Paper Menu >>
Journal Menu >>
 Natural Science, 2009, 1, 23-29 NS http://dx.doi.org/10.4236/ns.2009.11005 Copyright © 2009 SciRes. OPEN ACCESS Optimization of solvent extraction conditions for total carotenoids in rapeseed using response surface methodology Ling Wang1, Yun Liu1,* 1College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, P. R. China. Email: *liuyunprivate@sina.com Received 10 May 2009; revised 16 May 2009; accepted 19 May 2009. ABSTRACT The optimum total carotenoids (TC) extraction from rapeseed with solvent extraction method by UV-visible spectrophotometer determination was investigated by using response surface methodology (RSM). Extraction duration, re- peated extraction cycles, solvent-solid ratio and extraction temperature were assumed to be the most important factors affecting solvent extrac- tion for the determination of TC. Optimum sol- vent extraction conditions for maximizing the determination of TC were: extraction duration 7.3h, repeated extraction three times, ratio of solvent-solid (v/w, mL/mg) 29:1, extraction temperature 42°C. Under the optimal conditions, the yield of TC was up to 4.79 mg /100g. The model had a satisfactory coefficient of R2 (= 0.912) and verified experimentally. The results showed that the conditions were mild and use- ful for maximizing a quantitative spectropho- tometer determination of TC in rapeseed. Keywords: Carotenoids; Optimization; Solvent Ex- traction; Response Surface Methodology; Rape- seed 1. INTRODUCTION Carotenoids are a group of phytochemical bioactive compounds that are responsible for different colors of various plants and microorganisms but not animals [1]. It has been found that carotenoids can play an important role in the prevention of various types of cancer as well as other important ‘‘lifestyle- related’’ diseases, such as cardiovascular disease and age-related macular degen- eration due to their antioxidant activity [2,3,4]. In addi- tion to being potent antioxidants some carotenoids also contribute to provitamin A function [5]. Although the chemistry properties of carotenoids have been exten- sively studied their bioavailability, metabolism and bio- logical functions are only investigated recently [6,7]. In recent years the antioxidant properties of carotenoids have become the major focuses for researches, particu- larly focused on the role of lycopene in human health [8,9]. About 90% of the carotenoids in the diet and hu- man body are represented by β-carotene, α-carotene, lycopene, lutein and cryptoxanthin [10]. As the increasing of health-conscious and the demand for carotenoids, researchers shifted their attentions from chemical synthesis to natural products isolated from plants and microorganisms biological sources [11,12]. Rapeseed containing around 40% oil is one of the most important vegetable oil materials in the world. The total production of rapeseed plant all over the world was 46.2 Mt in 2005 [13]. Rapeseed contains rich carotenoids, such as β-carotein, α-carotein and lutein, which can con- tribute to prolong the rapeseed oil shelf life and increase oil nutrition [14,15]. Many effective methods for caro- tenoids extraction from biological sources have been intensively employed, such as solvent extraction, solid phase extraction (SPE) and supercritical fluid extraction (SFE) [12,16,17]. Above these methods, solvent extrac- tion method is universally application for extraction total carotenoids (TC) because solvent extraction method is relative simple and low cost [18,19,20,21]. However, the extraction of TC from rapeseed was rarely reported so far. The most commonly used techniques for TC detection are UV-visible spectrophotometry, mass spectrometry and hydrogen or carbon nuclear magnetic resonance spectroscopy (1H-NMR, 13C-NMR), coupled or not with chromatographic techniques [22,23]. Regardless of the technique utilized, carotenoid extraction is highly influ- enced by procedural variables such as type of sample, type and ratio of solvents, extraction duration, repeated extraction times, storage conditions, etc. Therefore, the objective of this work was to establish a solvent extrac- tion method for a quantitative spectrophotometer deter- mination of TC. Rapeseed was selected as representative due to its abundance all over the world. Response sur-  24 L. Wang et al. / Natural Science 1 (2009) 23-29 Copyright © 2009 SciRes. OPEN ACCESS face methodology (RSM) was employed to optimize the extraction conditions, which could maximize the deter- mination of TC in rapeseed. 2. MATERIALS AND METHODS 2.1 Materials Rapeseed (Brassica napus L.) was provided by Rapeseed Engineering Center of Huazhong University of Agricul- ture. The raw material consisted of moisture 2.9 ± 0.05%, kernel 73.4 ± 0.26%, and seed capsule 26.6 ± 0.02%. Hexamethylene, petroleum ether, chloroform, acetone and methanol were of analytic grade. 2.2 Experimental Design Six extraction solvents of hexamethylene, petroleum ether, chloroform, acetone, methanol and mixture of petroleum ether and acetone were tested to select the most optimum extraction solution for TC extraction. 3-5 g rapeseed was grinded into powder in a glass mortar and screened with 100 mesh sieve. Portions of ground rapeseed powder of 50mg (dry weight) were transferred to 40mL beakers added with 5mL extraction solvent and wrapped with aluminum foil. The samples were constantly agitated (Si- satom magnetic agitator) according to the extraction dura- tion, protected from light at room temperature (23°C). In the preliminary study, variables affecting TC extraction were solid-solvent ratio, extraction duration, extraction repeated cycles and extraction temperature. Response surface methodology (RSM) was used to optimize the above parameters. A four-factor- five-level centre com- posite design was adopted to optimize the extraction con- ditions for analysis of the TC in rapeseed. The quadratic response surface model fitted Eq.1: 0 bY + k i Xibi 1 + k i i Xbii 1 2+ji k i k ij ij XXb 1 +e (1) where Y standed for the total carotenoids yield, b0 de- noted the model intercept, i and j were the linear and quadratic coefficients, respectively, bi, bii and bij were the regression coefficient, k was the number of factors studied and optimized in the experiment and e was the random error. Statistical Analysis System (SAS Institute Inc, Cary, NC, USA) was used to fit the second order polynomial equation to the experimental data. The goodness of fit of the model was evaluated by the coefficient of determination (R2) and the analysis of variance (ANOVA). Quadratic polynomial equations were attained by holding one of the independent vari- ances at a constant value and changing the level of the other variables. 2.3 Total Carotenoids Quantification After extraction, the samples were filtered on filter paper, their volume was made up to 3mL, and they were stored in an amber flask (~10mL) filled with N2. To determine the amount of total carotenoids extracted, UV-visible spectrophotometer (UNICO UV-2802, USA) was used for a spectral window between 380 and 750 nm, in trip- licate. The absorbance value of carotenoids extract was monitored at 445 nm. The total carotenoids (TC) yield (mg/100g) was calculated according to the following Eq.2: wA mLyA gmgTC cm 1000 10)( )100/( % 1 6 (2) where A was the absorbance value of extract at 445 nm, y was the volume of extract, % 1cm A was the extinction coefficient of carotenoids, and w was the weight of rapeseed powder (g). 2.4 Verification of Model Optimizations of extraction conditions, including reac- tion temperature, solid-solvent ration, extraction dura- tion, and extraction repeated cycles for maximizing a quantitative UV-visible spectrophotometer determination of TC in the rapeseed were calculated by using the pre- dictive equation from RSM. The actual determination of TC was carried out by UV-visible spectrophotometer after extraction at the optimum conditions, and the result was compared to the predicted value. 3. RESULTS AND DISCUSSIONS 3.1. The Effect of Extraction Solvents on TC Yield Six different extraction solvents, hexamethylene, petro- leum ether, chloroform, acetone, methanol and petro- leum ether/acetone mixture, were employed to choose the most suitable solvent for TC extraction from rape- seed. The experimental results were shown in Table 1. It was indicated from Table 1 that the mixture solvent of petroleum ether and acetone (1:1, v/v) was the most effective solvent for TC extraction from rapeseed. As known, rapeseed contains polar carotenoid such as lutein, and nonpolar carotenoids such as β-carotene and carote- noids ester, the former is easily dissolved in polar sol- vent (e.g. acetone) while the latter is easily dissolved in nonpolar solvent (e.g. petroleum ether). Therefore, the Table 1. Effects of extraction solvent on TC yield. Extraction solvents TC yield (mg/100g) Hexamethylene 0.617±0.065 Petroleum ether 0.972±0.038 Chloroform 2.110±0.052 Acetone 2.438±0.063 Methanol 2.312±0.075 Petroleum ether/acetone mix- ture(1:1,v/v) 3.890±0.093 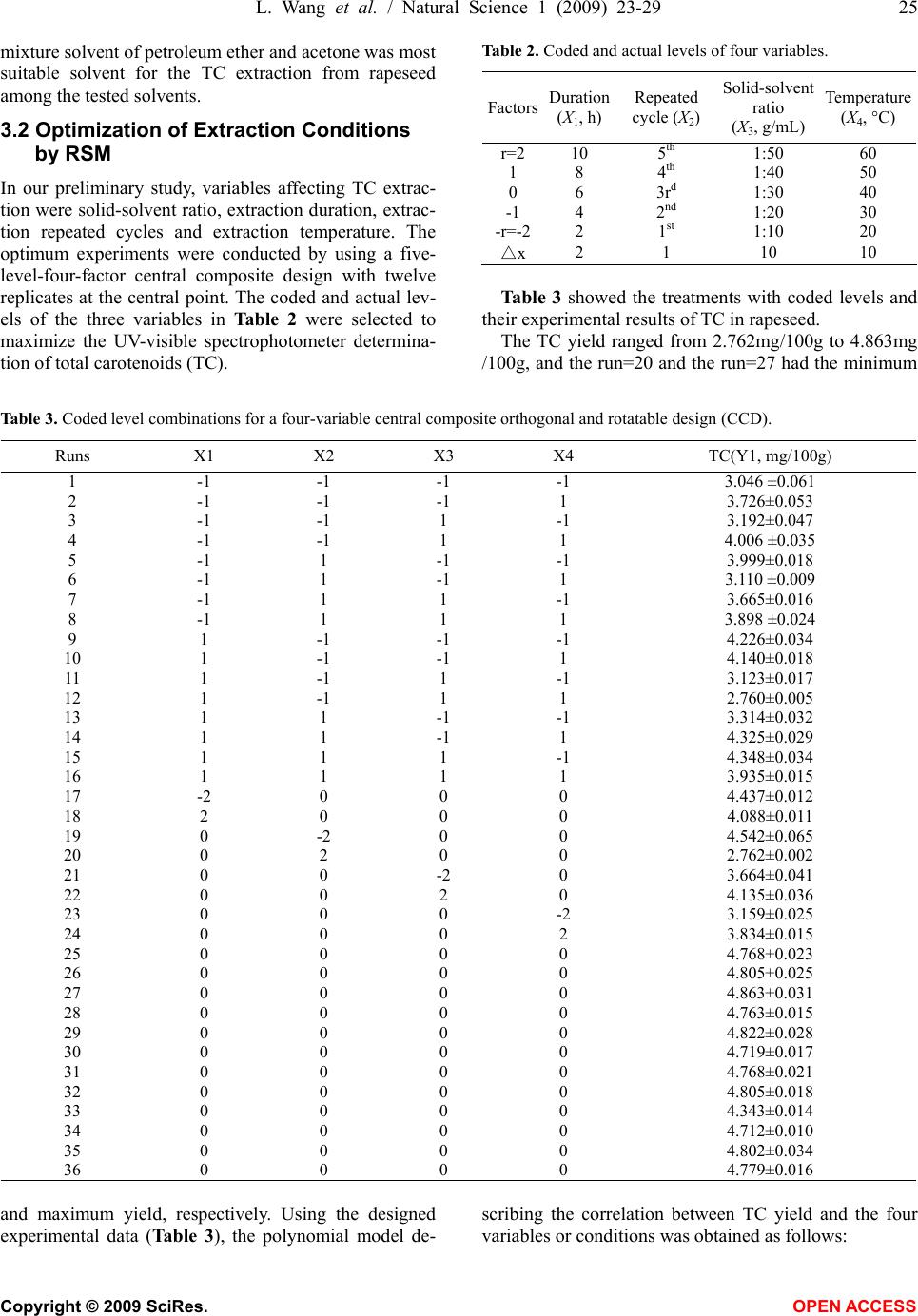 L. Wang et al. / Natural Science 1 (2009) 23-29 25 Copyright © 2009 SciRes. OPEN ACCESS mixture solvent of petroleum ether and acetone was most suitable solvent for the TC extraction from rapeseed among the tested solvents. 3.2 Optimization of Extraction Conditions by RSM In our preliminary study, variables affecting TC extrac- tion were solid-solvent ratio, extraction duration, extrac- tion repeated cycles and extraction temperature. The optimum experiments were conducted by using a five- level-four-factor central composite design with twelve replicates at the central point. The coded and actual lev- els of the three variables in Table 2 were selected to maximize the UV-visible spectrophotometer determina- tion of total carotenoids (TC). Table 2. Coded and actual levels of four variables. Factors Duration (X1, h) Repeated cycle (X2) Solid-solvent ratio (X3, g/mL) Temperature (X4, °C) r=2 10 5th 1:50 60 1 8 4th 1:40 50 0 6 3rd 1:30 40 -1 4 2nd 1:20 30 -r=-22 1st 1:10 20 △x 2 1 10 10 Table 3 showed the treatments with coded levels and their experimental results of TC in rapeseed. The TC yield ranged from 2.762mg/100g to 4.863mg /100g, and the run=20 and the run=27 had the minimum Table 3. Coded level combinations for a four-variable central composite orthogonal and rotatable design (CCD). Runs X1 X2 X3 X4 TC(Y1, mg/100g) 1 -1 -1 -1 -1 3.046 ±0.061 2 -1 -1 -1 1 3.726±0.053 3 -1 -1 1 -1 3.192±0.047 4 -1 -1 1 1 4.006 ±0.035 5 -1 1 -1 -1 3.999±0.018 6 -1 1 -1 1 3.110 ±0.009 7 -1 1 1 -1 3.665±0.016 8 -1 1 1 1 3.898 ±0.024 9 1 -1 -1 -1 4.226±0.034 10 1 -1 -1 1 4.140±0.018 11 1 -1 1 -1 3.123±0.017 12 1 -1 1 1 2.760±0.005 13 1 1 -1 -1 3.314±0.032 14 1 1 -1 1 4.325±0.029 15 1 1 1 -1 4.348±0.034 16 1 1 1 1 3.935±0.015 17 -2 0 0 0 4.437±0.012 18 2 0 0 0 4.088±0.011 19 0 -2 0 0 4.542±0.065 20 0 2 0 0 2.762±0.002 21 0 0 -2 0 3.664±0.041 22 0 0 2 0 4.135±0.036 23 0 0 0 -2 3.159±0.025 24 0 0 0 2 3.834±0.015 25 0 0 0 0 4.768±0.023 26 0 0 0 0 4.805±0.025 27 0 0 0 0 4.863±0.031 28 0 0 0 0 4.763±0.015 29 0 0 0 0 4.822±0.028 30 0 0 0 0 4.719±0.017 31 0 0 0 0 4.768±0.021 32 0 0 0 0 4.805±0.018 33 0 0 0 0 4.343±0.014 34 0 0 0 0 4.712±0.010 35 0 0 0 0 4.802±0.034 36 0 0 0 0 4.779±0.016 and maximum yield, respectively. Using the designed experimental data (Table 3), the polynomial model de- scribing the correlation between TC yield and the four variables or conditions was obtained as follows: 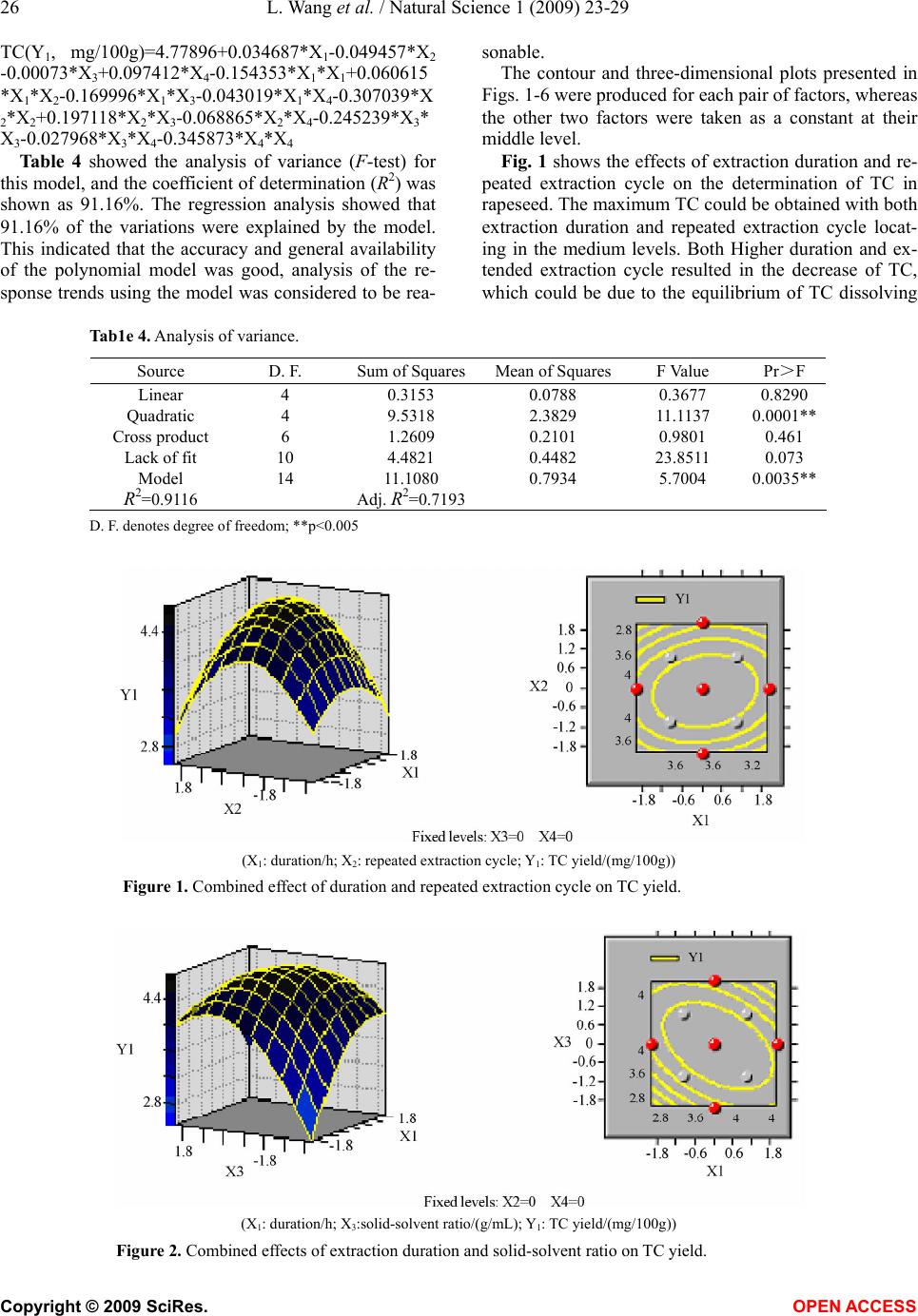 26 L. Wang et al. / Natural Science 1 (2009) 23-29 Copyright © 2009 SciRes. OPEN ACCESS TC(Y1, mg/100g)=4.77896+0.034687*X1-0.049457 *X2 -0.00073*X3+0 .097412* X4-0.15435 3*X1*X1+0.060615 *X1*X2-0.169996*X1*X3-0.043019*X1*X4-0.307039*X 2*X2+0.197118*X2*X3-0.068865*X2*X4-0.245239*X3* X3-0.027968*X3*X4-0.345873*X4*X4 Table 4 showed the analysis of variance (F-test) for this model, and the coefficient of determination (R2) was shown as 91.16%. The regression analysis showed that 91.16% of the variations were explained by the model. This indicated that the accuracy and general availability of the polynomial model was good, analysis of the re- sponse trends using the model was considered to be rea- sonable. The contour and three-dimensional plots presented in Figs. 1-6 were produced for each pair of factors, whereas the other two factors were taken as a constant at their middle level. Fig. 1 shows the effects of extraction duration and re- peated extraction cycle on the determination of TC in rapeseed. The maximum TC could be obtained with both extraction duration and repeated extraction cycle locat- ing in the medium levels. Both Higher duration and ex- tended extraction cycle resulted in the decrease of TC, which could be due to the equilibrium of TC dissolving Tab1e 4. Analysis of variance. Source D. F. Sum of SquaresMean of SquaresF Value Pr>F Linear 4 0.3153 0.0788 0.3677 0.8290 Quadratic 4 9.5318 2.3829 11.1137 0.0001** Cross product 6 1.2609 0.2101 0.9801 0.461 Lack of fit 10 4.4821 0.4482 23.8511 0.073 Model 14 11.1080 0.7934 5.7004 0.0035** R2=0.9116 Adj. R2=0.7193 D. F. denotes degree of freedom; **p<0.005 (X1: duration/h; X2: repeated extraction cycle; Y1: TC yield/(mg/100g)) Figure 1. Combined effect of duration and repeated extraction cycle on TC yield. (X1: duration/h; X3:solid-solvent ratio/(g/mL); Y1: TC yield/(mg/100g)) Figure 2. Combined effects of extraction duration and solid-solvent ratio on TC yield. 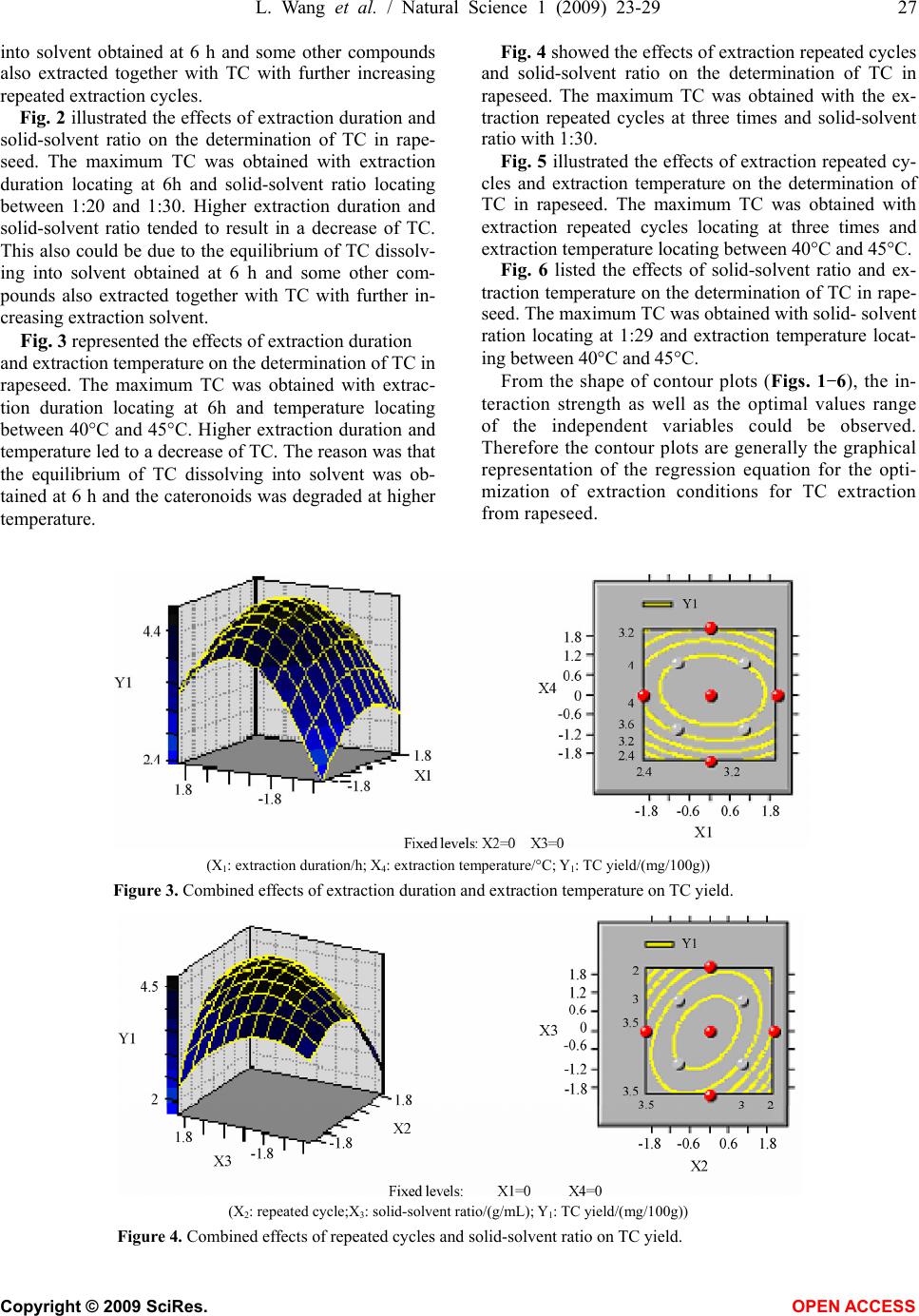 L. Wang et al. / Natural Science 1 (2009) 23-29 27 Copyright © 2009 SciRes. OPEN ACCESS into solvent obtained at 6 h and some other compounds also extracted together with TC with further increasing repeated extraction cycles. Fig. 2 illustrated the effects of extraction duration and solid-solvent ratio on the determination of TC in rape- seed. The maximum TC was obtained with extraction duration locating at 6h and solid-solvent ratio locating between 1:20 and 1:30. Higher extraction duration and solid-solvent ratio tended to result in a decrease of TC. This also could be due to the equilibrium of TC dissolv- ing into solvent obtained at 6 h and some other com- pounds also extracted together with TC with further in- creasing extraction solvent. Fig. 3 represented the effects of extraction duration and extraction temperature on the determination of TC in rapeseed. The maximum TC was obtained with extrac- tion duration locating at 6h and temperature locating between 40°C and 45°C. Higher extraction duration and temperature led to a decrease of TC. The reason was that the equilibrium of TC dissolving into solvent was ob- tained at 6 h and the cateronoids was degraded at higher temperature. Fig. 4 showed the effects of extraction repeated cycles and solid-solvent ratio on the determination of TC in rapeseed. The maximum TC was obtained with the ex- traction repeated cycles at three times and solid-solvent ratio with 1:30. Fig. 5 illustrated the effects of extraction repeated cy- cles and extraction temperature on the determination of TC in rapeseed. The maximum TC was obtained with extraction repeated cycles locating at three times and extraction temperature locating between 40°C and 45°C. Fig. 6 listed the effects of solid-solvent ratio and ex- traction temperature on the determination of TC in rape- seed. The maximum TC was obtained with solid- solvent ration locating at 1:29 and extraction temperature locat- ing between 40°C and 45°C. From the shape of contour plots (Figs. 1-6), the in- teraction strength as well as the optimal values range of the independent variables could be observed. Therefore the contour plots are generally the graphical representation of the regression equation for the opti- mization of extraction conditions for TC extraction from rapeseed. (X1: extraction duration/h; X4: extraction temperature/°C; Y1: TC yield/(mg/100g)) Figure 3. Combined effects of extraction duration and extraction temperature on TC yield. (X2: repeated cycle;X3: solid-solvent ratio/(g/mL); Y1: TC yield/(mg/100g)) Figure 4. Combined effects of repeated cycles and solid-solvent ratio on TC yield. 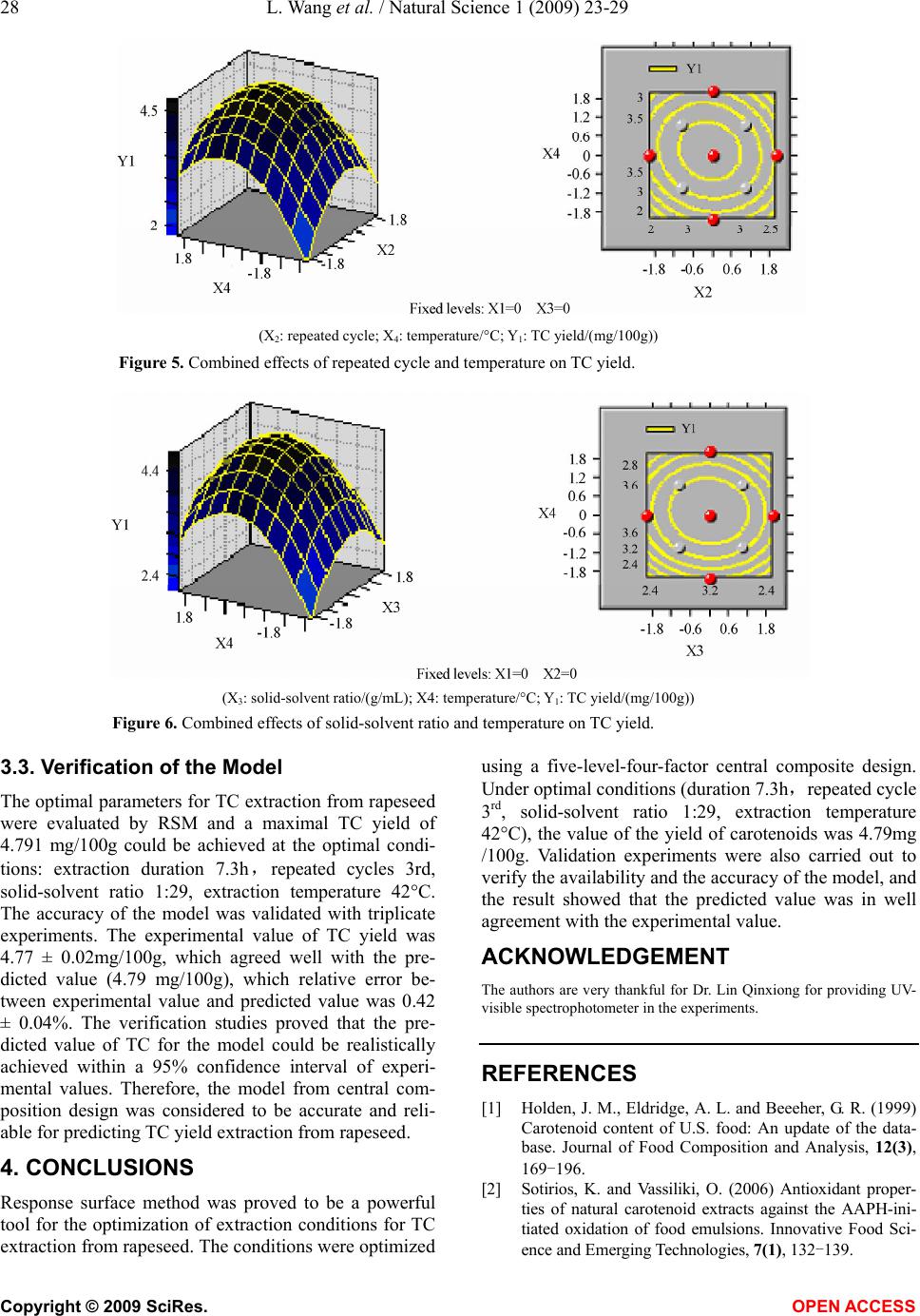 28 L. Wang et al. / Natural Science 1 (2009) 23-29 Copyright © 2009 SciRes. OPEN ACCESS (X2: repeated cycle; X4: temperature/°C; Y1: TC yield/(mg/100g)) Figure 5. Combined effects of repeated cycle and temperature on TC yield. (X3: solid-solvent ratio/(g/mL); X4: temperature/°C; Y1: TC yield/(mg/100g)) Figure 6. Combined effects of solid-solvent ratio and temperature on TC yield. 3.3. Verification of the Model The optimal parameters for TC extraction from rapeseed were evaluated by RSM and a maximal TC yield of 4.791 mg/100g could be achieved at the optimal condi- tions: extraction duration 7.3h,repeated cycles 3rd, solid-solvent ratio 1:29, extraction temperature 42°C. The accuracy of the model was validated with triplicate experiments. The experimental value of TC yield was 4.77 ± 0.02mg/100g, which agreed well with the pre- dicted value (4.79 mg/100g), which relative error be- tween experimental value and predicted value was 0.42 ± 0.04%. The verification studies proved that the pre- dicted value of TC for the model could be realistically achieved within a 95% confidence interval of experi- mental values. Therefore, the model from central com- position design was considered to be accurate and reli- able for predicting TC yield extraction from rapeseed. 4. CONCLUSIONS Response surface method was proved to be a powerful tool for the optimization of extraction conditions for TC extraction from rapeseed. The conditions were optimized using a five-level-four-factor central composite design. Under optimal conditions (duration 7.3h,repeated cycle 3rd, solid-solvent ratio 1:29, extraction temperature 42°C), the value of the yield of carotenoids was 4.79mg /100g. Validation experiments were also carried out to verify the availability and the accuracy of the model, and the result showed that the predicted value was in well agreement with the experimental value. ACKNOWLEDGEMENT The authors are very thankful for Dr. Lin Qinxiong for providing UV- visible spectrophotometer in the experiments. REFERENCES [1] Holden, J. M., Eldridge, A. L. and Beeeher, G. R. (1999) Carotenoid content of U.S. food: An update of the data- base. Journal of Food Composition and Analysis, 12(3), 169-196. [2] Sotirios, K. and Vassiliki, O. (2006) Antioxidant proper- ties of natural carotenoid extracts against the AAPH-ini- tiated oxidation of food emulsions. Innovative Food Sci- ence and Emerging Technologies, 7(1), 132-139.  L. Wang et al. / Natural Science 1 (2009) 23-29 29 Copyright © 2009 SciRes. OPEN ACCESS [3] Jamal, J. and Chieri, K. (2006) Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during post harvest storage. Post harvest Biol- ogy and Technology, 41(2), 151-155. [4] Rao, A. V. and Rao, L. G. (2007) Carotenoids and human health. Pharmacological Research, 55(3), 207-216. [5] Meléndez-Martínez, A. J., George, B. and Isabel, M. V. (2007) Relationship between the colour and the chemical structure of carotenoid pigments. Food Chemistry, 101(3), 1145-1150. [6] Meléndez-Martínez, A. J., Vicario, I. M. and Heredia, F. J. (2007) Review: Analysis of carotenoids in orange juice. Journal of Food Composition and Analysis, 20(7), 638-649. [7] Andrew, J. and Young, G. M. (2001) Antioxidant and prooxidant properties of carotenoids. Archives of Bio- chemistry and Biophysics, 385(1), 20-27. [8] Adetayo, O. O. and Aluko, R. E. (2005) The anti-car- cinogenic and anti-atherogenic effects of lycopene: A re- view. Trends Food Sci. Technol., 16(8), 344-350. [9] Cano, A., Acosta, M. and Arnao, M. B. (2003) Hydro- philic and lipophilic antioxidant activity changes during on-vine ripening of tomatoes (Lycopersicon esculentum Mill). Post-harvest Biol. Technol., 28(1), 59-65. [10] Akhtar, M. H. and Bryan, M. (2008) Extraction and quantification of major carotenoids in processed foods and supplements by liquid chromatography. Food Chem- istry, 111(1), 255-261. [11] Meléndez-Martínez, A. J., Vicario, I. M. and Heredia, F. J. (2007) Provitamin A, carotenoids and ascorbic acid con- tents of the different types of orange juices marketed in Spain. Food Chemistry, 101(1), 177-184. [12] Gua, Z. X., Chen, D. M., Han, Y. B., Chen, Z. G. and Gu, F. R. (2008) Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT-Food Science and Tech- nology, 41(6), 1082-1088. [13] FAO (2006) Statistical year book. Food and Agriculture Organization of the United Nations, 2/1,2/2, Rome. [14] Haila, H. and Heinonen, M. (1994) Action of β-carotene on purified rapeseed oil during light storage. Food Sci- ence and Technology, 27(6), 573-577. [15] Hornero-Méndez, D. and Mínguez-Mosquera, M. I. (2007) Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innovative Food Science & Emerging Technologies, 8(3), 407-412. [16] Rodriguez-Amaya, D. B., Kimura, M., Godoy, H. T., and Amaya-Farfan, J. (2008) Updated Brazilian database on food carotenoids: Factors affecting carotenoid composi- tion. Journal of Food Composition and Analysis, 21(6), 445-463. [17] Simkin, A. J., Moreau, H., Kuntz, M., Pagny, G., Chen- wei, L., Tanksley, Se., and McCarthy, J. (2008) An inves- tigation of carotenoid biosynthesis in Coffea canephora and Coffea Arabica. Journal of Plant Physiology, 165(10), 1087-1106. [18] Sachindra, N. M. and Mahendrakar, N. S. (2005) Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresource Technology, 96(10), 1195-1200. [19] Sheetal, M. C. and Laxmi (2007) Enzyme aided extrac- tion of lycopene from tomato tissues. Food Chemistry, 102(1), 77-81. [20] Huang, W., Li, Z. S., Niu, H., Li, D. and Zhang, J. (2008) Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response sur- face methodology. Journal of Food Engineering, 89(3), 298-302. [21] Mateose, R. and Garcia-Mesa, J. A. (2006) Rapid and quantitative extraction method for the determination of chlorophylls and carotenoids in olive oil by high-performance liquid chromatography. Analytical and Bioanalytical chemistry, 385(7), 1247-1254. [22] Rodriguez-Amaya, D. B. (2001) A guide to carotenoid analysis in foods. Washington, DC.: Ed. ILSI-Interna- tional Life Sciences Institute. [23] Schoefs, B. (2002) Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends in Food Science and Technolog., 13(11), 361-371. |

