Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 469-475 doi:10.4236/msa.2011.25063 Published Online May 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 469 Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions El Said Gouda1,2, Emad Ahmed1, NabihTawfik1 1Solid State Physics Department, Physics Division, National Research Center, Dokki, Giza, Egypt; 2Physics Department, Faculty of Science, Jazan University, Jazan, Saudi Arabia. Email: gouda.el73@yahoo.com Received May 1st, 2010; revised July 22nd, 2010; accepted May 11th, 2011. ABSTRACT In the present paper, the effect of Mg and Cu additions on melting, mechanical and corrosion behavior of Al-9Zn alloy was studied and analyzed. It was found that, addition of Cu and Mg led to form the Al-Cu, Al-Mg and Mg-Zn interme- tallic compounds. These compounds tend to increase the hardness values due to the precipitation strengthening har- dening mechanism of these compounds. Also corrosion behavior of the Cu and Mg content alloys measured by the weight loss method is better than those of the Al-9Zn alloy. Furthermore, corrosion resistance of the Cu content alloys is superior to that of the Mg content alloys. Keywords: Corrosion, Hardness, Melting Point, Aerospace Alloys 1. Introduction The study of the material strength is an important subject because it is the first characteristic comes in mind when used in industrial applications specially that subjected to shock loading. Steel is a good example for the most strength materials, but its high density restricts its uses. Recently, aluminum alloys are increasingly employed in many important manufacturing areas, such as the auto- mobile industry, aeronautics and the military [1]. Cur- rently, it offers the greatest potential for cost effective weight savings in automotive body structures and clo- sures. With a density of only 33% of that of steel and a greater strength-to-weight ratio, there is the possibility for a weight savings of 40% to 50%. Also, Mg alloys are very attractive materials for producing lightweight com- ponents for automobiles because they have densities that are 66% of Al alloys and 22% of steel. With their lower density and moderate strength, Al-Mg alloys are well suited for a number of applications, ranging from steer- ing wheels and instrument panels to engine and transmis- sion components. The mechanical properties of the Al- Mg plastically processed alloys depend on the content of magnesium in the alloy. With an increase of magnesium from 0.5% to 5% the properties increase; this rise is greater when magnesium increases from 3% to 6% [2]. There are many studies characterize the strength and mechanical properties of Al-based and Mg-based alloys with different elements [3-8]. The present paper aims to characterize corrosion and mechanical properties of the quaternary alloy Al-1.5Cu-9.5Zn-3Mg as an example for a high strength material. 2. Experimental Procedures Four alloys of chemical composition as illustrated in Ta- ble 1 were prepared in the as-cast condition. The re- quired quantities were weighted out and melted in an electrical furnace then thermally agitated to ensure the homogenization. The molten alloys were cast into gra- phite molds to produce rods of 25 mm length and 10 mm diameter. Then the rods were cut in to small discs of about 1 cm length. X-ray diffraction analysis was per- formed to identify the phases presented in these alloys using a 1390 Philips X-ray Diffractometer with Cu-ra- diation. DSC tests were carried out during heating using a heating rate of 10˚C/min to evaluate the melting beha- vior of these alloys. Corrosion behavior of these alloys was determined chemically by the weight loss method. The test pieces were cut into 1 × 1 cm and mechanically polished with emery paper and weighted. Then the sam- ples were suspended in a test solution of 1 mole of HCl with doubly distilled water. The measurements were car- ried out after suspension time of 15, 30, 45, 60 and 75 min. The average weight loss at the specific time was  Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions Copyright © 2011 SciRes. MSA 470 Table 1. Chemical composition of the used alloys. Sample Chemical composition alloy 1 Al-9Zn alloy 2 Al-9Zn-1.5Cu alloy 3 Al-9Zn-3Mg alloy 4 Al-9Zn-1.5Cu-3Mg calculated. Measurements of hardness were done using Vickers hardness tester with the test samples placed against a glass slide with a constant load of 4.9 N for 10 sec. 3. Results and Discussion 3.1. Phases Constitution Figure 1 shows the X-ray diffraction patterns of the Al- 9Zn, Al-9Zn-1.5Cu, Al-9Zn-3Mg, Al-9Zn-1.5Cu-3Mg alloys. All diffraction patterns contain peaks due to Al matrix phase. Accordingly, the Al-9Zn alloy shows the presence of only the Al phase, suggesting that all the Zn content has gone in to Al to form supersaturated solid solution. This is inferred from the shift of Al peaks to higher angular positions and consequently the Al solid solution phase had smaller lattice parameters than that of pure Al. The pattern of the Al-9Zn-1.5Cu alloy indicates two X-ray diffraction peaks at 2θ ≈ 43.25 and 43.96˚ refer to the AlCu4 and Al4Cu9 compounds, respectively. The pattern of the Al-9Zn-3Mg alloy indicates precipita- tion of Al2Mg compound at 2θ ≈ 36.9, 39.13 and 43.26˚, and MgZn2 compound at 2θ ≈ 45.18˚. This means adding of Mg decrease the solubility of Zn in Al matrix phase by forming the MgZn2 compound. The quaternary Al-9Zn- 1.5Cu-3Mg alloy indicates precipitations of Al12Mg17, MgZn2, Al2Mg and Al4Cu9 compounds as illustrated in Table 2. Increasing the amount of the intermetallic compound MgZn2 indicates that, the solubility of Zn in Al-matrix was decreased. 3.2. Melting Behavior DSC endothermic peaks of the Al-9Zn, Al-9Zn-1.5Cu, Al-9Zn-3Mg, Al-9Zn-1.5Cu-3Mg alloys during conti- nuous heating are demonstrated in Figure 2. It shows that, the DSC curve is not discernible exothermic/endo- thermic peaks occurred until the remelting temperature range was reached. In alloy 1 there is a large endothermic peak at 657.7˚C, corresponds to the melting reaction of the binary Al-9Zn alloy. DSC curve of alloy 2 consists of three endothermic peaks, as labeled 1, 2 and 3, respective- ly. Peak 1 is the smallest one with an onset temperature of about 532.7˚C followed by the second peak. Peak 2 ap- peared at 640.3˚C, while the third peak, peak 3 started at Table 2. Intermetallic compounds presented in Al-Zn-Cu- Mg alloys. Alloy Lattice parameter, a of Al matrix phase, Å Intermetallic compound 2θ degree alloy 14.050 - - alloy 24.044 AlCu4 Al4Cu9 43.25 43.96 alloy 34.063 Al2Mg MgZn2 36.95 39.13 43.26 45.18 alloy 44.059 Al2Mg Al12Mg17 MgZn2 Al4Cu9 37.33 40.52 39.66 65.15 41.25 45.73 43.75 677.5˚C and a peak temperature of about 697.8˚C. DSC curve of alloy 3 indicates a single endothermic peak due to the melting reaction observed at 643.44˚C. While, the DSC curve of alloy 4 indicates two endothermic sharp peaks, the first, which is the smallest one is observed at a temperature of about 480.5˚C, which may corresponding to the diffusion mechanism of low melting Al12Mg17 compound formed at this alloy composition. While, the second peak which is the largest one observed at a tem- perature of about 634.7˚C is related to the melting reac- tion of this alloy. 3.3. Hardness In solid mechanics, the material hardness is described as the resistance of deformation and should be independent from the applied load. So, it was measured using a fixed load of 4.9 N for 10 sec and the results are illustrated in Figure 3. It was observed that, the hardness with the corresponding values of the yield strength [9] is in- creased continually with the Cu or Mg content alloys. The maximum value obtained for alloy 4, can be attri- buted to the more precipitations of Al-Cu, Al-Mg and Mg-Zn IMCs, which act as hard inclusions in soft matrix. The continuous increase of Hv can be attributed to the precipitation hardening strengthening mechanisms of Al-Mg and Al-Cu and Mg-Zn compounds. 3.4. Corrosion Behavior Corrosion behavior of metals in an aqueous environment is characterized by the extent to which it dissolves in the solution. This can be quantified using the relationship W0= WB − WA, W0; weight lost in test solution, WB; weight before exposure, WA; weight after exposure. Fig- ure 4 shows weight loss of the samples versus the expo- sure time in min. It shows that, the weight loss increases as the exposure time increases by different amounts de- 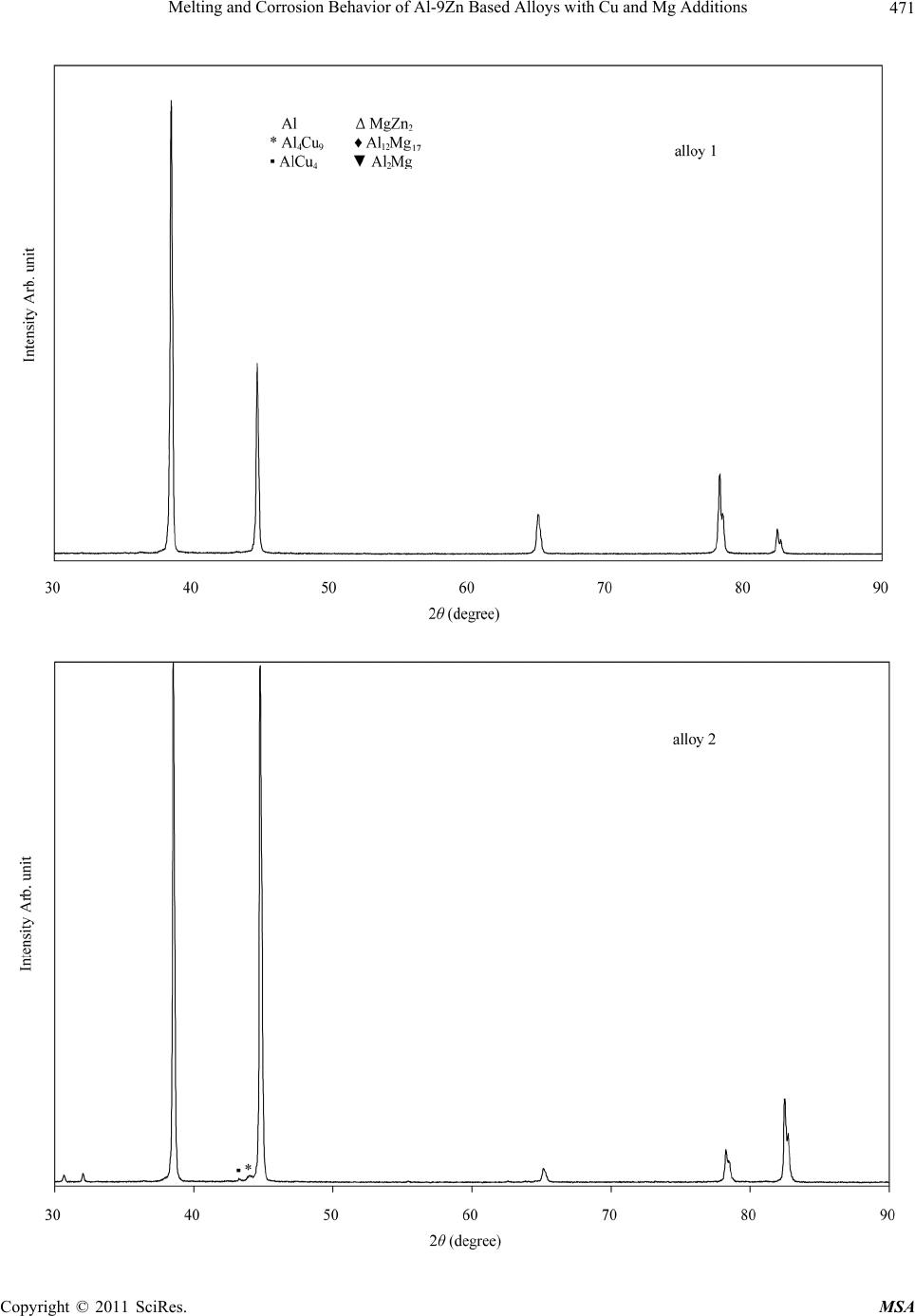 Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions Copyright © 2011 SciRes. MSA 471 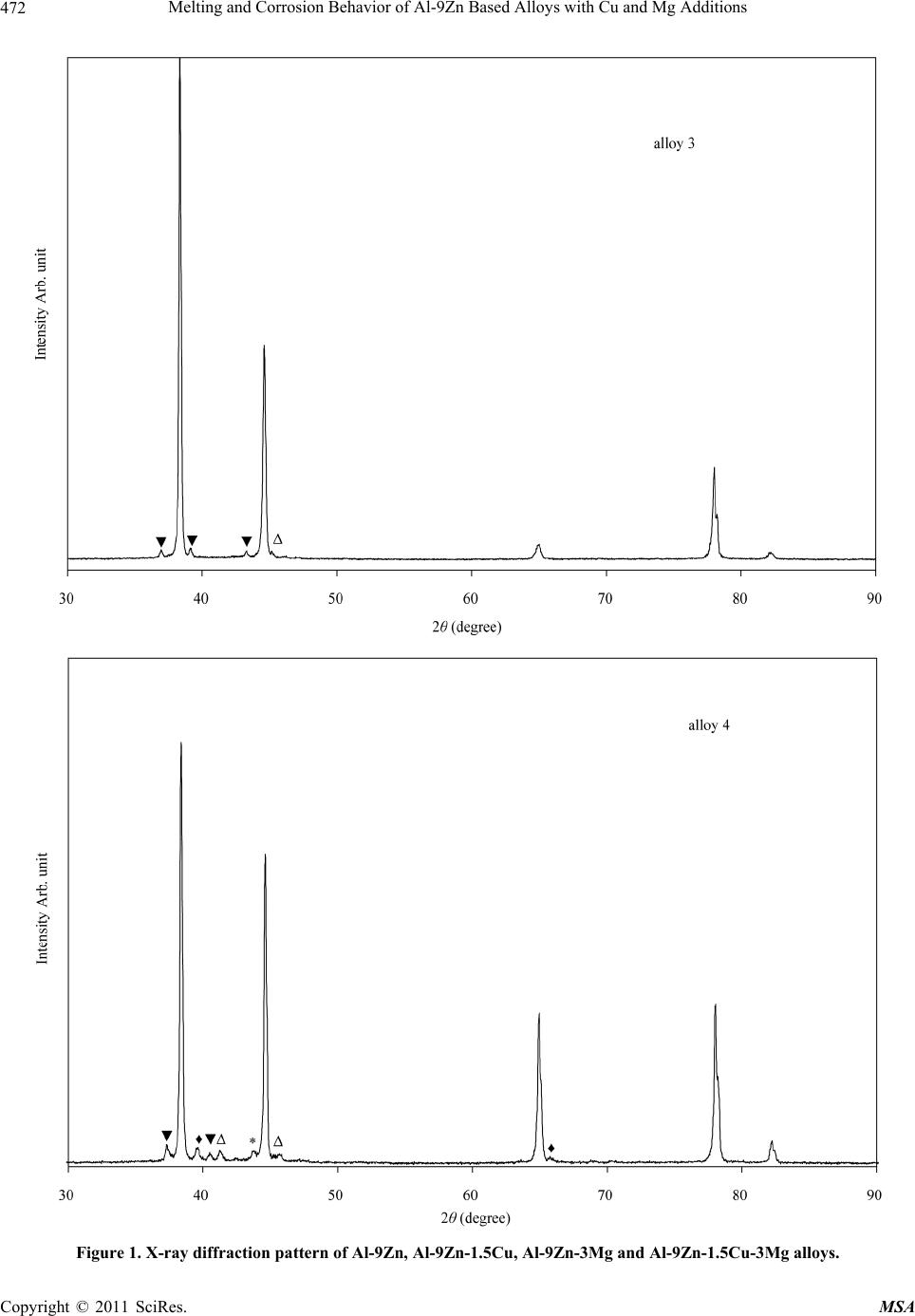 Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions Copyright © 2011 SciRes. MSA 472 Figure 1. X-ray diffraction pattern of Al-9Zn, Al-9Zn-1.5Cu, Al-9Zn-3Mg and Al-9Zn-1.5Cu-3Mg alloys.  Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions Copyright © 2011 SciRes. MSA 473 Figure 2. DSC endothermic peak of Al-9Zn, Al-9Zn-1.5Cu, Al-9Zn- 3Mg and Al-9Zn-1.5Cu-3Mg alloys. Figure 3. Hardness with the corresponding values of the yield strength of Al-Zn-Cu-Mg alloys. 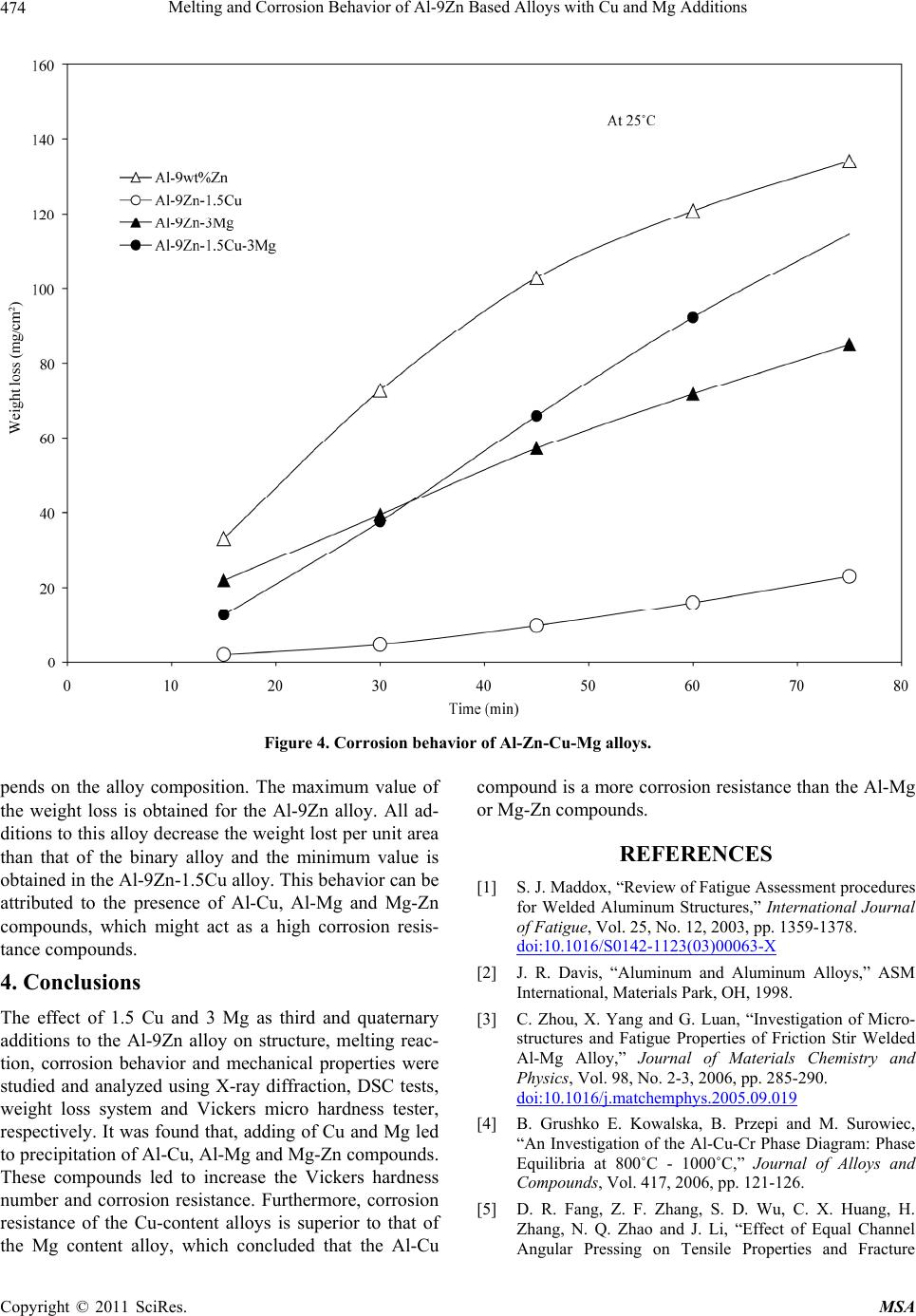 Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions Copyright © 2011 SciRes. MSA 474 Figure 4. Corrosion behavior of Al-Zn-Cu-Mg alloys. pends on the alloy composition. The maximum value of the weight loss is obtained for the Al-9Zn alloy. All ad- ditions to this alloy decrease the weight lost per unit area than that of the binary alloy and the minimum value is obtained in the Al-9Zn-1.5Cu alloy. This behavior can be attributed to the presence of Al-Cu, Al-Mg and Mg-Zn compounds, which might act as a high corrosion resis- tance compounds. 4. Conclusions The effect of 1.5 Cu and 3 Mg as third and quaternary additions to the Al-9Zn alloy on structure, melting reac- tion, corrosion behavior and mechanical properties were studied and analyzed using X-ray diffraction, DSC tests, weight loss system and Vickers micro hardness tester, respectively. It was found that, adding of Cu and Mg led to precipitation of Al-Cu, Al-Mg and Mg-Zn compounds. These compounds led to increase the Vickers hardness number and corrosion resistance. Furthermore, corrosion resistance of the Cu-content alloys is superior to that of the Mg content alloy, which concluded that the Al-Cu compound is a more corrosion resistance than the Al-Mg or Mg-Zn compounds. REFERENCES [1] S. J. Maddox, “Review of Fatigue Assessment procedures for Welded Aluminum Structures,” International Journal of Fatigue, Vol. 25, No. 12, 2003, pp. 1359-1378. doi:10.1016/S0142-1123(03)00063-X [2] J. R. Davis, “Aluminum and Aluminum Alloys,” ASM International, Materials Park, OH, 1998. [3] C. Zhou, X. Yang and G. Luan, “Investigation of Micro- structures and Fatigue Properties of Friction Stir Welded Al-Mg Alloy,” Journal of Materials Chemistry and Physics, Vol. 98, No. 2-3, 2006, pp. 285-290. doi:10.1016/j.matchemphys.2005.09.019 [4] B. Grushko E. Kowalska, B. Przepi and M. Surowiec, “An Investigation of the Al-Cu-Cr Phase Diagram: Phase Equilibria at 800˚C - 1000˚C,” Journal of Alloys and Compounds, Vol. 417, 2006, pp. 121-126. [5] D. R. Fang, Z. F. Zhang, S. D. Wu, C. X. Huang, H. Zhang, N. Q. Zhao and J. Li, “Effect of Equal Channel Angular Pressing on Tensile Properties and Fracture  Melting and Corrosion Behavior of Al-9Zn Based Alloys with Cu and Mg Additions Copyright © 2011 SciRes. MSA 475 Modes of Casting Al-Cu Alloys,” Materials Science and Engineering A, Vol. 426, No. 1-2, June 2006, pp. 305-313. doi:10.1016/j.msea.2006.04.044 [6] B. B. Straumal, B. Baretzky, A. A. Mazilkin, F. Phillipp, O.A. Kogtenkova, M. N. Volkov and R. Z. Valiev, “For- mation of Nanograined Structure and Decomposition of Supersaturated Solid Solution during High Pressure Tor- sion of Al-Zn and Al-Mg Alloys,” Acta Materialia, Vol. 52, No. 15, September 2004, pp. 4469-4478. doi:10.1016/j.actamat.2004.06.006 [7] A. Białobrzeski and E. Czekaj, “An Attempt to Use Alloy Synthesis in Evaluating the Corrosion Behaviour of Aland Mg-Based Alloys”, Journal of Materials Process- ing Technology, Vol. 175, No. 1-3, June 2006, pp. 27-32. doi:10.1016/j.jmatprotec.2005.04.028 [8] E. A. Hady, N. Tawfik and E.S. Gouda, “Mechanical Properties of Some Al-Based alloys with Heat Treat- ment,” Journal of Heat Treatment and Surface Engineer- ing, Vol. 8, No. 1-2, 2007, pp. 37-49. [9] M. Kamal and El Said Gouda, “New Lead-Containing Solder Alloy with Improved Properties,” Radiation Ef- fects and Defects in Solids, Vol. 161, No. 8, 2006, pp. 461-466. |

