washed with distilled water. The precipitation was dispersed in 200 mL of 0.3 M HNO3. The mixture was refluxed under vigorous stirring at 70˚C for 16 h as Titania sol was pre- pared. 25 mL of tetraethylorthosilicate was added drop  Sol-Gel Preparation of Nanoscale TiO2/SiO2 Composite for Eliminating of Con Red Azo Dye Copyright © 2011 SciRes. MSA 477 wise to the above sol and stirred at 70˚C. The resulting powder was filtered and washed with distilled water then dried at room temperature. The composite produced was denoted as TSR. In order to study phase transformation of prepared composite, it is calcined for 1 h at 800˚C and 1100˚C and the obtained samples were denoted as TS800 and TS1100, respectively. 2.3. Characterization Phase identification of the products was carried out by X-ray diffraction (XRD) obtained on Philips X-pert dif- fractometer using Cu Kα line radiation. The crystallite size of the samples was determined by Scherrer equation [19]. Thermogravimetry analysis (TGA) was performed using STA 150 Rhenometric Scientific unit. Measure- ment was taken with a heating rate of 10˚C/min from 25 to 800˚C in argon atmosphere. For the composition ana- lysis X-ray fluorescence spectroscopy (XRF) using Ox- ford ED 2000 was employed. Spectroscopic analysis of the nanocomposite was performed using a Fourier trans- form infrared (FT-IR) spectrometer (Perkin-Elmer 843) and UV-vis spectrophotometer (Shimadzu UV 2100). The morphology of the products was studied by trans- mission electron microscopy (TEM, Philips-EM208S electron). The specific surface area of the samples was determined through nitrogen adsorption using a surface area analyzer (CHEMBET3000). 2.4. Photoreactor Photocatalytic activity of the synthesized nanocompo- sites and commercial TiO2 were evaluated by the degra- dation of Congo Red. All of the experiments were con- ducted in an opened Pyrex vessel of 50 ml capacity and in identical conditions. A 30 W UV-C lamp was used as light source. The distance between the UV source and the vessels containing reaction mixture was fixed at 15 cm. Air was continuously bubbled into the solution in order to provide a constant source of dissolved oxygen. 0.025 g of photocatalyst was placed in a 50 mL aqueous solution of 5 ppm Congo Red. Prior to irradiation, the suspension was magnetically stirred in the dark for 30 min. Then the lamp was switched on to initiate the reaction. During irradiation, the suspension was sampled at regular inter- vals and immediately centrifuged to remove catalyst par- ticles. The photocatalytic degradation was monitored by measuring the absorbance of the solution samples with UV-vis spectrophotometer. 3. Results and Discussion 3.1. FT-IR Spectroscopy FT-IR spectrum of the as-synthesized composite (Figure 1) has three characteristic bands that appeared at around Figure 1. FT-IR spectrum of the as-synthesized TiO2/SiO2 nanocomposite (TSR). 1100 950, and 650 cm−1. The bands at around 650 and 1100 cm−1 are representative of TiO2 and SiO2 matrixes in nanocomposite. The band at around 950 cm−1 has been assigned to the stretching of the Si-O− species of Si-O-Ti or Si-O defect sites which are formed by the inclusion of Ti4+ ions into the SiO2 matrixes. Thus, the appearance of the band at around 950 cm−1 indicates that the TiO2 spe- cies are embedded into SiO2 matrixes within TiO2/SiO2 nanocomposite. the broad peak appearing at 3100 - 3600 cm−1 is assigned to the fundamental stretching vibration of hydroxyl groups (free or bonded) which is further confirmed by the weak band at about 1620 cm−1 [20-23]. 3.2. X-Ray Diffr ac ti on Figure 2 shows the XRD patterns for synthesized com- posite and calcined samples. It reveals that as-synthesi- zed TiO2/SiO2 nanocomposite (TSR) has crystalline ana- tase phase in amorphous silica matrix. Both calcined na- nocomposites TS800 and TS1100 have anatase phase TiO2 but in TS1100, amorphous silica transforms to crys- tobalite silica phase. Both the interactions Si-O-Ti and high dispersion of TiO2 in SiO2 prevent the crystalline transition to rutile [24,25]. The sizes of the anatase crys- tallites in the prepared TiO2/SiO2 nanocomposite samples measured according to the Scherrer equation are 5.0, 7.8, and 26.7 nm for RSR, TS800, and TS1100, respectively. Doping of SiO2 into TiO2 could effectively retard the growth of nanoparticles and thus reduce the particle size. This observation may have resulted from the formation of the Ti-O-Si bond and due to the presence of amor- phous SiO2 around TiO2, which would prevent the growth of TiO2 particles [26]. The particle size of TSR and TS800 are close together but at 1100˚C a clear jump in the particle size is shown due to transformation of amorphous silica to crystobalite. 3.3. Thermogravimetric Analysis The inset of Figure 2 shows thermogravimetric curve of TiO2/SiO2 composite (TSR). After the removal of water and organic residue up to 150˚C, no appreciable change 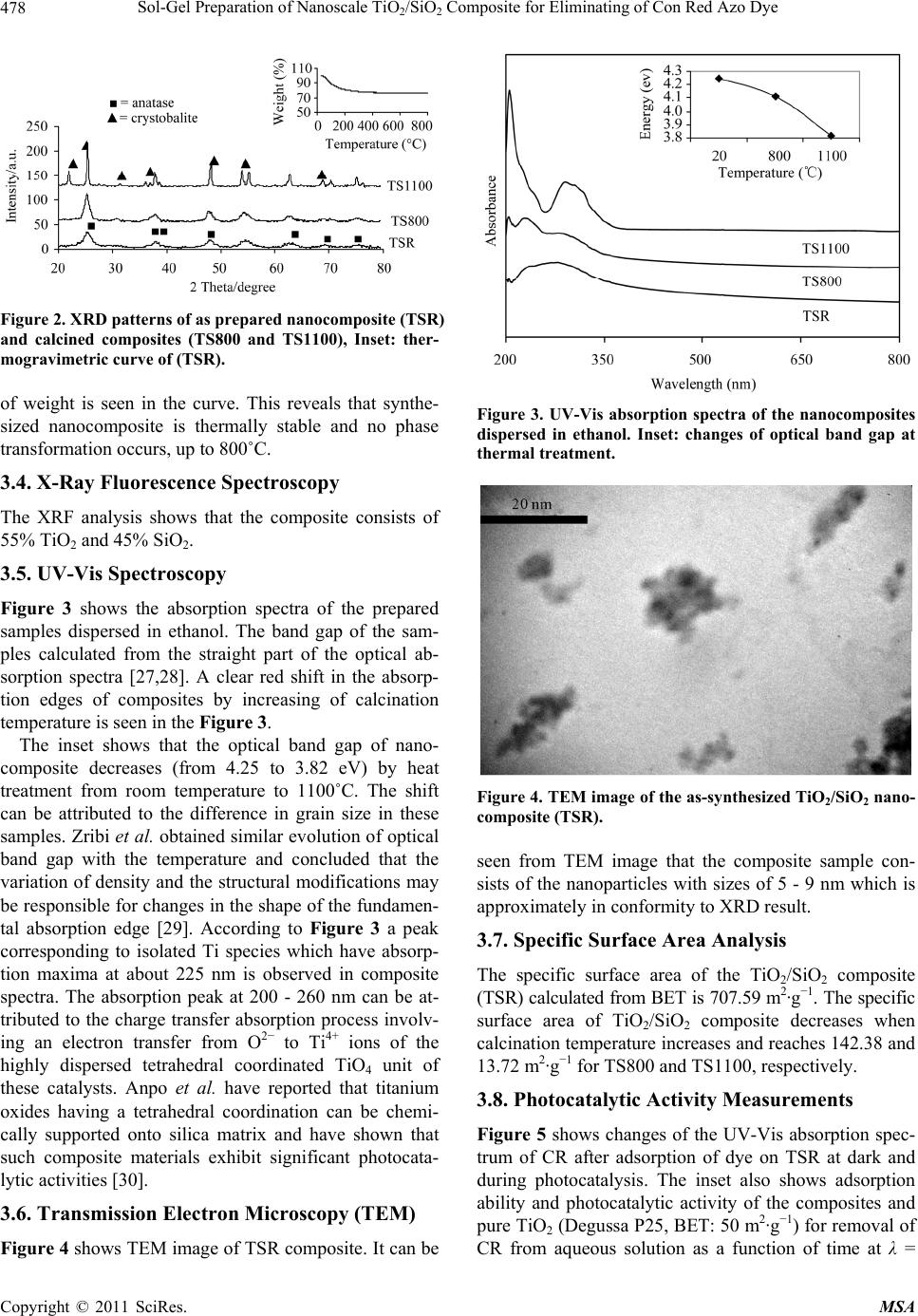 Sol-Gel Preparation of Nanoscale TiO2/SiO2 Composite for Eliminating of Con Red Azo Dye Copyright © 2011 SciRes. MSA 478 Figure 2. XRD patterns of as prepared nanocomposite (TSR) and calcined composites (TS800 and TS1100), Inset: ther- mogravimetric curve of (TSR). of weight is seen in the curve. This reveals that synthe- sized nanocomposite is thermally stable and no phase transformation occurs, up to 800˚C. 3.4. X-Ray Fluorescence Spectroscopy The XRF analysis shows that the composite consists of 55% TiO2 and 45% SiO2. 3.5. UV-Vis Spectroscopy Figure 3 shows the absorption spectra of the prepared samples dispersed in ethanol. The band gap of the sam- ples calculated from the straight part of the optical ab- sorption spectra [27,28]. A clear red shift in the absorp- tion edges of composites by increasing of calcination temperature is seen in the Figure 3. The inset shows that the optical band gap of nano- composite decreases (from 4.25 to 3.82 eV) by heat treatment from room temperature to 1100˚C. The shift can be attributed to the difference in grain size in these samples. Zribi et al. obtained similar evolution of optical band gap with the temperature and concluded that the variation of density and the structural modifications may be responsible for changes in the shape of the fundamen- tal absorption edge [29]. According to Figure 3 a peak corresponding to isolated Ti species which have absorp- tion maxima at about 225 nm is observed in composite spectra. The absorption peak at 200 - 260 nm can be at- tributed to the charge transfer absorption process involv- ing an electron transfer from O2− to Ti4+ ions of the highly dispersed tetrahedral coordinated TiO4 unit of these catalysts. Anpo et al. have reported that titanium oxides having a tetrahedral coordination can be chemi- cally supported onto silica matrix and have shown that such composite materials exhibit significant photocata- lytic activities [30]. 3.6. Transmission Electron Microscopy (TEM) Figure 4 shows TEM image of TSR composite. It can be Figure 3. UV-Vis absorption spectra of the nanocomposites dispersed in ethanol. Inset: changes of optical band gap at thermal treatment. Figure 4. TEM image of the as-synthesized TiO2/SiO2 nano- composite (TSR). seen from TEM image that the composite sample con- sists of the nanoparticles with sizes of 5 - 9 nm which is approximately in conformity to XRD result. 3.7. Specific Surface Area Analysis The specific surface area of the TiO2/SiO2 composite (TSR) calculated from BET is 707.59 m2·g−1. The specific surface area of TiO2/SiO2 composite decreases when calcination temperature increases and reaches 142.38 and 13.72 m2·g−1 for TS800 and TS1100, respectively. 3.8. Photocatalytic Activity Measurements Figure 5 shows changes of the UV-Vis absorption spec- trum of CR after adsorption of dye on TSR at dark and during photocatalysis. The inset also shows adsorption ability and photocatalytic activity of the composites and pure TiO2 (Degussa P25, BET: 50 m2·g−1) for removal of CR from aqueous solution as a function of time at λ =  Sol-Gel Preparation of Nanoscale TiO2/SiO2 Composite for Eliminating of Con Red Azo Dye Copyright © 2011 SciRes. MSA 479 Figure 5. UV-Vis spectrum of CR (5 ppm) using TSR photo- catalyst. Inset: Degree of decolorization by various photo- catalysts. 497 nm. The efficiency or degree of photodegradation (X) is given by: X = (C0 − C)/C0, where C0 is the initial con- centration of dye, and C the concentration of dye at time T. CR dye is strongly adsorbed on the surface of TSR so that more than 98% of dye decolorization performed af- ter 30 min adsorption in dark. Percent of decolorization due to sorption on the surface of Degussa, TS800 and TS1100 are obtained to be 16.7%, 22.3% and 3.5%, re- spectively. These results are in a good conformity to the BET surface area of the samples. It reveals that the as-prepared composite is the most effective sorbent and photocatalyst. The samples calcined at 800˚C and 1100˚C are weaker photocatalyst than the commercial P25. Finally, it can be deduced from the re- sults obtained that the well crystallized mixed crystalline structure (75% anatase and 25% rutile) of P25 would be responsible for the photocatalysis superiority in com- parison with the calcined nanocomposites, although the sample calcined at 800˚C has larger surface area. 4. Conclusion TiO2/SiO2 nanocomposite was synthesized via sol-gel process at room temperature. Formation of the Ti-O-Si bond and amorphous SiO2 in TiO2/SiO2 could effectively increase the stability of anatase TiO2, limit the growth of crystallites, and increase the surface area. Significantly, such an increase in the surface area and the existence of tetrahedrally coordinated TiO2, improves the photocata- lytic activities of the TiO2/SiO2 ceramic. REFERENCES [1] C. M. Visinescu, R. Sanjines, F. Levy, V. Marcuc and V. I. Parvulescu, “Tantalum Doped Titania Photocatalysts: Preparation by dc Reactive Sputtering and Catalytic Be- havior,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 174, No. 2, August 2005, pp. 106-110. doi:10.1016/j.jphotochem.2005.03.017 [2] M. H. Habibi, S. Tangestaninejad and B. Yadollahi, “Photocatalytic Mineralization of Mercaptans as Environ- mental Pollutants in Aquatic System Using TiO2 Suspen- sion,” Applied Catalysis. B: Environment, Vol. 33, No. 1, September 2001, pp. 57-61. doi:10.1016/S0926-3373(01)00158-8 [3] D. G. Shchukin and D. V. Sviridov, “Photocatalytic Processes in Spatially Confined Micro- and Nanoreac- tors,” Journal of Photochemistry and Photobiology A: Chemistry Review, Vol. 7, 2006, pp. 23-39. [4] R. Zhang, L. Gao and Q. Zhang, “Photodegradation of Surfactants on the Nanosized TiO2 Prepared by Hydroly- sis of the Alkoxide Titanium,” Chemosphere, Vol. 54, No. 3, January 2004, pp. 405-411. doi:10.1016/S0045-6535(03)00588-5 [5] K. Patterson, B. W. Lykins, J. C. Ireland, S. D. Richard- son, A. D. Thruston and T. W. Collett, “Identification of TiO2/UV Disinfection Byproducts in Drinking Water,” Environmental Science and Technology, Vol. 30, No. 11, 1996, pp. 3327-3334. doi:10.1021/es960142m [6] P. S. Awati, S. V. Awate, P. P. Shah and V. Ramaswamy, “Photocatalytic Decomposition of Methylene Blue Using Nanocrystalline Anatase Titania Prepared by Ultrasonic Technique,” Catalysis. Communications, Vol. 4, No. 8, August 2003, pp. 393-400. doi:10.1016/S1566-7367(03)00092-X [7] D. Beydoun, R. Amal, G. Low and S. McEvoy, “Role of Nanoparticles in Photocatalysis,” Journal of Nanoparticle Research, Vol. 1, 1999, pp. 439-458. doi:10.1023/A:1010044830871 [8] M. R. Prairie, L. R. Evans, B. M. Stange and S. L. Mar- linez, “An Investigation of TiO2 Photocatalysis for the Treatment of Water Contaminated with Metals and Or- ganic Chemicals,” Environmental Science and Techno- logy, Vol. 9, 1993, p. 27. [9] P. Chenga, C. Denga, M. Gub and A. X. Dai, “Effect of Urea on the Photoactivity of Titania Powder Prepared by Sol-Gel Method,” Materials Chemistry and Physics, Vol. 107, No. 1, January 2008, pp. 77-81. doi:10.1016/j.matchemphys.2007.06.051 [10] G. Calleja, D. P. Serrano, R. Sanz and P. Pizarro, “Me- sostructured SiO2-Doped TiO2 with Enhanced Thermal Stability Prepared by a Soft-Templating Sol-Gel Route,” Microporous and Mesopororous Materials, Vol. 111, 2008, p. 429. [11] C. Garzella, E. Comini, E. Bontempil, L. E. Deperol, C. Frigeri and G. Sberveglieri, “Nanostructured TiO2 and W: TiO2 Thin Films by a Novel Sol-Gel Processing for Al- cohol Sensing Devices,” Materials Research Society Symposium Proceedings, Vol. 638, 2001, p. 111. [12] Z. Wang, U. Helmersson and P. Kall, “Optical Properties of Anatase TiO2 Thin Films Prepared by Aqueous Sol- Gel Process at Low Temperature.” Thin Solid Films, Vol. 405, No. 1-2, February 2002, pp. 50-54. doi:10.1016/S0040-6090(01)01767-9 [13] T. Ivanova, A. Harizanova and M. Surtchev, “Formation and Investigation of Sol-Gel TiO2-V2O5 System,” Materi- als Letters, Vol. 55, 2002, pp. 327-333. [14] S. Dohshi, M. Takeuchi and M. Anpo, “Effect of the Lo-  Sol-Gel Preparation of Nanoscale TiO2/SiO2 Composite for Eliminating of Con Red Azo Dye Copyright © 2011 SciRes. MSA 480 cal Structure of Ti-Oxide Species on the Photocatalytic Reactivity and Photo-Induced Super-Hydrophilic Proper- ties of Ti/Si and Ti/B Binary Oxide Thin Films,” Cataly- sis Today, Vol. 85, No. 2-4, October 2003, pp. 199-205. doi:10.1016/S0920-5861(03)00387-0 [15] R. R. Gonçalves, Y. Messaddeq, M. Atik and S. J. L. Ribeiro, “Optical Properties of ZrO2, SiO2 and TiO2-SiO2 Xero gels and Coatings Doped with Eu3+ and Eu2+,” Jour- nal of Material Research, Vol. 2, No. 1, January 1999, pp. 11-15. [16] T. Nishide, M. Sato and H. Hara, “Crystal Structure and Optical Property of TiO2 Gels and Films Prepared from Ti-Edta Complexes as Titania Precursors,” Journal of Ma- terial Science, Vol. 35, 2000, pp. 465-469. [17] S. Cabrera, J. El Haskouri, A. Beltra’n-Porter, D. Bel- tra’n-Porter, M. D. Marcos and P. Amoro’s, “En- hanced Surface Area in Thermally Stable Pure Mesoporous TiO2,” Solid State Science, Vol. 2, No. 5, September 2000, pp. 513-518. doi:10.1016/S1293-2558(00)00057-1 [18] L. Zhou, S. Yan, B. Tian, J. Zhang and M. Anpo, “Pre- paration of TiO2-SiO2 Film with High Photocatalytic Ac- tivity on PET Substrate,” Materials Letters, Vol. 60, No. 3, February 2006, pp. 396-402. doi:10.1016/j.matlet.2005.08.065 [19] A. L. Patterson, “The Sherrer Formula for X-Ray Particle Size Determination,” Physical Review, Vol. 56, 1939, pp. 978-983. doi:10.1103/PhysRev.56.978 [20] Y. Gao, Y. Masuda, Z. Peng, T. Yonezawa and K. Kou- moto, “Light-Excited Super Hydrophilicity of Amorph- ous TiO2 Thin Films Deposited in an Aqueous Peroxoti- tanate Solution,” Journal of Material Chemistry, Vol. 13, 2003, pp. 608-613. doi:10.1039/b208105a [21] G. Calleja, D. P. Serrano, R. Sanz, P. Pizarro and A. Garcı´a, “Study on the Synthesis of High-Surface-Area Mesoporous TiO2 in the Presence of Nonionic Surfac- tants,” Indian Engineering Chemical Research, Vol. 43, No. 10, 2004, pp. 2485-2492. doi:10.1021/ie030646a [22] S. J. Kalita, S. Qiu and S. Verma, “A Quantitative Study of the Calcination and Sintering of Nanocrystalline Tita- nium Dioxide and Its Flexural Strength Properties,” Ma- terials Chemistry and Physics, Vol. 109, No. 2-3, June 2008, pp. 392-398. doi:10.1016/j.matchemphys.2007.12.031 [23] S. J. Kim, S. D. Park, C. K. Rhee, W. W. Kim and S. Park, “Photocatalytic Characteristics of Homogeneously pre- cipitated TiO2 Nano-Sized Powders,” Scripta Materialia, Vo. 44, 2001, pp. 1229-1233. doi:10.1016/S1359-6462(01)00854-5 [24] J. Aguado, M. Grieken, J. Lopez-Munoz and J. Marugan, “A Comprehensive Study of the Synthesis, Characteriza- tion and Activity of TiO2 and Mixed TiO2/SiO2,” .Applied Catalysis A: General, Vol. 312, September 2006, pp. 202- 210. doi:10.1016/j.apcata.2006.07.003 [25] K. Kabra, R. Chaudhary and R. L. Sawhney, “Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase,” Photocatalysis: A Review Indian Engi- neering Chemical Research, Vol. 43, 2004, pp. 7683- 7696. [26] M. Machida, K. Norimoto, T. Watanabe, K. Hashimoto and A. Fujishima, “The Effect of SiO2 Addition in Su- per-Hydophylic Property of TiO2 Photocatalyst,” Journal of Material Science, Vol. 34, 1999, pp. 2569-2574. doi:10.1023/A:1004644514653 [27] M. N. Kamalasanan and S. Chandra, “Sol-Gel Synthesis of ZnO Thin Films,” Thin Solid Films, Vol. 288, No. 1-2, November 1996, pp. 112-118. doi:10.1016/S0040-6090(96)08864-5 [28] P. Wang, S. M. Zakeeruddin, I. Exnarb and M Grätzel, “High Efficiency Dye-Sensitized Nanocrystalline Solar cells Based on Ionic Liquid Polymer Gel Electrolyte,” Chemical Communications, Vol. 111, 2002, pp. 2972- 2973. doi:10.1039/b209322g [29] M. Zribi, M. Kanzari and B. Rezig, “Structural, Morpho- logical and Optical Properties of Thermal Annealed TiO Thin Films,” Thin Solid Films, Vol. 516, 2008, pp. 1476-1482. doi:10.1016/j.tsf.2007.07.195 [30] H. Yamashita, Y. Ichihashi, M. Harada, M. Stewart, A. Fox and M. Anpo, “Photocatalytic Degradation of 1-Oc- tanol on Anchored Titanium Oxide and on TiO2 Powder Catalysts,” Journal of Catalysis, Vol. 158, 1996, pp. 97- 103. doi:10.1006/jcat.1996.0010 |

