Chinese Medicine
Vol.5 No.1(2014), Article ID:44280,13 pages DOI:10.4236/cm.2014.51005
Long-Term Treatment with an Herbal Formula MCC Ameliorates Obesity-Associated Metabolic Dysfunction in High Fat Diet-Induced Obese Mice: A Comparative Study among MCC and Various Combinations of Its Constituents
Pou Kuan Leong1, Hoi Yan Leung1, Hoi Shan Wong1, Ji Hang Chen1, Wing Man Chan1, Chung Wah Ma2, Yi Ting Yang2, Kam Ming Ko1*
1Division of Life Science, Hong Kong University of Science and Technology, Hong Kong, China
2Infinitus (China) Company Ltd., Guangzhou, China
Email: *bcrko@ust.hk
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 7 January 2014; revised 11 February 2014; accepted 24 February 2014
ABSTRACT
Obesity has been found to be associated with increased incidence of various metabolic disorders. Anti-obesity interventions are therefore urgently needed. An earlier study has demonstrated that treatment with an herbal formula MCC, which comprises the fruit of Momordica charantia (MC), the pericarpium of Citri reticulate (CR) and L-carnitine (CA), reduced the weight gain in high fat diet (HFD)-fed mice. In the present study, we investigated the effect of long-term treatment with MCC (6 g/kg/day × 40 doses) and various combinations of its constituents in HFD-fed female ICR mice. Body weight change was monitored during the course of the experiment. Total and differential adiposity, plasma lipid contents, metabolic enzyme activities and mitochondrial coupling efficiency in skeletal muscle were measured. Glucose homeostasis was also assessed. Results showed that HFD increased the body weight, total and differential adiposity, and plasma lipid contents as well as impaired metabolic status in skeletal muscle and glucose homeostasis. MCC and all combinations of its constituents reduced the weight gain in HFD-fed mice, which was accompanied with an improvement on glucose homeostasis. While MC, CA and CR independently suppressed the HFD-induced weight gain in mice, MC seems to be the most effective in weight reduction, all of which correlated with the induction of mitochondrial uncoupling in skeletal muscle. Only CA and CR, but not MC, significantly reduced the total adiposity and visceral adiposity as well as plasma cholesterol level. However, the two component combinations, MC + CR and MC + CA, decreased the degree of visceral adiposity and plasma cholesterol level, respectively. MCC treatment at 1.5 g/kg (but not a higher dose of 6 g/kg) suppressed visceral adiposity and induced mitochondrial uncoupling in skeletal muscle in HFD-fed mice. The finding suggests that MCC may offer a promising prospect for ameliorating the diet-induced obesity and metabolic disorders in humans.
Keywords:High Fat Diet; Obesity; Weight Control; Momordica Charantia; Citri Reticulata; L-Carnitine

1. Introduction
The worldwide epidemic of obesity has been found to be associated with increased incidence of cardiovascular diseases, type 2 diabetes as well as the metabolic syndrome, leading to severe economic burden on healthcare system in modern society. Body mass index (BMI), which indicates the ratio of body weight to height, is a conventional metrics for assessing the health risk arising from obesity. Recent studies have demonstrated an inverse relationship of obesity and mortality in cardiovascular diseases and diabetes, suggesting that BMI may be not a reliable benchmark of healthy individual [1] . Alternatively, the mass of visceral fat pad, which is the fat pad of intra-abdominal cavity, is causally related to metabolic diseases, presumably by virtue of its secretion of pro-inflammatory adipokines [2] . In addition to visceral fat mass, a high ratio of low-density lipoprotein cholesterol (LDL-C) to high-density lipoprotein cholesterol (HDL-C) in combination with hypertriglyceridemia correlates well with a high risk of coronary heart disease [3] . In this regard, anti-obesity interventions, which aimed at controlling not only body weight but also plasma lipids as well as visceral fat mass, are urgently needed.
Regular physical exercise combined with low calorie diet is a commonly used intervention for the treatment of obesity. However, only a small portion of obese individuals with intervention on lifestyle can maintain a prolonged and sustained weight control [4] . Therapeutic interventions, including the use of pancreatic lipase inhibitor and stimulator of central noradrenaline release, are alternative approach to treating obesity. Despite their efficacy of weight reduction, adverse effects, such as steatorrhoea and liver injury, were observed in some obese individuals [5] . Recently, Tseng et al. have proposed a novel target of obesity therapy by increasing cellular bioenergetics [6] . As such, the dissipation of proton gradient across the mitochondrial inner membrane (i.e. mitochondrial uncoupling) can cause an inefficient generation of cellular ATP, with a resultant increase in energy expenditure. In this connection, the induction of mitochondrial uncoupling, particularly in skeletal muscle, offers a potential treatment of obesity [7] .
In an effort to develop safe interventions for obesity, herbal medicine, which has a long history of use in weight reduction, has attracted a lot of interest. An herbal formula MCC, which is comprised of the fruit of Momordica charantia (MC, also called bitter melon), the pericarpium of Citri reticulata (CR) and L-carnitine (CA), was found to reduce the weight gain, visceral fat mass and hyperlipidemia in high fat diet (HFD)-induced obese mice [8] . However, the role of the three constituents of MCC formula in producing the pharmacological action remains to be investigated.
In the present study, we endeavored to define the role of the three constituents of MCC formula by comparing the weight reduction effect of MCC and various combinations of its constituents (i.e., 1- and 2-component combinations), using a mouse model of HFD-induced obesity. The body weight gain was monitored during the course of 8-week experiment. To examine the degree of adiposity and plasma lipid levels, the mass of gonadal fat, mesenteric fat and subcutaneous fat as well as various plasma lipid contents [triglyceride (TG), LDL-C and HDL-C] were measured after the 8-week HFD feeding. To investigate the glucose homeostasis of HFD-fed mice, plasma glucose levels after 24-h fasting as well as glucose intolerance and insulin sensitivity index in oral glucose tolerance test (OGTT) were measured. To assess the metabolic status of skeletal muscle, activities of myocellular phosphofructokinase (PFK), β-hydroxyacyl-Co A dehydrogenase (β-HAD), carnitine palmitoyl CoA transferase (CPT) and citrate synthase (CS), as well as state 3 and state 4 respiratory rates of isolated mitochondria of skeletal muscle were assayed. The mitochondrial coupling efficiency was then estimated by computing the ratio of state 3 to state 4 mitochondrial respiratory rate.
2. Materials and Methods
2.1. Chemicals
PicoLabÒ Rodent diet 20 (normal diet) was purchased from LabDietÒ (City, State, USA). The “Original” High Fat Diet (Diet-induced Obesity Formula D12492, 60% energy from fat) was purchased from Research Diets, Inc. (New Brunswick, NJ, USA). TG and cholesterol assay kits were purchased from Wako Pure Chemical Industries, Ltd (Okasa, Japan). HDL-C test kit was purchased from Wako Diagnostics (Richmond, VA, USA). Glucose assay reagent was obtained from Sigma Chemical Co. (St. Louis, MO, USA). ELISA kit for measuring mouse insulin was purchased from Crystal Chem Inc. (Downers Grove, USA). The water extracts of MC and CR as well as CA were manufactured and supplied by Infinitus (China) Company Ltd., Guangzhou, China. The MCC formula is comprised of MC, CR and CA with the mass ratio of MC:CR:CA being 1:3.5:10. All other chemicals were of analytical grade.
2.2. Animal Care
Female ICR mice (8 - 10 weeks old, 20 - 25 g) were maintained under a 12-hour dark/light cycle at about 22˚C, and allowed food and water ad libitum in the Animal and Plant Care Facilities at the Hong Kong University of Science and Technology (HKUST). All experimental protocols were approved by the University Committee on Research Practice at HKUST
2.3. Animal Treatment
In a preliminary study, MCC treatment was found to reduce the high fat diet-induced weight gain in both male and female ICR mice, with similar changes in the tested biochemical parameters. In the present study, only female ICR mice were used, which were randomly assigned to 10 groups, with 10 - 15 mice in each group: 1) Normal diet (ND, 13% energy from fat, PicoLab) control; 2) High fat diet (HFD, 60% energy from fat, Test Diet) control; 3) HFD, MC 0.17 g/kg; 4) HFD, CR, 0.6 g/kg; 5) HFD, CA, 1.71 g/kg; 6) HFD, MC + CR, 0.17 + 0.6 g/kg; 7) HFD, MC + CA, 0.17 + 1.71 g/kg; 8) HFD, CR + CA, 0.6 + 1.71 g/kg; 9) HFD, MCC, 1.5 g/kg; 10) HFD, MCC, 6.0 g/kg; 11) Emodin, 40 mg/kg. Doses of various combinations were equivalent to 6.0 g/kg of MCC. Mice were administered intragastrically at the indicated dose 5 days per week for 8 weeks. Control mice received water (vehicle) only. Twenty-four hours after the last dosing, blood samples were drawn from overnight fasted, phenobarbital-anesthetized mice by cardiac excision using syringes rinsed with 0.5% heparin in saline (w/v), and the mice were then sacrificed by cervical dislocation. Samples of gastrocnemius muscle and fat pads (gonadal, mesenteric and subcutaneous fat) were excised, and they were subjected to further analysis.
2.4. Preparation of Samples
Plasma samples were obtained by centrifuging whole blood samples at 1500 ´ g for 10 minutes at 4˚C. Plasma samples were then subjected to biochemical analysis.
Minced gastrocnemius muscle tissues were digested by collagenase solution [0.075% (w/v) in buffer] at 4˚C for 20 min. After removing the collagenase solution by centrifugation, the digested tissues were mixed with 20 mL of ice-cold homogenizing buffer (100 mM KCl, 50 mM MOPS, 10 mM EGTA, pH 7.2) and subjected to homogenization with a Teflon-glass homogenizer at 4000 rpm for 25 - 30 complete strokes. Then the homogenates were centrifuged at 600 ´ g for 10 min at 4˚C. The resultant supernatant was nucleus-free fraction. For measurements of β-HAD, CS and CPT activities, nucleus-free fraction was diluted with 0.2% Triton X-100 (w/v, in K2HPO4 buffer) [8] .
Mitochondrial pellets were prepared from nucleus-free fractions of muscle homogenates by centrifugation at 9200 ´ g at 4˚C for 30 min. The mitochondrial pellets were then resuspended in a buffer containing 250 mM sucrose, 50 mM Tris, pH 7.5 and constituted the mitochondrial fractions [8] .
2.5. Measurement of Body Weight and Fat Pad Weight
Body weight of mice was measured once a week during the 8-week course of experiment. Gonadal, mesenteric, and subcutaneous fat pads were weighed. The ratio of a particular fat pad weight to body weight was estimated and expressed as fat pad index [8] .
2.6. Biochemical Analysis
Plasma glucose levels were measured using an assay kit basing on coupled hexokinase-catalyzed and glucose-6- phosphate dehydrogenase-catalyzed reactions, with a resultant NAD reduction. Absorbance changes at 340 nm were monitored spectrophotometrically by Victor3 Multi-label Counter (Perkin Elmer, Turku, Finland). Plasma levels of TG, HDL-C, and TC levels were measured using assay kits. LDL-C level was estimated by Friedewald’s formula: 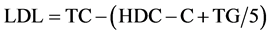 [9] . Plasma insulin level was measured using an ELISA kit. PFK, b-HAD, CS and CPT activities in skeletal muscle were measured using enzymatic methods, as described as Leong et al. and Colberg et al. [8] [10] .
[9] . Plasma insulin level was measured using an ELISA kit. PFK, b-HAD, CS and CPT activities in skeletal muscle were measured using enzymatic methods, as described as Leong et al. and Colberg et al. [8] [10] .
2.7. Oral Glucose Tolerance Test
After 8 weeks of experiment, oral glucose tolerance test (OGTT) was performed. Six hour post-fasting, blood samples were collected as initial levels of plasma glucose and insulin (i.e. time = 0). Then, glucose (2 g/kg) was orally administered to the mice. Blood samples were collected at 15, 30, 60 and 120 min post-dosing of glucose. Plasma glucose and insulin levels were measured. Glucose intolerance was estimated by computing the area under the curve (AUC) plotting plasma glucose level against time and expressed in arbitrary unit. Insulin sensitivity index (ISI) was estimated by following equation:
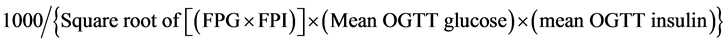 where FPG = fasting plasma glucose; FPI = fasting plasma insulin [10] .
where FPG = fasting plasma glucose; FPI = fasting plasma insulin [10] .
2.8. Measurement of Mitochondrial Respiration
Mitochondrial respiratory was measured polarographically by a Clark-type oxygen electrode (Hansatech Instruments Ltd., Norfolk, UK) at 30˚C. Mitochondrial fraction (~0.5 mg protein/mL) was incubated in a buffer containing 30 mM KCl, 6 mM MgCl2, 75 mM sucrose, 1 mM EDTA, 20 mM KH2PO4 and 0.1% (w/v) fatty acid-free BSA, pH 7.0. Substrate solution containing 10 mM glutamate and 2.5 mM malate was added, and after a stable state 2 respiration had been established, state 3 respiration (coupling) was initiated by the addition of ADP (final concentration 0.6 mM). When all of the added ADP was used up for ATP generation, oligomycin (ATP synthase inhibitor) was added to induce the state 4 respiration (uncoupling). The respiratory control ratio (RCR) was estimated by calculating the ratio of state 3 to state 4 respirations [11] .
2.9. Statistical Analysis
The time dependent changes in the body weight during the course of 8 week experiment was analyzed by mixed Analysis of Variance (mixed ANOVA). Other data were analyzed by one-way Analysis of Variance (ANOVA). Post-hoc multiple comparisons were performed using Least Significant Difference (LSD). P values < 0.05 were regarded as statistically significant.
3. Results
3.1. Effects of MCC and Various Combinations of Its Constituents on Body Weight Gain in HFD-Fed Mice
ND slightly but not significantly increased the body weight during the course of 8-week experiment in mice. HFD accelerated the body weight increase during the course of experiment in mice, with the degree of stimulation being 24%, when compared with that of ND (Figure 1). MCC and all combinations of its constituents were found to inhibit the HFD-induced body weight gain (Table 1). While MC (0.17 g/kg) completely abrogated the HFD-induced body weight gain, MC + CR (0.17 and 0.6 g/kg) caused the largest degree of suppression (91.6%) among the 2-component combinations (Table 1). The 3-component combination MCC (6.0 g/kg) also suppressed the HFD-induced body weight gain, with the extent of inhibition being 76% (Figure 1 and Table 1).
3.2. Effects of MCC and Various Combinations of Its Constituents on Various Fat Pad Indices in HFD-Fed Mice
The HFD-induced body weight gain was associated with an increase in total fat pad index (162%), as well as
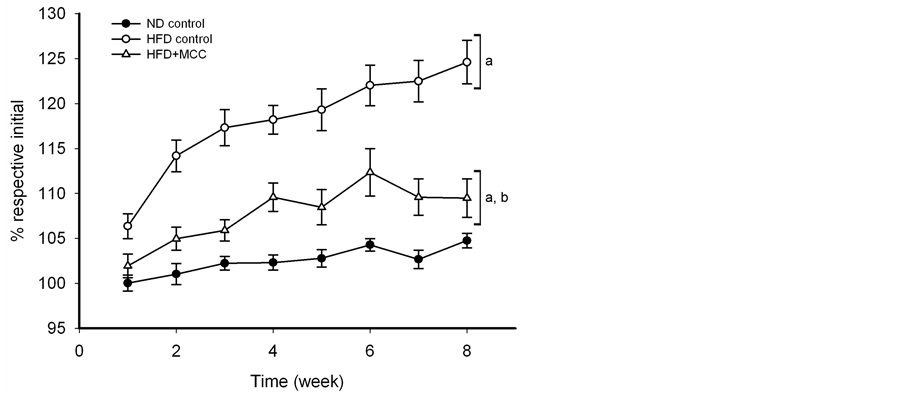
Figure 1. Effect of MCC on body weight gain during the course of 8-week experiment in HFD-fed mice. Mice were allowed food [ND (13% energy from fat) or HFD (60% energy from fat)] and water ad libitum, during the course of 8-week experiment. In the drug treatment groups, mice were intragastrically administered with MCC (6 g/kg/day) 5 days per week for 8 weeks (i.e. 40 doses), while control mice received water (vehicle) only. Body weights of mice were monitored by measuring the body weight of mice every week during the 8-week period. Data were expressed as % initial, by normalizing with initial body weight (i.e. time = week 0) of the respective mouse. Value given are means ± SEM, with n = 10 - 15. The initial values of body weight of each group were given as follows: 1) ND control = 33.1 ± 0.5 g; 2) HFD control = 33.2 ± 1.0 g; 3) HFD + 6 g/kg MCC = 36.1 ± 0.7 g. aSignificantly different from time-matched ND-fed control; bsignificantly different from time-matched HFD-fed control.
Table 1. Effects of MCC and various combinations of its constituents on body weight in HFD-fed mice.
aSignificantly different from ND-fed control; bsignificantly different from HFD-fed control.
differential increases in gonadal fat pad (184%), mesenteric fat pad (93%) and subcutaneous fat pad (162%) indices, when compared with the ND control (Table 2). Among the single-component treatments, MC and CR suppressed the HFD-induced increases in total fat pad index (39% and 36%, respectively) as well as gonadal fat pad index (47% and 38%), whereas CA reduced the gonadal fat pad index (46%) and mesenteric fat pad index (103%) in HFD-fed mice (Table 2). CR further suppressed the subcutaneous fat by 23%. Among the 2-component treatments, MC + CR and MC + CA decreased the total fat pad index (41% and 74%, respectively) and gonadal fat pad index (57% and 87%) in HFD-fed mice. In addition, MC + CA also suppressed increases in fat pad indices of total fat pad (64%) and subcutaneous fat pad (50%) of HFD-fed mice. CR + CA reduced the subcutaneous fat index (27%) in HFD-fed mice (Table 2). MCC (6.0 g/kg) did not produce detectable changes the total and differential fat pad indices.
3.3. Effects of MCC and Various Combinations of Its Constituents on Plasma Lipid Levels in HFD-Fed Mice
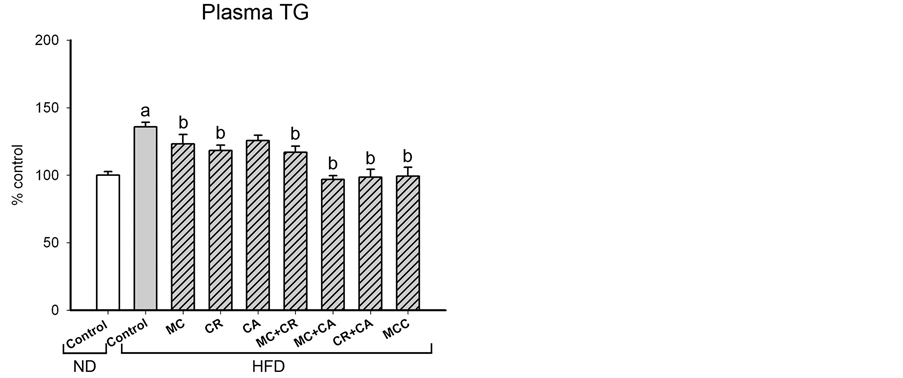
The HFD-induced increases in total and differential fat pad indices were paralleled by increases in plasma levels of TG and TC and (36% and 79%, respectively), as well as the ratio of LDL-C to HDL-C (27%) in HFD-fed mice (Figure 2). MCC (6.0 g/kg) and various combinations of its constituents differentially modulated the plasma lipid level in HFD-fed mice (Figure 2). The HFD-induced elevation in plasma TG level was decreased by MCC and all tested combinations (except CA), with complete suppression produced by MC + CA, CR + CA and MCC. MCC and all tested combinations reduced the plasma TC level in HFD-fed mice, with the extent of suppression afforded by CR + CA (77%) being most prominent, followed by MCC (71%). The HFD-induced increase in LDL-C/HDL-C ratio was suppressed by MCC and all tested combinations (except MC and MC + CA), with the complete inhibition produced by MCC.
3.4. Effects of MCC and Various Combinations of Its Constituents on Glucose Homeostasis in HFD-Fed Mice
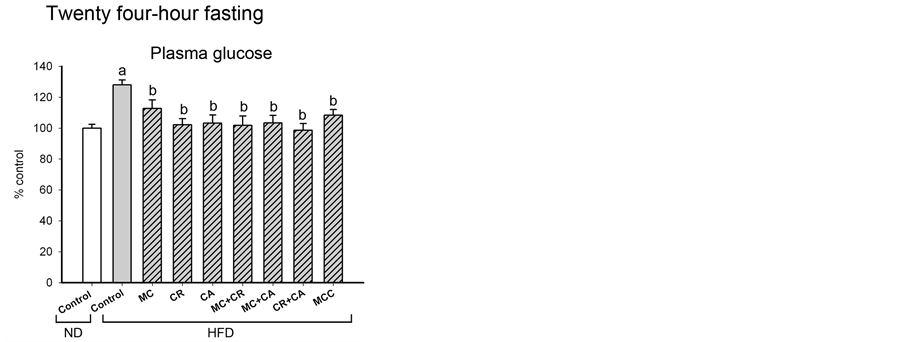
In addition to increases in plasma lipid levels, HFD also impaired the glucose homeostasis, as indicated by increases in fasting plasma glucose level (28%) and oral glucose intolerance (25%) as well as the decrease in insulin sensitivity (65%) (Figures 3(A)-(C)). MCC and all tested combinations caused reversal changes on fasting plasma glucose level, oral glucose intolerance and insulin sensitivity in HFD-fed mice. Treatment with CR, MC + CR or CA + CR caused complete normalization of fasting plasma glucose level in HFD-fed mice (Figure 3 (A)). In the oral glucose tolerance test, MC, MC + CR, MC + CA, CR + CA and MCC completely reversed the glucose intolerance, while CA, MC + CR, MC + CA and MCC completely restored the insulin sensitivity in HFD-fed mice.
3.5. Effects of MCC and Various Combinations of Its Constituents on Various Metabolic Enzyme Activities of Skeletal Muscle in HFD-Fed Mice
The HFD-induced impairment of glucose homeostasis was associated with the reduced PFK activity (20%) of
Table 2 . Effects of MCC and various combinations of its constituents on various fat pad indices in HFD-fed mice.
aSignificantly different from ND-fed control; bsignificantly different from HFD-fed control.
 (a)
(a)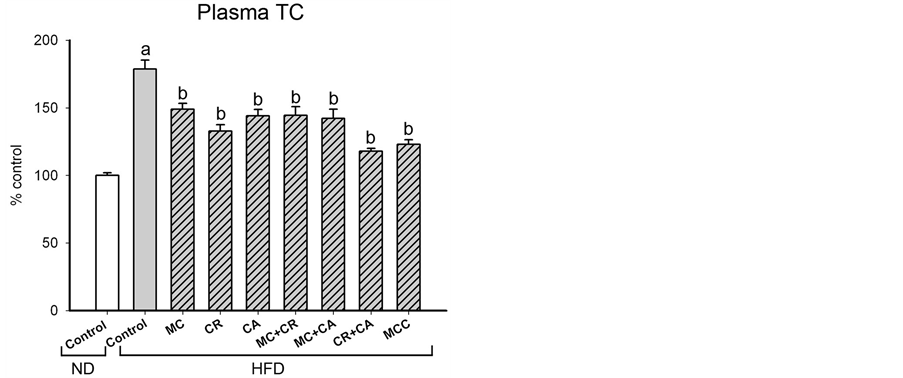 (b)
(b)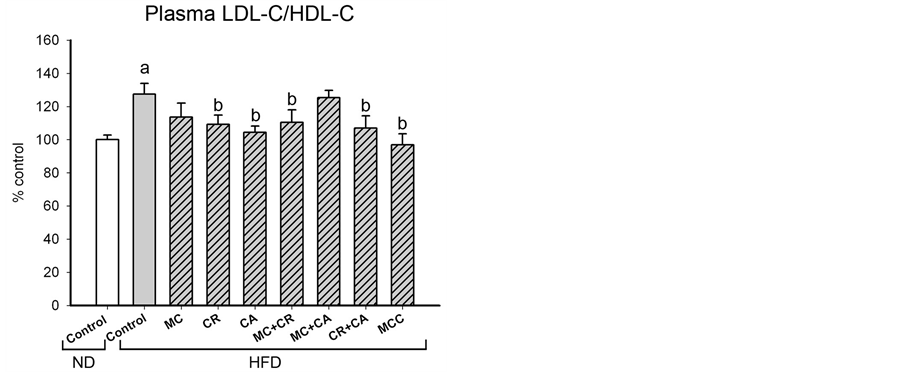 (c)
(c)
Figure 2. Effects of MCC and various combinations of its constituents on plasma lipid levels in HFD-fed mice. Mice were sacrificed at 24 h after the last dosing of MCC as described in Materials and Methods. Plasma triglyceride (TG) level (ND control = 59.0 ± 2.5 mg/dL), total lipoprotein cholesterol (TC) level (ND control = 146.3 ± 5.2 mg/dL) and ratio of low density lipoprotein cholesterol (LDL-C) to high density lipoprotein cholesterol (HDL-C) (ND control = 1.4 ± 0.11) were measured, as described in Materials and methods. Data were expressed as % control by normalizing with ND control. Value given are means ± SEM, with n = 10 - 15. aSignificantly different from ND control; bSignificantly different from HFD control.
skeletal muscle (Table 3). In addition, HFD increased the β-HAD activity (24%) and CPT activity (18%) of skeletal muscle in HFD-fed mice (Table 3 ). No detectable changes in CS activity of skeletal muscle in HFD-fed mice were observed. While MC, CR, CA and MC + CA partially reversed the HFD-induced reduction in PFK activity, CR, CA, MC + CR and MC + CA suppressed the CPT activity in HFD-fed mice.
3.6. Effects of MCC and Various Combinations of Its Constituents on Mitochondrial Coupling Efficiency of Skeletal Muscle in HFD-Fed Mice
HFD did not cause detectable change in mitochondrial coupling efficiency of skeletal muscle in HFD-fed mice. All single-component (MC, CR and CA) treatments decreased the coupling efficiency (36%, 19% and 31%, respectively), with the extent of reduction afforded by MC being most prominent (Figure 4). Among the 2-component combinations, only MC + CR and MC + CA decreased the mitochondrial coupling efficiency (28% and 20%, respectively) of skeletal muscle in HFD-fed mice. MCC did not affect the mitochondrial coupling effi-
 (A)
(A)  (B)
(B)
Figure 3. Effects of MCC and various combinations of its constituents on glucose homeostasis in HFDfed mice. (A) Mice were sacrificed at 24 h after the last dosing of MCC as described in Materials and Methods. Plasma glucose level (ND control = 80.7 ± 4.2 mg/dL) was measured. (B) Mice were objected to oral glucose tolerance test at 24 h after the last dosing of MCC as described in Materials and Methods. Blood samples were collected at 15, 30, 60 and 120 min post-dosing of glucose. Plasma glucose and insulin levels were measured. Glucose intolerance was estimated by computing the area under the curve (AUC) plotting plasma glucose level against time and expressed in arbitrary unit (ND control = 1030 ± 40). Insulin sensitivity index (ISI) was estimated (ND control = 2623 ± 14), as described in Materials and Methods. Data were expressed as % control, by normalizing with ND control. Value given are means ± SEM, with n = 10 - 15. aSignificantly different from ND control; bsignificantly different from HFD control.
ciency of skeletal muscle.
3.7. Effects of a Low Dose of MCC on Various Parameters in HFD-Fed Mice
The effects of MCC treatment at a low dose of 1.5 g/kg were also investigated in HFD-fed mice (Table 4). MCC (1.5 g/kg) reduced the HFD-induced body weight gain, with the degree of suppression being 64%. In contrast to the that of high dose of 6.0 g/kg, the weight reduction caused by the low dose of MCC was associated with decreases in total fat pad index (22%) as well as subcutaneous and mesenteric fat pad indices (36% and 66%, respectively) in HFD-fed mice. Consistent with this, MCC treatment also reduced the HFD-induced elevations of plasma TG and TC levels (by 65% and 72%, respectively) as well as plasma LDL-C to HDL-C ratio (127%). MCC also improved the glucose homeostasis in HFD-fed mice, as evidenced by the reversal of changes in fasting plasma glucose level (83%), glucose intolerance (100%) and insulin sensitivity (29%). Despite the fact
Table 3 . Effects of MCC and various combinations of its constituents on various metabolic enzymes of skeletal muscle in HFD-fed mice.
aSignificantly different from ND control; bsignificantly different from HFD control.
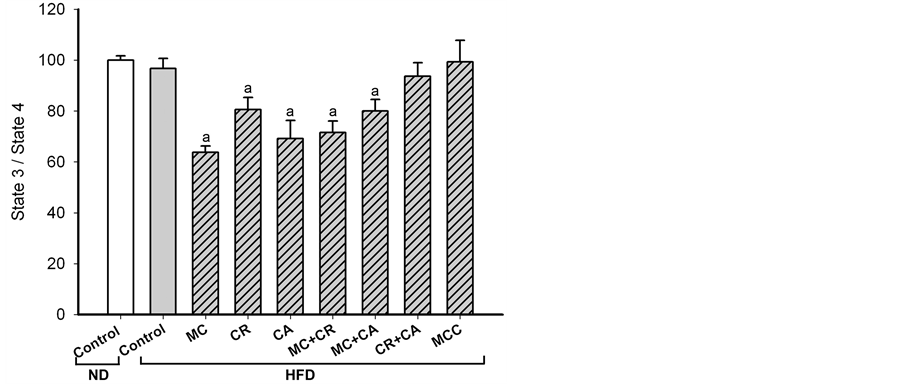
Figure 4. Effects of MCC and various combinations of its constituents on mitochondrial coupling efficiency of skeletal muscle in HFD-fed mice. Mice were sacrificed at 24 h after the last dosing of MCC as described in Materials and Methods. Mitochondrial respiratory of skeletal muscle was measured polarographically by a Clark-type oxygen electrode at 30˚C. The respiratory control ratio (RCR) was estimated by calculating the ratio of state 3 to state 4 respirations. Data were expressed as % control, by normalizing with ND control. Values given are means ± SEM, with n = 10 - 15. aSignificantly different from ND control; bsignificantly different from HFD control.
that MCC (1.5 g/kg) did not produce detectable changes in various metabolic enzyme activities (PFK, β-HAD and CS) of skeletal muscle, MCC decreased the CPT activity (56%) as well as the mitochondrial coupling efficiency (28%) of skeletal muscle in HFD-fed mice.
4. Discussion
HFD-fed mice, as observed in the present and other studies [12] , showed increases in body weight, the degree of
aSignificantly different from ND control; bsignificantly different from HFD control.
adiposity and plasma lipid contents, as well as impairments in glucose homeostasis and metabolic status of skeletal muscle. It has been shown that HFD caused an increase in free fatty acid uptake in skeletal muscle, with a resultant insulin resistance [13] , which is a well-established risk factor of diabetes and metabolic syndromes. In the present study, MCC and various combinations of its constituents reduced the weight gain in HFD-fed mice, which was accompanied with an improvement on glucose homeostasis. Our earlier study has suggested that the weight loss effect produced by MCC is likely due to a modulation of fatty acid absorption and/or metabolism [8] . In the present study, emodin, which was found to ameliorate metabolic dysfunctions in HFD-induced obese mice [14] , was used as a positive control. Consistent with the recent findings, emodin reduced the weight gain, adiposity and plasma lipid contents in HFD-fed mice (data not shown), suggesting the validity of the mouse model of HFD-induce obesity in the present study. Varied effects of MCC and its constituents or their combinations on total and differential adiposity, plasma lipid contents and metabolic status of skeletal muscle were observed. We therefore sought to define the role of each constituent of MCC in producing the weight loss effect and the associated metabolic changes in HFD-induced obese mice.
Despite the fact that all constituents of MCC (MC, CA and CR) independently suppressed the HFD-induced weight gain in mice, MC seems to be the most effective. The MC/CA/CR-induced weight reduction in HFD-fed mice may be attributed to the improvement in metabolic status of skeletal muscle, as indicated by as a reversal increase in PFK activity and an induction of mitochondrial uncoupling, with the degree of improvement produced by MC being larger than those of CA and CR. However, only CA and CR, but not MC, significantly reduced the total adiposity and visceral adiposity (as assessed by mesenteric fat pad index) as well as plasma LDL-C to HDL-C ratio. On the other hand, MC, when being combined with CR or CA, could reduce the degree of visceral adiposity or the LDL-C to HDL-C ratio respectively, in addition to decreasing body weight. Given that an increased visceral adiposity and an elevated plasma LDL-C to HDL-C ratio are predisposing factors of metabolic disorders [2] , the combination of MC with CR or CA may be beneficial for lowering the risk of metabolic disorders in obese individuals. This postulation was strengthened by the observation that among the 2- component combinations, MC + CA and MC + CR treatments were more effective than that of CA + CR in weight reduction, possibly due to the induction of mitochondrial uncoupling in skeletal muscle of mice. While MC + CA reduced the extent of visceral adiposity and stimulated the PFK activity of skeletal muscle, MC + CR decreased the plasma LDL-C/HDL-C ratio. Conceivably, MCC treatment may produce a multiple effect on weight reduction, visceral adiposity suppression and plasma LDL-C/HDL-C regulation in HFD-induced obese mice. Unexpectedly, the weight reduction afforded by MCC (6.0 g/kg) was not accompanied with the suppression of visceral adiposity and induction of mitochondrial uncoupling in skeletal muscle in HFD-induced obese mice. Since an inducer of mitochondrial uncoupling has been shown to cause a dose-dependent and biphasic response in round spermatids [15] , the failure of MCC to induce mitochondrial uncoupling in skeletal muscle may be related to the high dose of treatment. This postulation is supported by the finding that the weight reduction afforded by MCC treatment at a lower dose (1.5 g/kg) was associated with an induction of mitochondrial uncoupling in skeletal muscle as well as a suppression of visceral adiposity and a reduction of LDL-C/HDL-C ratio in HFD-induced obese mice. While the ability of MCC to modulate the metabolism of cholesterol remains unclear, the suppression of visceral adiposity is likely causally related to mitochondrial uncoupling in skeletal muscle, which oxidizes excessive fatty acids in HFD-fed mice. As visceral adipose tissue contains a large number of mitochondria [16] , we cannot exclude the possibility that MCC may also induce mitochondrial uncoupling in visceral adipose tissue, with a resultant mobilization of lipid reservoir in adipose tissue. As an excessive accumulation of visceral adipose tissue is associated with metabolic abnormalities such as insulin resistance and high blood lipid contents [17] , the reduction of fat mass of visceral adipose tissue is therefore beneficial for preventing metabolic diseases such as metabolic syndromes and type 2 diabetes [18] . In this regard, MCC treatment was found to improve glucose tolerance and insulin sensitivity in HFD-fed mice.
Contemporary anti-obesity approaches have been targeted at the reduction of intestinal absorption [19] , the enhancement of fatty acid metabolic status in skeletal muscle [20] and the induction of mitochondrial uncoupling in skeletal muscle [21] . An earlier study on the weight loss effect of MCC revealed that MCC treatment (5.1 g/kg) decreased the weight gain in HFD-fed mice, presumably due to the reduction of intestinal lipid absorption in HFD-fed mice [8] . In the present study, we have further demonstrated that a lower dose of MCC (1.5 g/kg) induces mitochondrial uncoupling in skeletal muscle of HFD-fed mice and thus provides an extended view on the biochemical mechanism underlying weight loss effect of MCC. In this connection, an induction of mitochondrial uncoupling in skeletal muscle was found to augment the energy expenditure in uncoupling protein 1 up-regulated mice [22] .
5. Conclusion
In conclusion, MCC treatment at a dose of 1.5 g/kg was found to reduce the HFD-induced weight gain and the associated metabolic abnormalities in HFD-fed mice. The finding suggests that MCC may offer a promising prospect for ameliorating the diet-induced obesity and the associated metabolic disorders in humans.
References
References
Ahima, R.S. and Lazar, M.A. (2013) The Health Risk of Obesity-Better Metrics Imperative. Science, 341, 856-858. http://dx.doi.org/10.1126/science.1241244
Fontana, L.J., Eagon, C., Trujillo, M.E., Scherer, P.E. and Klein, S. (2007) Visceral Fat Adipokine Secretion Is Associated with Systemic Inflammation in Obese Humans. Diabetes, 56, 1010-1013. http://dx.doi.org/10.2337/db06-1656
Manninen, V., Tenkanen, L., Koskinen, P., Huttunen, J.K., Mänttäri, M., Heinonen, O.P. and Frick, M.H. (1992) Joint Effects of Serum Triglyceride and LDL Cholesterol and HDL Cholesterol Concentrations on Coronary Heartdisease Risk in the Helsinki Heart Study. Implications for Treatment. Circulation, 85, 37-45. http://dx.doi.org/10.1161/01.CIR.85.1.37
Wing, R.R. and Phelan, S. (2005) Long-Term Weight Loss Maintenance. The American Journal of Clinical Nutrition, 82, 222S-225S.
Caveney, E., Caveney, B.J., Somaratne, R., Turner, J.R. and Gourgiotis, L. (2011) Pharmaceutical Interventions for Obesity: A Public Health Perspective. Diabetes, Obesity and Metabolism, 13, 490-497. http://dx.doi.org/10.1111/j.1463-1326.2010.01353.x
Tseng, Y., Cypess, A.M. and Kahn, C.R. (2010) Cellular Bioenergetics as a Target for Obesity Therapy. Nature Reviews Drug Discovery, 9, 465-482. http://dx.doi.org/10.1038/nrd3138
Thrush, A.B., Dent, R., McPherson, R. and Harper, M.E. (2013) Implications of Mitochondrial Uncoupling in Skeletal Muscle in the Development and Treatment of Obesity. FEBS Journal, 280, 5015-5029. http://dx.doi.org/10.1111/febs.12399
Leong, P.K., Leung, H.Y., Wong, H.S., Chen, J., Ma, C.W., Yang, Y. and Ko, K.M. (2013) Long-Term Treatment with an Herbal Formula MCC Reduces the Weight Gain in High Fat Diet-Induced Obese Mice. Chinese Medicine, 4, 63-71. http://dx.doi.org/10.4236/cm.2013.43010
Siddiqua, M., Hamid, K., Rashid, M.H.A., Akther, M.S. and Choudhuri, M.S.K. (2010) Changes in Lipid Profile of Rat Plasma after Chronic Administration of Laghobanondo Rosh (LNR)—An Ayurvedic Formulation. Biology and Medicine, 2, 58-63.
Colberg, S.R., Simoneau, J.A., Thaete, F.L. and Kelley, D.E. (1995) Skeletal Muscle Utilization of Free Fatty Acids in Women with Visceral Obesity. The Journal of Clinical Investigation, 95, 1846-1853. http://dx.doi.org/10.1172/JCI117864
Crescenzo, R., Mainieri, D., Solinas, G., Montani, J.P., Seydoux, J., Liverini, G., Iossa, S. and Dulloo, A.G. (2003) Skeletal Muscle Mitochondrial Oxidative Capacity and Uncoupling Protein 3 Are Differently Influenced by Semistarvation and Refeeding. FEBS Letters, 544, 138-142. http://dx.doi.org/10.1016/S0014-5793(03)00491-5
Wang, C.Y. and Liao, J.K. (2012) A Mouse Model of Diet-Induced Obesity and Insulin Resistance. Methods in Molecular Biology, 821, 421-433. http://dx.doi.org/10.1007/978-1-61779-430-8_27
Martins, R., Nachbar, R.T., Gorjao, R., Vinolo, M.A., Festuccia, W.T., Lambertucci, R.H., Cury-Boaventura, M.F., Silveira, L.R., Curi, R. and Hirabara, S.M. (2012) Mechanisms Underlying Skeletal Muscle Insulin Resistance Induced by Fatty Acids: Importance of the Mitochondrial Function. Lipids in Health and Disease, 11, 30. http://dx.doi.org/10.1186/1476-511X-11-30
Feng, Y., Huang, S.L., Dou, W., Zhang, S., Chen, J.H., Shen, Y., Shen, J.H. and Leng, Y. (2010) Emodin, a Natural Product, Selectively Inhibits 11beta-Hydroxysteroid Dehydrogenase Type 1 and Ameliorates Metabolic Disorder in Diet-Induced Obese Mice. British Journal of Pharmacology, 161, 113-126. http://dx.doi.org/10.1111/j.1476-5381.2010.00826.x
Nakamura, M., Ikeda, M., Suzuki, A., Okinaga, S. and Arai, K. (1988) Metabolism of Round Spermatids: Gossypol Induces Uncoupling of Respiratory Chain and Oxidative Phosphorylation. Biology of Reproduction, 39, 771-778. http://dx.doi.org/10.1095/biolreprod39.4.771
Kraunsøe, R., Boushel, R., Hansen, C.N., Schjerling, P., Qvortrup, K., Støckel, P., Mikines, K.J. and Dela, F. (2010) Mitochondrial Respiration in Subcutaneous and Visceral Adipose Tissue from Patients with Morbid Obesity. The Journal of Physiology, 588, 2023-2032. http://dx.doi.org/10.1113/jphysiol.2009.184754
Girard, J. and Lafontan, M. (2008) Impact of Visceral Adipose Tissue on Liver Metabolism and Insulin Resistance. Part II: Visceral Adipose Tissue Production and Liver Metabolism. Diabetes & Metabolism, 34, 439-445. http://dx.doi.org/10.1016/j.diabet.2008.04.002
Knight, J.A. (2011) Diseases and Disorders Associated with Excess Body Weight. Annals of Clinical & Laboratory Science, 41, 107-121.
Caveney, E., Caveney, B.J., Somaratne, R., Turner, J.R. and Gourgiotis, L. (2011) Pharmaceutical Interventions for Obesity: A Public Health Perspective. Diabetes, Obesity and Metabolism, 13, 490-497. http://dx.doi.org/10.1111/j.1463-1326.2010.01353.x
Goto, T., Teraminami, A., Lee, J.Y., Ohyama, K., Fu-nakoshi, K., Kim, Y.I., Hirai, S., Uemura, T., Yu, R., Takahashi, N. and Kawada, T. (2012) Tiliroside, a Glycosidic Flavo-Noid, Ameliorates Obesity-Induced Metabolic Disorders via Activation of Adiponectin Signaling Followed by Enhancement of Fatty Acid Oxidation in Liver and Skeletal Muscle in Obese-Diabetic Mice. The Journal of Nutritional Biochemistry, 23, 768-776. http://dx.doi.org/10.1016/j.jnutbio.2011.04.001
Costford, S., Gowing, A. and Harper, M.E. (2007) Mitochondrial Uncoupling as a Target in the Treatment of Obesity. Current Opinion in Clinical Nutrition & Metabolic Care, 10, 671-678. http://dx.doi.org/10.1097/MCO.0b013e3282f0dbe4
Adjeitey, C.N., Mailloux, R.J., Dekemp, R.A. and Harpe, M.E. (2013) Mitochondrial Uncoupling in Skeletal Muscle by UCP1 Augments Energy Expenditure and Glutathione Content While Mitigating ROS Production. American Journal of Physiology—Endocrinology and Metabolism, 305, E405-E415. http://dx.doi.org/10.1152/ajpendo.00057.2013
List of Abbreviations
NOTES

*Corresponding author.


