Health
Vol.08 No.03(2016), Article ID:63584,21 pages
10.4236/health.2016.83026
LDL-Related Intolerance to Glucose, Diastolic Hypertension and Additive Effects of Smoking Were Found with Three Female Study Groups
Ruth-Maria Korth
Practice and Research in General Medicine FiDA, Munich, Germany

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 9 December 2015; accepted 16 February 2016; published 19 February 2016
ABSTRACT
Initial prodiabetic risk profiles were invented here with three female study groups consisting of primarily healthy women (A1: 1990-1999, n = 160; A2: 2009, n = 88; A: n = 248, 36 ± 14 years; B: 2014: n = 65, aged 37± 11 years). Significantly higher blood pressure was found comparing intolerance versus tolerance to glucose (p < 0.05, IGTT, 22 of 68). High LDL-cholesterol (LDL-C) showed additive effects as LDL-related intolerance was further related with rise of blood pressure (p < 0.05), of triglycerides (p = 0.02), of fasting blood glucose (p = 0.07) and of urine pathology (p = 0.07). High LDL-C of women who reported smoking at baseline was correlated with diastolic hypertension whereby alcohol problems overlapped (p = 0.036, A). Unhealthy combinations were found consisting of LDL-related intolerance to glucose, LDL-related smoking, of alcohol-related hypertriglyceridemia or of combined drinking and smoking testing urine pathology over the course of time. Obese women were at direct risk for hypertension in the presence of high LDL-C and submaximal ratio of serum albumin to triglycerides (Alb/Trig). Obese women reacted highly sensitive to critical alcohol con- sumption showing then macroalbuminuria. Current participants who disowned daily alcohol con- sumption showed healthy morning urines and normal fasting blood glucose. Mild decrease of HDL-C was observed during heavy smoking of relatively young women who had normal biomarkers. Women with intolerance to glucose were at direct risk for hypertension whereby high LDL-C and/or smoking triggered prodiabetic risk profiles. Obese women had elevated LDL-C during hypertension and reacted highly sensitive to alcohol-related proteinuria and/or hematuria.
Keywords:
Combined Telemedical Care: Women’s Health, Obesity, LDL-Intolerance to Glucose, Diastolic Hypertension, Ratio of Serum Albumin to Triglycerides (Alb/Trig), Albuminuria

1. Introduction
Individual and combined risk factors characterized here middle aged women selected out of three consecutive study groups. Coded biomarkers were enrolled using equivalent criterions to determine initial risk factors of non-diabetic women over indicated time periods since 1990. Intolerance to glucose was compared to controls and dependable risk factors characterized then risk profiles for diastolic hypertension and critical glucose profiles. High LDL-C and/or self-reported smoking were evaluated with blood pressure, metabolic profiles and morning urines of primairily healthy women.
Several local study groups have provided evidence before that men and/or women with LDL-related hypertriglyceridemia have significantly higher blood pressure compared to normolipidemic persons [1] - [3] . Critical alcohol consumption of hyperlipidemic persons aggravates the risk for diastolic hypertension, significant rise of LDL/HDL, lowering of HDL-cholesterol (HDL-C) and rise of urine pathology compared to hyperlipidemic women reporting healthy lifestyle [1] . Obesity, hypertriglyceridemia and critical alcohol consumption were related with significantly lower serum albumin to triglycerides ratio (Alb/Trig) compared to appropriate controls [1] - [4] . Proteinuria or hematuria were not directly related with smoking or with rise of blood pressure perhaps because evaluated women were relatively young and the majority showed normal biomarkers [1] .
The clinical study program was originally based on recognized pathways of alcohol-related phosphocholines as those specifically activate human cells [4] - [6] . Chemically defined alkyl-acyl-(long-chain)-phosphocholines (AAGPC, LA-paf) are released from cells together with apoprotein B [7] - [9] . Apoprotein B and related lipoproteins carry lipophilic phosphocholines to explain at least in part why LDL- or VLDL-particles upregulate human cells and make human endothelial cells sticky [7] - [9] . Phospholipases on outer membranes of human endothelial cells trigger release deacylated phosphocholines (lysopaf) and serum albumin incorporates lysopaf to some degree [9] - [12] . Functional serum albumin was invented here based on opposite relationships between serum albumin and triglycerides (Alb/Trig). Endogenous transcytosis of albumin-bound lysopaf across dysfunctional endothelium barriers has been shown before with cerebrospinal fluids (CSF) whereby upregulatory potency of lysopaf is clinically shown with significant increase of psychotic symptoms [13] [14] .
Purified serum albumin protects human cells and completes specific antagonists of phosphocholines, so-called Ginkgoloides [9] [15] - [18] . Pure serum albumin further protects human cells against unhealthy formation and release of acetylhydrolases, phospholipases A2 (PLA2) [13] . Opposite unfavourable effects of human LDL-par- ticles trigger accelerated formation of phospholipases to be then expressed on outer membranes of human endothelial cells, monocytic cells and platelets [10] [12] [13] . Receptor-dependent transcytosis of paf-like phosphocholines is confirmed with immune histology of large endosomal compartments showing that paf receptors are expressed in/on endothelial cells [19] . Early animal models show transcytosis of labeled serum albumin or of LDL-particles across endothelium of isolated organs [20] .
Clinical studies correlate thickening of arterial intima media during insulin resistance, mild hypertension and increased plasma levels of antibodies against lipoprotein-associated phospholipids (LA-paf) [21] . Biomedical reports support migration of smooth muscle cells into the arterial intima whereby combined signalling of neighbouring cells is based on several growth factors such as insulin-like growth factor, vascular endothelial growth factor and/or angiotensin II [22] [23] .
Tobacco-related rise of urine pathology has been found with morning urines of men but alcohol consumption overlapped [2] [3] . Reviews summarize various tobacco-related aldehydes suggesting conjugation of proteins whereby radicals in arterial plaques impair lipoprotein-associated phospholipases during late arterial disorders [24] - [28] . High prevalence of smoking is correlated with type 2 diabetes of elderly persons in a Finnish study suggesting that smoking impairs regeneration of pancreatic betacells [29] . Impaired filtration of renal endothelium is shown with insulin, thyroid hormones and angiotensinogen in urinary compartments of diabetic persons [30] .
Clinical observations show enhanced exsudation of labeled albumin into tissues during obesity and insulin resistance of men while no relevant exsudation of labeled albumin has been found of persons during isolated hypertension [31] . Clinical follow-up studies establish the predictory values of persistent albuminuria/proteinu- ria showing high prevalence of sustainable hypertension, stroke and/or cardiovascular disorders among elderly US persons [32] . Hypertension and proteinuria predict renal failure as shown with a 24 year follow up study of 1462 women in Sweden [33] . The present study invented critical glucose profiles and mild hypertension at baseline of middle-aged women who often reported smoking at baseline.
Physicians know that persistent hypertension needs pharmacotherapy in the presence of additive risk factors and that sustainable hypertension needs early antihypertensive pharmacotherapy with diuretics and/or antagonists against adrenergic receptors, angiotensins or elevated aldosterone (≥150/≥100 mmHg). Dietary supplements are adapted to complete antihypertensive pharmacotherapy and benefit is shown with treated hypertension of abstinent former alcohol abusers [6] [17] .
Next, hyperlipidemic persons are at risk for hypertension and react highly sensitive to critical alcohol consumption [1] - [3] . Alcohol-free liquids are developed to replace alcohol use and to reduce uptake of cholesterol, saturated fat, sugar alcohol, adverse carbohydrates, sodium, transformed phospholipids to neutralize lipid-related problems [17] . Skilled persons know that transformed food components contain unhealthy transformed fatty acids and that some dairy products contain unfavourable glycated albumin so that only certified low fat dairy products and certified oils from ecologically grown plants were recommended as healthy supplements [17] [34] [35] . Lipid lowering strategies aim to reduce high triglycerides and balance insulin-related uptake of glucose. Biomedical pathways of high triglycerides are shown with cells as triglycerides trigger insulin resistance, translocation of glucose transporters and activation of proteinkinases [36] .
Physicians know that abdominal fat of younger persons triggers insulin resistance and that metabolic syndromes predict arterial lesions at 21 to 39 years of age [37] . Rise of childhood obesity over the past 30 years leads to higher quality of school lunch, improves dietary education of children and skills healthy food early in life [38] . Dietary strategies recommend obese persons to reduce uptake of salty food, of saturated fat and of adverse carbohydrates to neutralize insulin resistance [39] .
Higher risk of obese women for thrombotic events must be considered during oral uptake of contraceptives as obese families often show endothelial-derived thrombotic risk factors [40] [41] . Obese women are at higher risk for antiphospholipid syndromes based on endogenous formation of antibodies against adverse carrier proteins initiating then a dramatic inflammatory cross talk between endothelium and smooth muscle cells with accelerated risk for thrombotic events [42] [43] .
Classical antihypertensive diets recommend low fat dairy products and fresh vegetables to reduce uptake of sodium, fat and sugar [44] . These recommendations are originally based on animal models teaching that high glucose of aging rats impairs endothelium-related glycoprotein matrix leading to disturbed filtration [45] . In addition, perfusion of the aortic cannula of rats with high doses of lysophospholipids triggers arrythmia during accelerated excretion of lysophospholipids into the cardial tissue [46] . Moderate intensity of exercising lowers expression of voltage-dependent calcium channels whereby these voltage-gated channels sensitize rats to adverse renal responses during intake of high sodium [47] . Insulin resistance and higher sensitivity to hypertension might share vulnerable regions of chromosome 4 at least of rats [48] . Recent dietary strategies monitor 24 h urines to reach healthy relationships of urinary sodium and potassium and recommend to reduce uptake of sodium and to increase uptake of potassium e.g. with vegetables [49] - [51] .
Local internet presentations of the practice provide comprehensible stategies in good time against alcohol-related hypertriglyceridemia and related albuminuria (www.fida-aha.com). Telemedical informations are combined here with personal counseling and physical examinations recommend healthy food, lifestyle and exercising (www.fidabus.com) [1] - [6] . Coded case reports motivated as abstinent former alcohol abusers overcome hypertension during treatment with antihypertensive pharmacotherapy and healing of hematuria during complementary treatment with purified extracts from Ginkgo biloba [6] [17] [52] . Elderly hypercholesterolemic women who report heavy smoking overcome mild diabetes and hematuria during long lasting cessation of smoking providing prescriptions to lower cholesterol combined with Ginkgoloides to repair renal endothelium barriers [17] [53] - [55] . Low fat dairy products were supplemented with low doses of vitamin D considering that high doses of steroid derivatives can enhance the risk for hypertension [17] [56] . Plasmatic potassium, thyroidea hormones, C-reactive proteins, uric acids and glomerular filtration rates were tested and normal values widely excluded here hormonal, disorders, renal hypertension and silent kidney disorders at baseline [56] - [58] .
The present study invented here the defense potency of opposite plasma compartments (LDL/HDL, Alb/Trig) testing blood pressure and morning urines. Combined monitoring aimed to improve self assessment of lifestyle problems as critical alcohol cosumption was often reported. Intolerance to glucose of non-pregnant women was invented here as independent risk factor of middle-aged women using two statistical methods. Interrelationships of dependable variants were then characterized to be neutralized. Isolated or combined risk factors can be neutralized to protect against progression of hypertension, prodiabetic risk profiles or disturbed renal endothelium barriers.
2. Subjects and Methods
Three female study groups were enrolled based on equivalent criterions using medical standard procedures as shown before [1] - [3] . The local ethical authority has approved the study program with self control documents and informed written consent of participants (BLÄK-EK No. 02088). The study was conducted with coded biomarkers of primairily healthy women who initially attended the practice of General Medicine since 1990 (FiDA-practice).
Participants did not suffer of known disorders as women were not included who had at least one of the below mentioned disorders at baseline (A: n = 923, B: n = 73). Complete blood counting excluded hematological disorders at baseline. Women with primairily known diabetes mellitus were not included (HBA1 ≤ 6%). Unexpected diabetes was treated at baseline and were not evaluated here. Patients with known inflammatory, urological, cerebral or neoplastic disorders were not included. Women with hepatitis or liver cirrhosis were excluded. Women using pharmaceutical treatment such as lipid-lowering and/or antidiabetic were not included. Sustainable systolic hypertension had to be treated at baseline (>160 mmHg, not shown here) and treated persons were not included. Women attending the practice only for vaccination were not included. Urine testing was not performed during menstruation. Women with known pregnancy were not included here.
Coded biomarkers were divided into three study groups enrolled over the course of time. First/early (A1: 1990-1999: n = 160, aged 30 ± 10 years) and second/recent study groups (A2-2009: n = 88, aged 37 ± 16 years) were compared. Equivalent risk factors were indicated and/or pooled (A1 & A2, n = 248 (A), aged 36 ± 14 years). Currently scored women were also characterized with coded biomarkers (B: 2010-2014, n = 65, aged 37 ± 11 years).
Age, personal history, intake of oral contraceptives, daily exercising and family disorders were initially documented. Self-reported risky/critical alcohol consumption was stated (≥20 g ethanol/day) in accordance with the Official German alcohol guidelines [59] . The number of daily cigarettes was confidentially documented as well. Physical examinations were provided at baseline in the practice of General Medicine (hereinafter Fida- practice) whereby body weight, blood pressure and morning urines were enrolled. Body mass index classified normal weight (BMIn: <25 kg/m2), overweight (BMI1: ≥25 kg/m2) or obesity (BMI2: ≥29 kg/m2). Blood pressure was determined during initial examination after 10 min resting. Hypertension was stated with systolic or diastolic blood pressure (140 - 160 or ≥90 mmHg, stage 1). Pro-hypertension (135 - 139/85 - 89 mmHg) or normal blood pressure (<135/<85 mmHg) were indicated.
Venous blood was initially taken after 12 h fasting and metabolic profiles were determined at baseline. Clinical chemistry was performed using medical standard procedures. C-reactive proteins (CRP < 0.5 mg/dl), thyroid stimulating hormones (normal TSH: 0.3 - 3.5 µU/ml), serum potassium (normal 3.5 - 5.0 mmol/l) and liver values were indicated. Normal plasma creatinine (<1.3 mg/dl) and healthy glomerular filtration rates (GFR/MDRD- formula) were indicated excluding here silent kidney disorders (101 ± 19 > 90 ml/min/1.7 m2) [60] . Uric acids were in the normal range (4.4 ± 1.0 < 6 mg/dl). Lipases were in the normal range as well (40 ± 12 < 60 U/l).
Fasting blood glucose values were fully enrolled. Critical fasting and postprandial blood glucose were invented here as “prodiabetic risk profiles” (FG: 90-119 mg/dl, 21% of A (n = 53); 14% of B (n = 9)). Normal (LDL-C < 150) or elevated LDL-C (≥150 mg/dl) classified fasting and postprandial blood glucose (1 h pp). Tolerance to glucose was tested one hour after oral uptake of 100 g glucose to compare intolerance versus normal tolerance to glucose (C out of A1 & A2 & B, n = 68). Intolerance to glucose (IGTT: 1 hpp 172 ± 40, 2 hpp 138 ± 22 mg/dl, 22 out of 68, aged 41 ± 15 years) or normal tolerance were found (NormGTT: 1 hpp < 140 mg/dl, 46 out of 68, aged 38 ± 19 years). Two of these women with intolerance to glucose showed elevated HBA1c (6% - 6.5%) and one case with diabetes was found (FG: 345 mg/dl, HbA1c: 12.5%). These women were treated and not evaluated here.
Direct comparison of mild intolerance versus tolerance of glucose was performed in the presence of critical (FG 90-119 mg/dl, D) or normal fasting blood glucose (FG < 90 mg/dl, E). Intolerance to glucose was characterized during high LDL-C and so-called “LDL-related intolerance to glucose” was evaluated using multivariate analysis. Smoking and high LDL-C further characterized intolerance to glucose (A5: IGTT & NIC, n = 13, LDL-C: 146 ± 25 mg/dl, aged 41 ± 21 years) compared to nomal LDL-C (A6: IGTT & NIC, LDL-C < 150 mg/dl: 135 ± 4 mg/dl, n = 3). Blood pressure of non-smoking IGTT women with high LDL-C was also tested (A7: NoNic-IGTT, LDL-C: 167 ± 47 mg/dl, n = 10).
High LDL-cholesterol characterized previously and currently scored biomarkers (LDL-C ≥ 150 mg/dl, 11% of 248 (A), aged 35 ± 15 years; 17% out of 65 (B), aged 49 ± 6 years). High triglycerides and high cholesterol were related (p = 0.06, Trig ≥ 170 mg/dl, 15% of A, aged 41 ± 16 years, chol ≥ 200 mg/dl, 17% of A, aged 40±19 year). Mixed hyperlipidemia and higher blood pressure were correlated before (p < 0.05, LDL + Trig: 10% of A, aged 35 ± 15 years versus normolipidemia 18% of A, aged 31 ± 11 years) [1] . Current participants were characterized with LDL-C, with high triglycerides (B: Trig ≥ 160 mg/dl: 9% of 65, aged 51 ± 11 years) or with normolipidemia (n = 54 out of B, 83% of 65, aged 33 ± 10 years).
Divisions of heavy or moderate smoking were previously observed testing adverse cholesterol distribution (LDL/HDL ≥ 3; ≥20 cig: 62% of 60 or <20 cig/day: 38% of 60 (A)). HDL-C currently characterized self-re- ported smoking (≥20 cig: 12% or <20 cig: 23% of B). Submaximal ratio of serum albumin to triglycerides (Alb/Trig < 40: 22% of B, aged 48 ± 16 years) or low HDL-C (HDL-C < 50 mg/dl: 23% of 65 (B), aged 38 ± 12 years) determined dyslipidemic risk profiles of currently scored women.
2.1. Urine Testing
Morning urines were fully screened over the course of time and urine pathology was tested as described [1] . Test strips were initially used to determine proteinuria and/or hematuria and to exclude leukocyturia (Combur of Roche). Women with leukocyturia were initially excluded. Urinary albumin and urinary creatinine were tested using first morning urines (Microalbustix from Bayer, Germany). Proteinuria, hematuria or albuminuria were confirmed in a collaborative laboratory (Synlab Munich). Urine microscopy confirmed intact red cells and excluded pathological casts. Proteinuria and albuminuria were confirmed using protein analysis to exclude pathological proteins. Normal glomerular filtration rates excluded silent kidney disorders [60] . Total urinary calcium in morning urines (n = 26, mmol/l) was tested with Arsenazo III method [61] in the collaborative laboratory (Olympus, photometric Calcium Arsenazo, normal value 1.5 - 6 mmol/l).
2.2. Statistical Methods
Medical data were coded and indicated here with means ± standard deviations. Blinded biomarkers were divided into time-related study groups. Intolerance and tolerance to glucose were compared (C: n = 68). It is noted that only one case of intolerance to glucose was currently found (B). Dependable variants were tested with multivariate ranking to classify biomarkers with critical fasting blood glucose, rise of blood pressure, hypertriglyceridemia, serum albumin, urine pathology, high LDL-C or self-reported drinking and/or smoking (Table 1). Alcohol-related urine pathology and triglyceride-related hypertension have been evaluated before using two statistical methods [1] .
Direct comparison of blinded cohorts were evaluated by statistical experts using Tukey’s tests for pairwise comparisons, controlling type I error rate from a generalized linear model (GLM, SAS-V8.2, PROC GLM, estimates, Augsburg, Germany). The multiple logistic regression evaluated related symptoms (SAS V8.2, PROC LOGIST “multivariate analysis”).
2.3. Combined Telemedical Monitoring
Self control documentations were initially offered in the practice and women provided informed, written consent to initiate combined monitoring. Home testing of body weight, blood pressure, morning urines was combined with medical examinations [1] - [3] . Telemedical informations improved self assessment to overcome adverse alcohol consumption and to improve the quality of food and lifestyle. Healthy exercising was recommended, for example to walk at least 30 minutes per day. Healthy prescriptions were provided (www.fidabus.com, webstart 05/01/2001, www.fida-aha.com, webstart 10/31/2002), Germany). Non-pregnant participants were motivated
Table 1. Three female study groups had overlapping risk profiles, Alcohol use and hyperlipidemia (AHA, A) were related with hypertension (<0.05). High LDL-C, obesity, LDL-related hypertriglyceridemia and diastolic were also currently found (B).
Drinking, smoking and hyperlipidemia overlapped and were related with diastolic hypertension (p < 0.05, A1&A2) and with urine pathology (p = 0.005) compared to hyperlipidemic women reporting healthy lifestyle [1] . Alcohol-relate dyslipidemia triggered hematuria and hypertension (122 ± 20/94 ± 5 mmHg; Alb/Trig 35±12, HDL: 46 ± 9; 6 out of A2). Alcoholic mixed hyperlipidemia (AHA) was correlated with diastolic hypertension (p = 0.04) and with high LDL/HDL (3.6 ± 1, p = 0.001). The second study group (A2 was at highest risk for hematuria. Current benefit was based on lower alcohol use (14% (A), 6% (B). Values are means ± 1 S.D.
with coded case reports reporting benefit of healthy lifestyle. For example, pregnant women initiated and continued cessation of smoking and overcome mild intolerance to glucose without hypertension (www.fidabus.com). Consenting women continued medical monitoring combined with home testing of blood pressure, body weight, morning urines (not shown here).
Non-pregnant women with proteinuria and/or hematuria were treated with diluted purified extracts from Ginkgo biloba to repair renal endothelium during settled cessation periods as shown before [6] [9] [10] [15] - [17] . Benefit and safety of the specifically adapted Fida-compositions have been shown before with longitudinal studies over five years in follow (not shown here) [17] .
3. Results
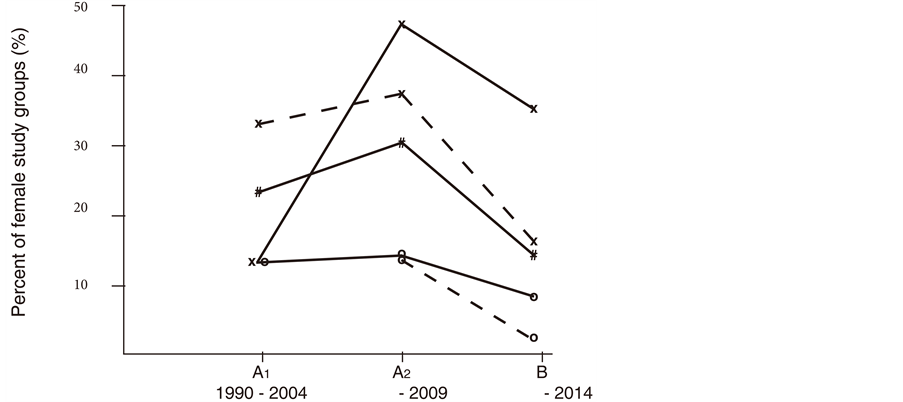
Individual risk factors overlapped at baseline and three study groups were characterized over the course of time in Table 1. Better rates of critical fasting blood glucose and of diastolic hypertension were found and currently scored women showed better risk profiles as the majority disowned daily alcohol consumption (Figure 1). Better rates of diastolic hypertension were paralleled with decrease of LDL-related smoking as women with high LDL-C currently more often reported healthy lifestyle behaviour (Figure 1). In addition, better rates of urine pathology were paralleled with decreased rates of combined drinking and smoking, with better rates of hypertriglyceridemia or with better cholesterol distribution (LDL/HDL in Figure 2). Healthy morning urines confirmed benefit of abstinent periods in a reliable manner.
Figure 1. Female cohorts were characterized (A1: n = 160; A2: n = 88; B: n = 65). Better rates of diastolic hypertension (x- - -x, ≥90 mmHg); of critical fasting blood glucose (#__#, 90-119 mg/dl), of alcohol consumption (O__0, ≥20 g ethanol/day) or of LDL-related smoking (o- - -o) were found with current participants (out of B, aged 37 ± 11 years). Self-reported smoking (X__X) failed relevant benefit. The characteristics of three study groups were shown here in the methods section and in Table 1.
Figure 2. Better rates of urine pathology (X−−X, A2: 26% of 88 (A2), B: 9% of 65 (B)) were paralleled with better cholesterol distribution (LDL/HDL≥3, *___*, 25% of A2:, 8% of B) and with decrease of hypertriglyceridemia (O−−O). Better rates of self-reported alcohol problems (#−−#, ≥20 g/day, 16% of A2, 6 % of B) and of combined drinking and smoking (#- - -#, 19% of A2, 6% of B) were paralleled with decrease of hematuria (x- - - x, 15% of A2, n = 1 in B). Characteristics and urine pathology of three study groups were shown in Table 1.
Rates of self-reported smoking were high and self-assessment of smoking was reliable (Figure 1). Additive effects of self-reported smoking were invented here testing glucose profiles, blood pressure and morning urines (Table 2 and Table 3).
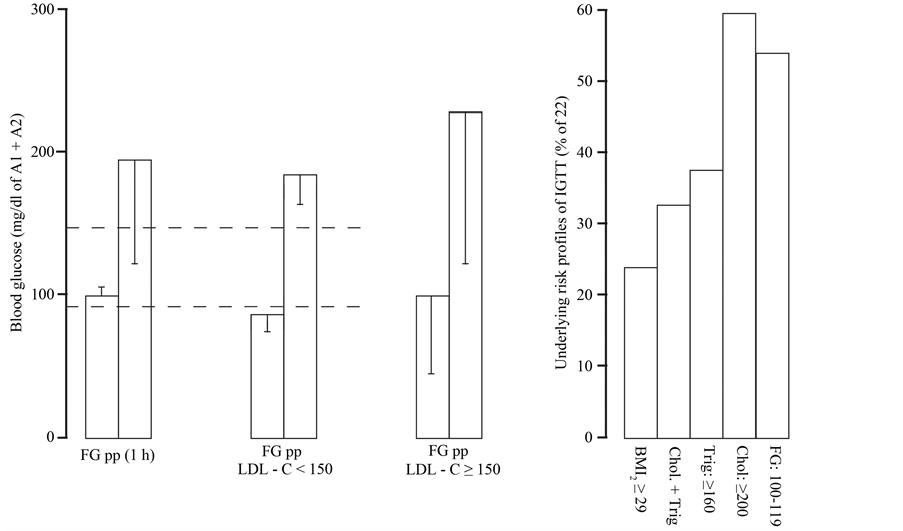
The first and second study groups showed equivalent proportions of high LDL-cholesterol and/or obesity while underlying risk profiles changed over the course of time (Table 3 and Table 4). Critical family history indicated age-related rise of vascular disorders, hypertension and diabetes (19% of A or B).
The current study group tended to normal glucose profiles, normal triglycerides and better rates of dependable variants evaluating here indicated risk profiles of non-pregnant women who often reported smoking.
3.1. Critical Glucose Profiles and Hypertension
Rates of critical glucose profiles currently decreased and rates of diastolic hypertension decreased as well (Figure 1, Table 1). Better rates of critical fasting blood glucose were currently found of women at higher age and these women often reported smoking (Table 2). Normal blood pressure was found with women having normal tolerance to glucose (Table 2).
Critical fasting and postprandial blood glucose overlapped with obesity, high triglycerides and mild increase of cholesterol overlapped with critical fasting blood glucose (Figure 3). LDL-related intolerance to glucose was then evaluated to overcome high variances of indicated prodiabetic risk profiles (Figure 3).
The direct comparison of intolerance versus tolerance to glucose determined raised blood pressure as major dependable variant (Figure 4, Table 2). Indeed, intolerance versus tolerance to glucose showed significantly
Table 2. Intolerance versus tolerance to glucose showed higher blood pressure (IGTT vs. NormGTT, p < 0.05**, C out of A + B).
Risky fasting blood glucose (FG) or intolerance to glucose were sensitive to higher age or smoking (IGTT, 1 hpp: 172 ± 40 mg/dl). Smoking or high LDL-C during IGTT triggered hypertension of women with overweight and high triglycerides overlapped. Alcohol use and obesity triggered hypertension and albuminuria (AHA: Alb/Trig: 31 ± 8, 28 ± 5g ethanol/day, 75 ± 3 mg/l U-albumin). One case of diabetes with hematuria was found among obese women during alcohol-related hyperlipidemia (see result section). Values are means ± 1 S.D.
Figure 3. Critical fasting (FG) and postprandial (1 h pp) blood glucose were characterized with normal LDL-C (<150135 ± 4 mg/dl, n = 3) or high LDL-C (≥150 mg/dl; n = 13). High variances indicated inhomogenous cohorts as obesity (BMI2: ≥29 kg/m2), hyperlipidemia (Chol + Trig), moderate increase of cholesterol (≥200 mg/dl) overlapped with critical fasting blood glucose (FG). Characteristics of women with indicated glucose profiles were shown in Table 2. Values are means ± 1 S.D.
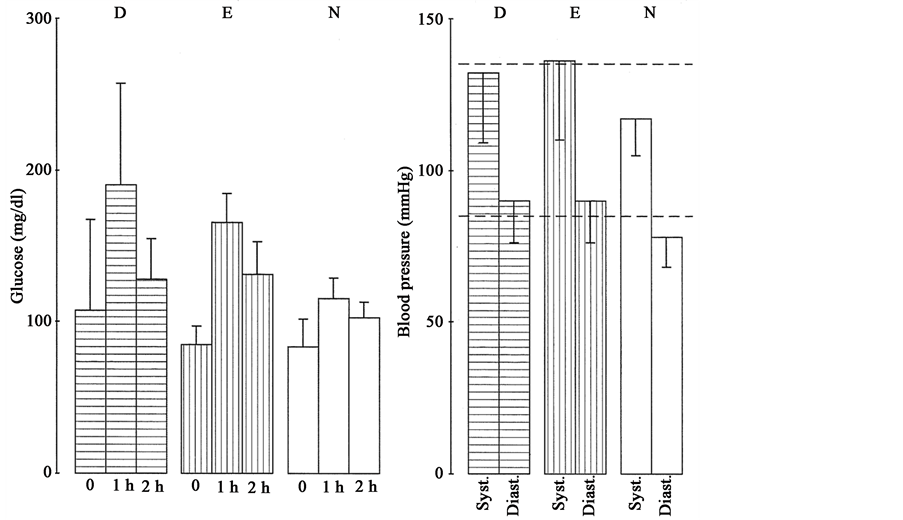
Figure 4. Blood glucose was determined preprandial or 1 h, 2 h after oral uptake of 100 g glucose and rise of blood pressure was found comparing women with intolerance versus normal tolerance to glucose (p < 0.05). Women with intolerance to glucose (IGTT) showed significantly higher blood pressure in the presence of critical (D, FG: 90 - 119 mg/dl, 1hpp: 191 ± 67 mg/dl, n = 10) or normal fasting blood glucose (E, FG < 90 mg/dl, 1hpp: 172 ± 40 mg/dl, n = 12) compared to normal glucose tolerance testing (N < 140 mg/dl, n = 46 of 68). Characteristics of indicated glucose profiles were shown in Table 2. Values are means ± 1 S.D.
Table 3. Diastolic hypertension of recent (A2) and current (B) groups showed high LDL-C, obesity and/or mixed hyperlipidemia and often reported smoking while the amount of reported alcohol consumption currently decreased (16 ± 9 g ethanol/day).
LDL-related hypertriglyceridemia versus normolipidemia showed here again higher blood pressure (LDL & Trig, p ≤ 0.05, [1] ). Currently scored diastolic hypertension was paralleled with LDL-related obesity or with LDL-related hypertriglyceridemia. HDL-C was lower during heavy smoking (HDL-C: 48 ± 2 mg/dl, 22 ± 8 cig/day: 6 of B) showig normal biomarkers. Low Alb/Trig and hypertension were currently paralleled (Alb/Trig 31 ± 8, 136 ± 12/98 ± 18 mmHg, n = 4, 29 ± 4 kg/m2). Normal serum potassium (B: 4.4. ± 0.1 mmol/l) and heal t h y glomerular filtration rates (101 ± 19 ml/min/1.7m2) were found.
Table 4. Obesity, high LDL-C, hypertension, low Alb/Trig and high sensitivity to alcohol consumption were found. Women with normal weight often smoked (BMIn), with overweight (BMI1) showed progress while obese women showed indicated risk profiles.
Obese women reacted highly sensitive to alcohol problems showing then macrolbuminuria, high LDL-C, low ratio of serum albumin to triglycerides (Alb/Trig 25 ± 5 < 30, Trig: 176 ± 49 mg/dl, Alb: 3.96 ± 0.24 ≤ 4 g, GammaGT: 16 ± 11 U/l, CRP-BMI2: 0.46 ± 0.2 mg/dl). Moderate smoking failed relevant effects while heavy smoking lowers HDL-C (n = 8 ≥ 20 cig vs n = 15 < 20 cig/day of B). Values are means ± 1 S.D.
higher systolic and diastolic blood pressure comparing intolerance versus tolerance to glucose in the presence of critical fasting blood glucose (Figure 4, IGTT-D: FG: 100-119 mg/dl, p=0.0029, p=0.002). Intolerance versus tolerance to glucose showed significantly higher blood pressure also in the presence of normal fasting blood glucose (Figure 4, IGTT-E: p = 0.0146, p = 0.0007). Intolerance to glucose showed a moderate relationship with critical fasting blood glucose (p = 0.07) and with diastolic hypertension using multivariate analysis (p = 0.07).
LDL-related intolerance to glucose was then more directly related with diastolic blood pressure (p = 0.047) and with high triglycerides (p = 0.02). Urine pathology (p = 0.07) was related with alcohol problems (p < 0.05) and then with critical fasting blood glucose of women with LDL-related intolerance to glucose (p = 0.07, Table 1).
Next, women with intolerance to glucose often reported smoking and a weak relationship was confirmed between intolerance to glucose and smoking using multivariate analysis (p = 0.09, Table 2). Smoking women with IGTT showed systolic and diastolic hypertension (Figure 5(A5), Table 2). Smoking IGTT-women with normal LDL-C also showed systolic and diastolic hypertension (Figure 5(A6), Table 2). The motivating message was that non-smoking women with intolerance to glucose had normal blood pressure even in presence of high LDL-C (Figure 5(A7), Table 2). Thus, smoking more than high LDL-C aggravated systolic and diastolic hypertension during intolerance to glucose (IGTT & NIC: Table 2).
Altogether, intolerance to glucose was a direct risk factor for diastolic hypertension and smoking and/or high LDL-C were major additive risk factors for hypertension or critical fasting blood glucose. Overall, women with LDL-related intolerance to glucose who smoked had to perceive that smoking during LDL-related intolerance to glucose placed them into an even higher risk class for sustainable hypertension whereby alcohol problesm further triggered urine pathology and critical fasting blood glucose. These data implicated cessation of smoking and drinking to overcome moderate intolerance to glucose with healthy food and lifestyle.
3.2. Diastolic Hypertension and LDL-Cholesterol
Better rates of diastolic hypertension were paralleled here with decrease of LDL-related smoking keeping in mind that the pooled cohort (A1 & A2) with LDL-related hypertriglyceridemia was correlated with diastolic hypertension [1] . The recently scored women with diastolic hypertension showed imbalance of plasma compartments (LDL/HDL ≥ 3, Alb/Trig < 40) while HDL-C was in the normal range (A2 in Table 3).
Currently scored women with diastolic hypertension and/or with high LDL-C showed equivalent risk factors at higher age (B, Table 3). Hypertriglyceridemia currently decreased of women who were about ten years older (Table 1). Half of the currently scored women with diastolic hypertension showed obesity and high LDL-C and
Figure 5. Systolic and diastolic blood pressure or urine pathology were found during intolerance to glucose (A5: IGTT & NIC n = 13, LDL: 146 ± 25 mg/dl). Three smoking IGTT-women with normal LDL-C showed hypertension as well (A6: IGTT & NIC, LDL: 135 ± 4 mg/dl, n = 3). Non-smoking women with IGTT showed normal blood pressure and normal morning urines in the presence of high LDL-C (A7: NoNIC-IGTT, n = 10, LDL: 167 ± 47, n = 10). Urine pathology was not found with IGTT-women who disowned alcohol consumption (A6, A7). Heavy smoking impaired cholesterol distribution (LDL/HDL) with high variances. Characteristics of indicated glucose profiles were shown in Table 2. Values were means ± 1 S.D.
the other half had LDL-related hypertriglyceridemia and often reported alcohol problems and smoking (LDL & Trig, Table 3).
Women with submaximal ratio of serum albumin to triglycerides (Alb/Trig < 40) recently and currently showed diastolic hypertension, often reported drinking and smoking and often showed urine pathology (Table 3). Low ALB/Trig was paralleled with hypertension of women with overweight or obesity whereby high triglycerides rather than low serum albumin triggered hypertension as serum albumin was still in the normal range (Table 3).
Smoking and high LDL-C previously overlapped with alcohol consumption forming then a higher-risk combination for diastolic hypertension (p = 0.036) and for critical morning urines (p = 0.06). Better rates of diastolic hypertension were currently found as women with high LDL-C disowned smoking and drinking (Table 3). Isolated smoking or isolated rise of LDL-C of abstinent women failed direct relationships with urine pathology using multivariate analysis (p > 0.1, Table 4).
Obese women previously and currently showed significantly higher blood pressure compared to women with normal weight (p ≤ 0.05, Table 1). Currently scored obese women with diastolic hypertension had high LDL-C with submaximal ratio of serum albumin (Table 4). Half of the currently scored obese women reported alcohol problems showing then macroalbuminuria (Table 1 and Table 4).
Obese women often reported different lifestyle problems so that obesity cohorts were not pooled here (Table 4). It was clinically observed that half of the currently scored obese women reported alcohol consumption show- ing then macroalbuminuria and hypertension (Table 4). The recent obesity cohort often reported smoking without alcohol problems showing then diastolic hypertension with normal morning urines (Table 4). The first obesity cohort often reported alcohol use and showed hypertension with proteinuria and/or hematuria (Table 1).
Next, oral contraceptives did not modulate blood pressure as shown with multivariate analysis (p > 0.1, 20% of A). Obese women who reported uptake of oral contraceptives showed hypertension with normal morning urines (140 ± 20/95 ± 15 mmHg, n = 3 of A). The majority of the current participants had normal blood pressure and normal LDL-cholesterol during oral uptake of contraceptives (27 ± 7 years, LDL-C: 127 ± 36 mg/dl, 117 ± 13/82 ± 9 mmHg, 22 ± 4 kg/m2 of A).
The motivating message was that women at risk were currently about ten years older (Tables 1-4). The currently scored women with overweight often reported healthy lifestyle and tended to normal lipid profiles, normal blood pressure and healthy morning urines (BMI1 Table 4). The majority of the participants had normal weight and normal biomarkers while relatively young women with normal weight currently often reported smoking (BMIn, Table 4).
Women with obesity or overweight reacted highly sensitive to hypertension in the presence of LDL-related hypertriglyceridemia whereby smoking during critical alcohol consumption aggravated urine pathology (Table 3). Obese women currently showed the major risk profile consisting of high LDL-C, diastolic hypertension, submaximal Alb/Trig and albuminuria (Table 4). Obese women reacted highly sensitive to alcohol-related albuminuria implicating to cede daily alcohol consumption and/or to prepare antihypertensive pharmacotherapy.
3.3. HDL-Related Female Defense System and Smoking
Better rates of LDL/HDL were paralleled with reduced alcohol consumption and healthy morning urines were often found (Figure 2). Alcohol consumption decreased while proportions of smoking remained relevant (Figure 1). Normolipidemic women who reported drinking and/or smoking had normal HDL-C compared to normolipidemic women with healthy lifestyle (p > 0.1).
Significant increase of smoking was found comparing the first versus second study groups (A2 vs A1, p = 0.003, Figure 1) but no effects were found with LDL/HDL or with HDL-C comparing the first and second study groups (p > 0.1). Multivariate analysis further argued against direct relationhships of isolated smoking and HDL-C (p > 0.1). Nevertheless, it was observed that heavy smoking previously triggered high variances of LDL/HDL (Figure 5).
Current participants who reported heavy smoking showed a mild decrease of HDL-C here for the first time. Heavy smoking in the presence of low HDL-C further decreased HDL-C (HDL-C: 48 ± 2 < 50 mg/dl, 23 ± 1 kg/m2, 22 ± 8 cig./day, n = 5 out of Table 3). Moderate smoking showed normal HDL-C and normal biomarkers (n = 6, HDL-C: 62 ± 15 mg/dl, 7 ± 4 cig/day). In short, self-reporting smoking failed significancy testing isolated smoking among relatively young women but might impair cholesterol efflux to some degree.
Altogether, mild decrease of HDL-C was observed during heavy smoking of current participants in the presence of normal blood pressure and normal morning urines. These data implicated to cede smoking and to repair cholesterol efflux during settled cessation periods with healthy alcohol free liquids comprising supplements obtained from ecologically grown food such as appropriate vitamins, minerals and appropriate constituents.
3.4. Hematuria, Proteinuria, Albuminuria, Urinary Calcium Excretion
Better morning urines were currently paralleled with normal LDL/HDL and rates of initially reported alcohol problems also decreased (Figure 2). Healthy morning urines and better rates of urine pathology were paralleled here with decrease of combined drinking and smoking (Figure 2). In short, rates and amount of daily alcohol consumption currently decreased (6% of B: 16 ± 9 g/day ethanol (B), Table 1).
The recent study group showed the highest rate of alcohol-mediated hematuria and/or proteinuria during mixed hyperlipidemia with high LDL/HDL and low ratio of albumin to triglycerides (LDL/HDL: 3.6 ± 1, Alb/Trig 26 ± 8). Hematuria overlapped with drinking and smoking and LDL-related mixed hyperlipidemia triggered hypertension and adverse outcome of alcohol-related dyslipidemia was shown with recently and currently scored women as alcohol problems of hyperlipidemic women were again paralleled with urine pathology (Table 3).
Urine pathology was not directly related with hypertension using multivariate analysis (p > 0.1). However, alcohol consumption of hyperlipidemic women was related with urine pathology (p = 0.07). Thus, low HDL-C indicated degradation of HDL-particles in the presence of activated renal endothelium (LDL & Trig in Table 3). It has been shown before that alcohol use mediated urine pathology (p = 0.04) and that smoking aggravated alcohol-related urine pathology of hyperlipidemic women (p = 0.001, A [1] ).
Reliable benefit was currently reached with women who disowned alcohol use showing then better morning urines (Figure 2). Alcohol-related hypertriglyceridemia currently decreased and better rates of morning urines were currently found (Table 1).
Obese women were currently at higher risk as half of these women reported critical alcohol consumption and showed macroalbuminuria (75 ± 23 mg/l U-albumin, Table 2). Women with obesity or diastolic hypertension tended to mild albuminuria in general (30 ± 29 mg/l or 23 ± 10 mg/l urinary-albumin). The motivating message was that glomerular filtration rates were in the normal range (107 ± 29 ml/min/1.7 m2).
Next, urinary calcium was in the normal range indicating normal tubular reabsorption of calcium even during obesity or hypertension (1.7 ± 0.9, 1.7 ± 0.9 mmol/l). Mild increase of urinary calcium was found in the first morning urines only of smoking women (2.1 ± 0.8 mmol/g, n = 3) compared to non-smoking women (1.3 ± 1.1 mmol/g urinary creatinine, n = 14, aged 36 ± 7 years). Normal levels of total urinary calcium were found during oral uptake of contraceptives (1.5 ± 0.9 mmol/l; 1.7 ± 1 mmol/l).
Altogether, hematuria was paralleled with alcohol-related dyslipidemia of recently scored women who had high LDL/HDL, low HDL-C, low Alb/Trig and hypertension. Alcohol-related dyslipidemia or alcohol-related obesity triggered proteinuria and/or hematuria. Obese women reacted highly sensitive to critical alcohol consumption showing then macroalbuminuria.
Medical counseling recommended to cede drinking and smoking and to combine low fat dairy products with certified plant oils comprising healthy lecithins. Alcohol-free liquids were recommended comprising healthy albumin, supplements, minerals and constituents to repair alcohol-related dysfunction of renal endothelium barriers during abstinent periods.
4. Discussion
Women with intolerance to glucose reacted here highly sensitive to hypertension compared to controls whereby elevated LDL-C or smoking showed additive effects. LDL-related intolerance to glucose was associated with diastolic hypertension and with critical fasting blood glucose independently of age. The majority of the current participants disowned daily alcohol consumption showing then dependable benefit with lower rates of diastolic hypertension, of critical glucose profiles and more often healthy morning urines. Overall, alcohol-related hypertriglyceridemia decreased as shown with currently scored women attending the Fida-practice at baseline.
Better rates of urine pathology were paralleled with better rates of alcohol use, alcohol-related smoking, alcohol-related hyperlipidemia (LDL/HDL ≥ 3) or alcohol-related hypertriglyceridemia of current participants who often disowned daily alcohol consumption. The motivating message was that alcohol consumption was reduced of currently scored women and healthy biomarkers were found at baseline.
Intolerance to glucose was compared to controls and dependable variants characterized here primairily non-diabetic women who tended to mild hypertension, critical fasting blood glucose and/or elevated LDL-C. Unhealthy combinations of indicated risk factors were found with currently scored women having LDL-related intolerance to glucose, LDL-related smoking, alcohol-related smoking, alcohol-related obesity and/or triglyceride-related hypertension. Better rates of alcohol problems were found but self-reported smoking was still relevant.
High LDL-C or smoking were additive risk factors placing here women into a higher risk class for sustainable hypertension during intolerance to glucose, smoking, alcohol problems and/or obesity. The present study evaluated opposite plasma compartments (LDL/HDL, Alb/Trig) to better distinguish adverse risk profiles. Alcohol use specifically mediated urine pathology with and without second risk factors here and before [1] -[6] .
Evidence has been provided before that combined alcohol-related hyperlipidemia triggered hypertension, critical fasting blood glucose and urine pathology [1] - [6] . Hyperlipidemic versus normolipidemic women show significant rise of diastolic blood pressure (p = 0.004), alcohol use or smoking (p = 0.0001, p = 0.0019), urine pathology (p = 0.005) and lower HDL-cholesterol (p = 0.01) with higher LDL/HDL (p = 0.001) [1] . Hypertriglyceridemia is correlated with hypertension (p = 0.05) and with LDL-related intolerance to glucose (p = 0.021) whereby urine pathology (p = 0.07) overlapped with critical fasting blood glucose (p = 0.07) of primairily non-diabetic women who reported alcohol consumption. Urine pathology is associated with critical alcohol consumption (p = 0.04) and smoking aggravates alcohol-related urine pathology (p = 0.001) [1] . Overlapping risk factors were distinguished here to overcome high variances of isolated risk factors such as fasting blood glucose, of postprandial blood glucose, of high LDL-C, of serum albumin or of triglycerides. In short, alcohol-related hypertriglyceridemia decreased and better risk profiles were currently found.
Medical counseling has motivated middle aged women in good time to overcome mild risk profiles as additive effects were explained of independent risk factors and dependable variants showing here additive effects. Individual counseling informed about personal risk profiles initiating home testing of body weight, blood pressure and morning urines in good time to improve the quality of food and lifestyle (www.fidabus.com, www.fida-aha.com). Healthy exercising was recommended, for example to walk at least 30 minutes per day.
Rather unselected women currently showed here better self assessment at baseline indicating that current participates were initially motivated to cede adverse alcohol consumption. Self assessment was the condition in which combined monitoring was initiated in the Fida-practice. The selection of the current study group was correct and without bias as men currently failed any benefit and did not reduce adverse alcohol consumption [2] [3] . The combination of personal monitoring with telemedical informations increased the value of medical counseling and informed women who aimed to overcome healthcare problems at baseline. Mortality studies can scare and overestimated isolated risk factors can confuse keeping in mind that pooled study groups consisting of elderly ill persons can easily undervalue progress of younger persons [62] . Personal problems were more carefully debated here and informed middle class women translated medical recommendations into the practical life and often asked for combined monitoring at baseline.
The present study confirmed that obesity was an independent risk factor for mild hypertension indicating that obesity impaired dilatory adaptation of small vessels. Pharmacotherapy had to be considered in cases of sustainable, nocturnal hypertension [6] [52] . Unexpected cases of diabetes were found with obese woman to be treated and not evaluated here. Known diabetes and/or nocturnal, sustainable hypertension were initially treated and treated persons were not followed here.
Skilled persons know that adipocytes store lipophilic ether-linked phosphocholines [63] . HDL-particles incorporate triglycerides during hypertriglycridemia and LDL particles express phospholipases on outer endothelial cells so that lipidated HDL-particles were degraded by endothelial lipases and/or by phospholipases in accordance with underlying biomedical pathways [10] [63] - [66] . The motivating message was that hematuria and/or albuminuria can heal as shown before with abstinent former alcohol abusers during antihypertensive pharmacotherapy [52] . Elderly hypercholesterolemic women had to cede smoking to overcome mild diabetes and hematuria and age-related rise of cholesterol needed then pharmacotherapy to reduce LDL-cholesterol in the presence of several risk factors. Regeneration of healthy endothelium barriers was supported with low fat dairy products, certified plant oils comprising lecithins and Ginkgoloides [52] - [54] . The combination of dairy products and certified plant oils formed micelles to increase intestinal absorption of pure albumin with uptake of prehomones and constituents to supplement mild increase of urinary calcium as shown here with smoking women at baseline [17] [67] .
Obese women reacted highly sensitive to alcohol-related macroalbuminuria indicating disturbed filtration of renal endothelium barriers and/or declined defense potency of plasma albumin. Hematuria indicated thin basement membranes during alcohol-related dyslipidemia so that long cessation periods were needed without alcohol consumption, without sugar alcohol, saturated fat and transformed phospholipids. Women with albuminuria had to perceive that about 40% of excreted albumin is degraded during glomerular transcytosis and cannot be detected [60] [68] [69] . Urinary albumin cannot be reabsorbed and can trigger tubular fibrosis with disturbed tubular re-adsorption [60] . Other reports suggest that increase of urinary calcium predicts tubular problems as shown here with smoking women and elsewhere with offspring animals after gestational diabetes [68] - [70] . Women with hematuria had to give up drinking and smoking to prevent progression of glomerular filtration problems, of tubular fibroses and/or thin renal basement membranes [60] [71] . Thin basement nephropathy can indicate vulnerable DNA regions of rare cases with critical family history [72] .
Dyslipidemia was found here and before with persons who reported drinking and/or smoking during high LDL/HDL, low Alb/Trig and hypertension. Submaximal ratio of serum albumin to triglycerides indicated here overloaded albumin in the presence of obesity or declined potency of albumin during hypertriglyceridema. Steroids or insulin trigger accelerated synthesis and expression of phospholipases/lipases on outer endothelial cells [1] - [6] [10] [12] . Submaximal Alb/Trig triggered hypertension and alcohol-related dyslipidemia impaired the balance of renal endothelium barriers. Women at risk had to give up drinking and smoking to repair defects of renal endothelium barriers in good time.
Rating of risky/critical alcohol problems corresponded here with Official rates (A: 12% of 248: 30 ± 5 g ethanol/day). Middle aged men report higher alcohol consumption and react more sensitive to tobacco-related urine pathology [2] [3] [59] . Consenting women currently reached here a reliable progress as shown with normal plasma compartments, healthy morning urines and normal blood pressure. It is noted that healthy plasma albumin can endogenously strengthen the glycoprotein matrix covering then the entire luminal surface of human endothelium to modulate filtration of plasma compartments [73] .
Healthy alcohol free drinks were adapted comprising pure albumin and healthy lecithins to enhance intestinal absorption of pure serum albumin [17] . Low fat goat milk products are recommended comprising healthy phospholipases/acetylhydrolase to increase intestinal excretion of adverse lipid mediators [17] [50] . Ginkgoloides can protect renal endothelium during settled cessation of drinking and smoking composing alcohol-free drinks therewith to overcome urine pathology and healing was shown with abstinent former alcohol abusers and elderly women who had to cede smoking to overcome hematuria and mild diabetes at baseline [6] [52] - [54] . Obese or hyperlipidemic persons had to give up drinking and/or smoking and had to reduce uptake of sodium, cholesterol, saturated fat and adverse carbohydrates during abstinent periods [6] [52] - [54] .
Long time incubation of cells with hormones such as insulin or steroids differentiate human endothelial cells and express adhesion molecules [9] [10] . More recent reports confirm that hormones such as insulin and steroids trigger proliferative effects based on signal transduction pathways of cells [74] . Pleiotropic phenotypes indicate vulnerable DNA regions of insulin response elements as those are highly sensitive to hypertriglyceridemia and insulin promotor genes can modulate lipases and/or phospholipases [75] . Families can have favourable gene variants of apoproteins as healthy variants of apoB or of apoC3 reacting then about 39% lowering of postprandial triglycerides and 22% increase of HDL-cholesterol with 11% lowering of LDL-cholesterol compared to controls and these persons show 40% reduced prevalence of cardiovascular disorders [76] [77] . Other DNA regions encode variants of lipases/phospholipases and these regions are sensitive to triglyceride-related metabolic problems [78] . Asian persons with mutations of phospholipases/acetylhydrolases are at risk for hypertension and stroke [79] . Mutants of phospholipases A2 (PNPLA3) of obese persons trigger non-alcoholic fatty liver disease or non- alcoholic hypertriglyceridemia as variable phenotype expression [80] . Vulnerable genotypes of alcohol dehydrogenases are related with alcohol liver cirrhosis and pancreatitis of Caucasians persons at younger age [81] .
The rates of LDL-C did not change here over the course of time while hypercholesterolemic women with diastolic hypertension were currently about ten years older and needed phramcotherapy in the presence of additive risk factors in respect to the medical guidelines [82] . Statins can then inhibit cholesterol synthesis (e.g. to 70 - 100 mg/dl) and also accelerated synthesis of phospholipases as shown with benefit during diabetes [82] [83] . Ezetimibe or plant-derived oxysterols can inhibit intestinal adsorption of cholesterol [84] [85] . Combinations with fibrates can further protect persons against small vessel disease showing additive beneficial effects of statins and fibrates [82] . Fibrates inhibit peroxisome proliferator-activated receptors in cells interacting with long- chain fatty acids esterified of triglycerides and/or phospholipids such as alkyl-acyl-long-chain-glycerophos- phocholines (LA-paf) [86] [87] .
Self-reported smoking was a problem at baseline here and abroad [88] . Smoking aggravated here diastolic hypertension and formed additive risk combinations during critical alcohol consumption. Smoking women with high LDL-C reacted here highly sensitive to diastolic hypertension during prodiabetic glucose profiles. Relatively young women needed more pertinent informations to cede smoking as these women were indifferent to known mortality studies. These non-pregnant women who reported smoking had to perceive that smoking agravated alcohol-related small vessel disease and LDL-related hypertension. Smoking and drinking of hyperlipidemic persons triggered prodiabetic risk profiles independently of age. Heavy smoking directly impaired here cholesterol loading of HDL-particles in the presence of normal lipid profiles, normal blood pressure and normal morning urines for the first time. Other clinical reports confirm with Chinese men who smoke more than 20 cigarettes per day having then coronary heart disease showing then dysfunctional monocytes with disturbed expression of ATP-binding cassette transporters [89] . Women were currently motivated to cede drinking and smoking as shown with pregnant women who did not smoke and who overcome mild intolerance to glucose without hypertension (www.fidabus.com).
Background art discloses that smoking triggers enzymatic beta-oxidation in mitochondrial compartments and degradation of the cellular defense system is shown with alveolar epithelial carcinoma cells [90] . Individual history of depression and/or dosage of psychopharmaca had to be carefully considered to guide persons at risk during long cessation periods [91] . Healthy alcohol free liquids were adapted with xanthines, healthy albumin, calcium, prehormones and constituents to replace smoking and drinking, to repair the female defense system, to equilibrate the mind and to supplement urinary calcium excretion as well as required constituents [17] .
The major limitation of the study was that modified phospholipids were not measured in indicated compartments and that pre- or postprandial insulin values were not tested. It is noteworthy that the testing was reimbursed by assurances so that medical standard procedures had to be used in accordance with medical guidelines. Coded medical raw data provided here pertinent testing without undue experiments so that physicians can better find and neutralize mild diastolic hypertension at baseline.
The underlying biomedical pathways of ether-linked phosphocholines are included by citation [1] - [17] . Dehydrated alcohol, so-called aldehydes form ether groups in position 1 and aldehydes further conjugate esterified fatty acids in position 2 of glycerophospholipids [7] . Tobacco-related metabolites enhance betaoxidation of AAGPC leading then to biologically active alkyl-acyl-(short-chain)-glycerophosphocholines [7] . Endogenous transcytosis of albumin-bound lysopaf across endothelium is clinically shown with elevated albumin in cerebrospinal fluids during leakage of endothelial blood brain barriers (CSF: 0.245 ± 0.05 > 0.15 mg albumin in 500 µl with 68 ± 7 ng lyso paf per mg albumin n = 3) compared to healthy blood brain barriers of persons without psychiatric symptoms (0.1 ± 0.05 < 0.15 mg albumin in 500 µl CSF with 26 ± 7 ng lyso paf per mg albumin, n = 3) [14] . Plasmatic albumin of healthy volunteers bind lysopaf (116 ± 22 ng lysopaf/mg serum albumin). Urinary albumin carries then at least 26 ng lysopaf per mg urinary albumin.
Imbalance of plasma compartments have been shown before with two mixed study groups, two male study groups and three female study groups [1] -[6] . The currently scored study group showed better rates of critical alcohol consumption, more often healthy morning urines and the majority widely eliminated adverse lipid mediators in good time. The current participants showed better rates of LDL-related smoking because women with high LDL-C less often smoked. The relevant proportion of self-reported smoking was the current problem of relatively young women.
5. Conclusion
Alcohol consumption currently decreased in a reliable manner and hematuria decreased of women who currently attended the practice of General Medicine. Hyperlipidemia and/or obesity were related with high LDL-C, low Alb/Trig and hypertension and these risky women reacted highly sensitive to critical alcohol consumption showing then albuminuria. The majority of current participants disowned daily alcohol consumption showing then healthy morning urines. Relatively young women often reported smoking and tended to mild decrease of HDL-C without reaching significancy. Smoking showed additive effects during intolerance to glucose, high LDL-C and/or alcohol-related urine pathology. Obese or hyperlipidemic women reacted highly sensitive to urine pathology and had to give up adverse alcohol consumption. Healthy alcohol-free liquids were adapted to repair dysfunction of endothelium barriers and the defense potency of healthy serum albumin.
Acknowledgements
The study was presented during the NAVBO meeting in Boston, 2015.
Conflict of Interests
The author has no conflict of interests.
Cite this paper
Ruth-MariaKorth, (2016) LDL-Related Intolerance to Glucose, Diastolic Hypertension and Additive Effects of Smoking Were Found with Three Female Study Groups. Health,08,230-250. doi: 10.4236/health.2016.83026
References
- 1. Korth, R.M. (2014) Women with Overweight, Mixed Hyperlipidemia, Intolerance to Glucose and Diastolic Hypertension. Health, 6, 454-467.
http://dx.doi.org/10.4236/health.2014.65064 - 2. Korth, R.M. (2006) Gender, Obesity, Alcohol Use, Hyperlipidemia, Hypertension and Decline of Renal Endothelial Barriers. Journal Men’s Health & Gender, 3, 279-289.
http://dx.doi.org/10.1016/j.jmhg.2005.08.006 - 3. Korth, R.M. (2012) Two Male Study Groups with Adiposity and Hypertriglyceridemia Were at Risk for Hypertension. Health, 4, 1390-1395.
http://dx.doi.org/10.4236/health.2012.412A201 - 4. Korth, R.M. (2007) Obesity Mediated Hypertension While Alcohol Use Declined Renal Endothelial Barriers of Women. The FASEB Journal, 21, A1361.
- 5. Korth, R.M. (2002) AHA-Syndromes. Chemistry and Physics of Lipids, 118, 96-97.
- 6. Korth, R.M. (2009) Gender Dyslipidemia and Ether Phospholipids. The FASEB Journal, 19, 109.
- 7. Korth, R., Zimmermann, K. and Richter, W. (1994) Lipoprotein-Associated paf (LA-paf) Was Found in Washed Human Platelets and Monocyte/Macrophage-Like U 937 Cells. Chemistry and Physics of Lipids, 70, 109-119.
http://dx.doi.org/10.1016/0009-3084(94)90079-5 - 8. Korth, R.M. (1997) VLDL and PAF Binding to Human Endothelial Cells. Chemistry and Physics of Lipids, 88, 134.
- 9. Korth, R. (1999) Treatment and Prevention of Disorders Mediated by LA-paf or Endothelial Cells. United States Patent Publication No. 5895785.
- 10. Korth, R.M., Hirafuji, M., Benveniste, J. and Russo-Marie, F. (1995) Human Umbilical Vein Endothelial Cells: Specific Binding of Platelet-Activating Factor and Cytosolic Calcium Flux. Biochem Pharmacol, 49, 1793-1799.
http://dx.doi.org/10.1016/0006-2952(95)00025-U - 11. Korth, R.M. (1997) Specific Binding Sites for 1-O-Alkyl-sn-Glyceryl-3-Phosphorylcholine on Intact Human Blood Neutrophils. International Archives of Allergy and Immunology, 113, 460-464.
http://dx.doi.org/10.1159/000237623 - 12. Korth, R. and Middeke, M. (1991) Long Time Incubation of Monocytic U 937 Cells with LDL Increase Specific PAF-Acether Binding and the Cellular Acetylhydrolase Activity. Chemistry and Physics of Lipids, 59, 207-213.
http://dx.doi.org/10.1016/0009-3084(91)90020-C - 13. Korth, R., Bidault, J., Palmantier, R., Benveniste, J. and Ninio, E. (1993) Human Platelets Release a Paf-Acether: Acetylhydrolase Similar to That in Plasma. Lipids, 28, 193-199.
http://dx.doi.org/10.1007/BF02536639 - 14. Korth, R.M. (2000) Comparison of Phosphocholines in Plasma and Cerebrospinal Fluids (CSF). The FASEB Journal, 14, A72.
- 15. Korth, R. and Benveniste, J. (1987) BN 52021 Displaces [3H]paf-Acether from and Inhibits Its Binding to Intact Human Platelets. European Journal of Pharmacology, 142, 331-341.
http://dx.doi.org/10.1016/0014-2999(87)90071-9 - 16. Korth, R., Nunez, D., Bidault, J. and Benveniste, J. (1988) Comparison of Three paf-Acether Receptor Antagonists Ginkgolides. European Journal of Pharmacology, 152, 101-110.
http://dx.doi.org/10.1016/0014-2999(88)90840-0 - 17. Korth, R.M. (2014) Novel compositions against alkyl-acyl-GPC, the derivatives and products thereof. European Patent Publication No. 1965791B1.
- 18. Meade, C.J., Heuer, H. and Kempe, R. (1991) Commentary: Biochemical Pharmacology of Platelet-Activating Factor (and paf Antagonists) in Relation to Clinical and Experimental Thrombocytopenia. Biochemical Pharmacology, 41, 657-668.
- 19. Ihida, K., Predescu, D., Czekay, R.P. and Palade, G.E. (1999) Platelet Activating Factor Receptor (PAF-R) Is Found in a Large Endosomal Compartment in Human Umbilical Vein Endothelial Cells. Journal of Cell Science, 112, 285-295.
- 20. Nistor, A. and Simionescu, M. (1986) Uptake of Low Density Lipoproteins by the Hamster Lung. Interactions with Capillary Endothelium. The American Review of Respiratory Disease, 134, 1266-1272.
- 21. Wu, R., Lemne, C., De Faire, U. and Frostegard, J. (1997) Antibodies to Platelet-Activating Factor Are Associated with Borderline Hypertension, Early Atherosclerosis and the Metabolic Syndrome. Journal of Internal Medicine, 246, 389-397.
http://dx.doi.org/10.1046/j.1365-2796.1999.00570.x - 22. Me.G., Richard, G. and White, T.W. (2007) Gap Junctions: Basic Structure and Function. Journal of Investigative Dermatology, 127, 2516-2524.
http://dx.doi.org/10.1038/sj.jid.5700770 - 23. Louis, S.F. and Zaharadka, P. (2010) Vascular Smooth Muscle Cell Motility: From Migration to Invasion. Experi- mental & Clinical Cardiology, 15, e75-e85.
- 24. Pizzimenti, S., Ciamporcero, E., Daga, M., Pettazzoni, P., Arcaro, A. Cetrangolo, G., Minelli, R., Dianzani, C., Lepore, A., Gentile, F. and Barrera, G. (2013) Interaction of Aldehydes Derived from Lipid Peroxidation and Membrane Proteins. Frontiers in Physiology, 4, 242.
- 25. van der Vusse, G.J. (2009) Albumin as Fatty Acid Transporter. Drug Metabolism and Pharmacokinetics, 24, 300-307.
http://dx.doi.org/10.2133/dmpk.24.300 - 26. Ambrosio, G., Oriente, A., Napoli, C., Palumbo, G., Chiarello, P., Marone, G., Conorelli, M., Chiariello, M. and Trigginai, M. (1994) Oxygen Radicals Inhibit Plasma Acetylhydrolase, the Enzyme That Catabolized Platelet Activting Factor. Journal of Clinical Investigation, 93, 2408-2416.
http://dx.doi.org/10.1172/JCI117248 - 27. Silva, I.T., Mello, A.P.Q. and Damasceno, N.R.T. (2011) Antioxidant and Inflammatory Aspects of Lipoproteins-Associated Phospholipases A2 (LP-PLA2): A Review. Lipids in Health and Disease, 10, 170-175.
http://dx.doi.org/10.1186/1476-511X-10-170 - 28. Gregson, J., Stirnadel-Farrant, H.A., Doobaree, I. and Koro, C. (2012) Variation of Lipoprotein Associated Phosphoplipases A2 across Demographic Characteristics and Cardiovascular Risk Factors: A Systematic Review of Literature. Atherosclerosis, 225, 11-21.
http://dx.doi.org/10.1016/j.atherosclerosis.2012.06.020 - 29. Patja, K., Jousilahti, P., Hu, G., Valle, T., Quiao, Q. and Tuomilehto, J. (2005) Effects of Smoking, Obesity and Physical Activity on the Risk of Type 2 Diabetes in Middle-Aged Finnish Men and Women. Journal of Internal Medicine, 258, 356-362.
http://dx.doi.org/10.1111/j.1365-2796.2005.01545.x - 30. Saito, T., Urushihara, M., Kotani, Y., Kagami, S. and Kobori, H. (2009) Increased Urinary Angiotensinogen Is Precedent to Increased Urinary Albumin in Patients with Type 1 Diabetes. The American Journal of the Medical Sciences, 338, 478-480.
http://dx.doi.org/10.1097/MAJ.0b013e3181b90c25 - 31. Dell’Omo, G., Penno, G., Pucci, L., Mariani, M., Del Prato, S. and Pedrinelli, R. (2014) Abnormal Capillary Permeability and Endothelial Dysfunction in Hypertension with Comorbid Metabolic Syndrome. Atherosclerosis, 172, 383-389.
http://dx.doi.org/10.1016/j.atherosclerosis.2003.11.013 - 32. Barzilay, J.J., Peterson, D., Cushman, M., Heckbert, S.R., Cao, J.J., Blaum, C., Tracy, R.P., Klein, R. and Herrington, D.M. (2004) The Relationship of Cardiovascular Risk Factors to Microalbuminuria in Older Adults with or without Diabetes Mellitus or Hypertension: The Cardiovascular Health Study. American Journal of Kidney Diseases, 44, 25-34.
http://dx.doi.org/10.1053/j.ajkd.2004.03.022 - 33. Kristjansson, K., Ljungmann, S., Bengtsson, C., Bjorkel, C. and Sigurdsson, J.A. (2001) Microproteinuria and Long-Term Prognosis with Respect to Renal Function and Survival in Normotensive and Hypertensive Women. Scandinavian Journal of Urology and Nephrology, 35, 63-70.
http://dx.doi.org/10.1080/00365590151030868 - 34. Uribarri, J., Woodruff, S., Goodman, S., Cai, W., Chen, X., Pyzik, R., Yong, A., Striker, G.E. and Vlassara, H. (2010) Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. Journal of the Academy of Nutrition and Dietetics, 110, 911-916.
http://dx.doi.org/10.1016/j.jada.2010.03.018 - 35. Faure, P., Troncy, L., Lecomte, M., Wiernsperger, N., Lagarde, M., Ruggerio, D. and Halimi, S. (2005) Albumin Antioxidant Capacity Is Modified by Methylglyoxal. Diabetes & Metabolism, 31, 169-177.
http://dx.doi.org/10.1016/S1262-3636(07)70183-0 - 36. Muoio, D.M. (2010) Intramuscular Triacylglycerol and Insulin Resistance: Guilty as Charged or Wrongly Used. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 180, 281-288.
http://dx.doi.org/10.1016/j.bbalip.2009.11.007 - 37. Berenson, G.S., Srinivasan, S.R., Bao, W., Newmann, W.P., Tracy, R.E. and Wattigney, W.A. (1998) Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. The New England Journal of Medicine, 338, 1650-1656.
http://dx.doi.org/10.1056/NEJM199806043382302 - 38. Woo Baidal, J.A. and Taveras, E.M. (2014) Protecting Progress against Childhood Obesity—The National School Lunch Program. The New England Journal of Medicine, 371, 1862-1865.
http://dx.doi.org/10.1056/nejmp1409353 - 39. Volek, J.S., Fernandez, M.L., Feinman, R.D. and Phinney, S.D. (2008) Dietary Carbohydrate Restricition Induces a Unique Metablic State Positively Affecting Atherogenic Dyslipidemia, Fatty Acid Partioning, and Metabolic Syn- drome. Progress in Lipid Research, 47, 307-318.
http://dx.doi.org/10.1016/j.plipres.2008.02.003 - 40. Shufelt, C.J. and Merz, N.B. (2009) Contraceptive Hormone Use and Cardiovascular Disease. State-of-the-Art-Paper. Journal of the American College of Cardiology, 53, 221-231.
http://dx.doi.org/10.1016/j.jacc.2008.09.042 - 41. Georgieva, A.M., Cate, H.T., Keulen, E.T.P., van Oerle, R., Govers-Riemslag, J.W.G., Hamulyak, K., van der Kallen, C.J.H., van Greevenbrock, M.M.J. and de Bruin, T.W.A. (2004) Prothrombotic Markers of Familial Combined Hyperlipidemia. Evidence of Endothelial Cell Activation and Relation to Metabolic Syndrome. Atherosclerosis, 175, 345-351.
http://dx.doi.org/10.1016/j.atherosclerosis.2004.04.006 - 42. Heydarkhan-Hagvall, S., Helenius, G., Johansson, B.R., Li, J.Y., Mattson, E. and Risberg, B. (2003) Co-Culture of Endothelial Cells and Smooth Muscle Cells Affects Gene Expression of Angioneic Factors. Journal of Cellular Biochemistry, 89, 1250-1259.
http://dx.doi.org/10.1002/jcb.10583 - 43. Canaud, G., Bienaime, F., Tabarin, F., Bataillon, G., Seilhean, D., Noel, L.H, Dragon-Durey, M.L., Snanoudi, R., Friedlander, G., Halbwacgs-Mecarelli, L., Legendre, C. and Terzi, F. (2014) Inhibition of the mTORC Pathway in the Antiphospholipid Syndrome. The New England Journal of Medicine, 371, 303-312.
http://dx.doi.org/10.1056/NEJMoa1312890 - 44. Sacks, F.M., Svetkey, L.P., Vollmer, W.M., Appel, L.J., Bray, G.A., Harsha, D., Obarzanek, E., Conlin, P.R., Miller, E.R., Simons-Morton, D.G., Karanja, N., Lin, P.-H., et al., DASH-Sodium Collaboration Research Group (2001) Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. The New England Journal of Medicine, 344, 3-10.
http://dx.doi.org/10.1056/NEJM200101043440101 - 45. Salmon, A.H.J., Ferguson, J.K., Burford, J.L., Gevorgyan, H., Nakiano, D., Harper, S.J., Bates, D.O. and Peti-Peterdi, J. (2012) Loss of the Endothelial Glycocalyx Links Albuminuria and Vascular Dysfunction. Journal of the Amercian Society of Nephrology, 23, 1339-1350.
http://jasn.asnjournals.org - 46. Man, R.Y.K. (1988) Lysophosphatidylcholine-Induced Arrhythmias and Its Accumulation in the Rat Perfused Heart. British Journal of Pharmacology, 93, 412-416.
http://dx.doi.org/10.1111/j.1476-5381.1988.tb11448.x - 47. Chen, Y., Zhang, H., Zhang, Y., Lu, N., Zhang, L. and Shi, L. (2015) Exercise Intensity-Dependent Reverse and Adverse Remodeling of Voltage-Gated Ca2+ Channels in Mesenteric Arteries from Spontaneously Hypertensive Rats. Hypertension Research, 38, 656-665.
http://dx.doi.org/10.1038/hr.2015.56 - 48. Dominiczak, A.F., Negrim, D.C., Clark, J.S., Brosnan, J., McBride, M.W. and Alexander, M.Y. (2000) State-of-the- Art Lectures: Genes and Hypertension. Hypertension, 35, 164-172.
http://dx.doi.org/10.1161/01.HYP.35.1.164 - 49. Nebte, A., O’Donnell, M.J., Rangarajan, S., McQueen, M.J., Poirier, P., et al. (2014) Association of Urinary Sodium and Potassium Excretion with Blood Pressure. The New England Journal of Medicine, 371, 601-611.
http://dx.doi.org/10.1056/NEJMoa1311989 - 50. Furukawa, M., Narahara, H., Yasuda, K. and Johnston, J. (1993) Presence of Platelet-Activating Factor-Acetylhydro- lase in Milk. The Journal of Lipid Research, 34, 1603-1609.
- 51. Fruhwirth, B. and Hermetter, A. (2007) Seeds and Oil of the Syrian Oil Pumpkin: Components and Biological Activities. European Journal of Lipid Science and Technology, 109, 1128-1140.
http://dx.doi.org/10.1002/ejlt.200700105 - 52. Korth, R.M. (2003) Alcohol, Obesity, Hypertension and Endothelial Irritation. The FASEB Journal, 17, 4-5.
- 53. Korth, R.M. (2006) Association of Intolerance to Glucose with Rising Blood Pressure or Proteinuria/Hematuria. Journal of Vascular Research, 43, S90.
- 54. Korth, R.M. (2007) PO3-59 Smoking, Borderline LDL Levels and Renal Small Vessel Disease of Women with Overweight. Atherosclerosis Supplements, 8, 32-33.
http://dx.doi.org/10.1016/S1567-5688(07)71069-3 - 55. Korth, R.M. (2008) Aging Women with LDL-Related Borderline Syndromes. Chemistry and Physics of Lipids, 154, S47-S48.
http://dx.doi.org/10.1016/j.chemphyslip.2008.05.129 - 56. Chopra, S., Cherian, D. and Jacob, J.J. (2011) The Thyroid Hormone, Parathyroid Hormone and Vitamin D Associated Hypertension. Indian Journal of Endocrinology and Metabolism, 15, S354-S360.
- 57. Evans, R. (1988) The Steroid and Thyroid Hormone Receptor Superfamily. Science, 240, 889-895.
http://dx.doi.org/10.1126/science.3283939 - 58. Visser, M., Bouter, L.M., McQuillan, G.M., Wener, M.H. and Harris, T.B. (1990) Elevated C-Reactive Protein Level in Overweight and Obese Adults. JAMA, 282, 2131-2135.
- 59. Kraus, A.R. (2008) Alkoholkonsum, alkoholbezogene Probleme und Trends. Ergebnisse des epidemiologischen Suchtsurvy 2003. Robert Koch Institut, Gesundheitsberichterstattung des Bundes 2008, Heft 40.
- 60. Go, A.S., Chertow, G.M., Fan, D., McCulloch, C.E. and Hsu, C.Y. (2004) Chronic Kidney Disease and the Risks of Death, Cardiovascular Events and Hospitalization. The New England Journal of Medicine, 351, 1296-1305.
http://dx.doi.org/10.1056/NEJMoa041031 - 61. Sava, L., Pillai, S., More, U. and Sontakke, A. (2005) Serum Calcium Measurement: Total versus Free (Ionized) Calcium. Indian Journal of Clinical Biochemistry, 20, 158-161.
http://dx.doi.org/10.1007/BF02867418 - 62. Cook, N.R. and Ridker, P.M. (2014) Further Insight into Cardiovascular Risk Calculator: The Roles of Statins, Revascularizations and Underascertainment in the Women’s Health Study. JAMA Internal Medicine, 174, 1964-1971.
http://dx.doi.org/10.1001/jamainternmed.2014.5336 - 63. Chapman, M.J., Ginsberg, H.N., Amareno, P., et al. (2011) Triglyceride-Rich Lipoproteins and High-Density Lipoprotein Cholesterol in Patients at High Risk of Cardiovascular Disease: Evidence and Guidance for Management. European Heart Journal, 32, 1345-1361.
http://dx.doi.org/10.1093/eurheartj/ehr112 - 64. Bartz, R., Li, W.H., Venable, B., Zehmer, J.K., Welti, M.R., Aderson, R.G.W., Liu, P. and Chapman, K.D. (2007) Lipidomics Reveal That Adiposome Store Ether Lipids and Mediate Phospholipid Traffic. The Journal of Lipid Research, 48, 837-847.
http://dx.doi.org/10.1194/jlr.M600413-JLR200 - 65. Vergeer, M., Holleboom, A.G., Kastelein, J.J.P. and Kuivenhoven, J.A. (2010) The HDL Hypothesis: Does High-Density Lipoprotein Protect from Atherosclerosis. The Journal of Lipid Research, 51, 2058-2073.
http://dx.doi.org/10.1194/jlr.R001610 - 66. Lamarche, B. and Paradi, M.E. (2007) Endothelial Lipase and the Metabolic Syndrome. Current Opinion in Lipidology, 18, 298-303.
http://dx.doi.org/10.1097/MOL.0b013e328133857f - 67. Slattery, C., Zhang, L.A., Kelly, D.J., Thorn, P., Nikolic-Paterson, D.J., Tesch, G.H. and Poronnik, P. (2008) In Vivo Visualization of Albumin Degradation in the Proximal Tubule. Kidney International, 74, 1480-1486.
http://dx.doi.org/10.1038/ki.2008.463 - 68. Osicka, T.M., Panagiotopoulos, S., Jerums, G. and Comper, W.D. (1997) Fractional Clearance of Albumin Is Influenced by Its Degradation during Renal Passage. Clinical Science, 93, 557-564. http://dx.doi.org/10.1042/cs0930557
- 69. Bond, H., Sibley, C.P., Balment, R.J. and Ashton, N. (2005) Increased Renal Tubular Reabsorbation of Calcium and Magnesium by the Offspring of Diabetic Rat Pregnancy. Pediatric Research, 57, 890-895.
http://dx.doi.org/10.1203/01.PDR.0000157720.50808.97 - 70. Savige, J., Rana, K., Tonna, S., Buzza, M., Daghar, H. and Wang, Y.Y. (2003) Thin Basement Membrane Nephropathy. Kidney Inernational, 64, 1169-1178.
http://dx.doi.org/10.1046/j.1523-1755.2003.00234.x - 71. Collar, J.R., Ladva, S., Cairns, T.D. and Cattell, V. (2001) Red Cell Traverse through Thin Glomerular Basement Membranes. Kidney International, 59, 2069-2072.
- 72. Jacob, M., Bruegger, D., Rehm, M., Stoeckelhuber, M., Welsch, U., Conzen, P. and Becker, B.F. (2007) The Endothelial Glycocalyx Affords Compatibility of Starling’s Principle and High Cardiac Interstitial Albumin Levels. Cardiovascular Research, 73, 575-586.
http://dx.doi.org/10.1016/j.cardiores.2006.11.021 - 73. Shukla, A., Grisouard, J., Ehemann, V., Hermani, A., Enzmann, H. and Mayer, D. (2009) Analysis of Signaling Pathways Related to Cell Proliferation Stimulated by Insulin Analogs in Human Mammary Epithelial Cell Lines. Endocrine-Related Cancer, 16, 429-441.
http://dx.doi.org/10.1677/ERC-08-0240 - 74. Waterworth, D.M., Talmud, P.J., Luan, J., Flavell, D.M., Byrne, C.E., Humphries, S.E. and Wareham, N.J. (2003) Variants in the APOC3 Promoter Insulin Responsive Element Modualte Secretion and Lipids in Middle-Aged Men. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1637, 200-206.
http://dx.doi.org/10.1016/S0925-4439(03)00021-8 - 75. The TG and HDL Working Group of the Exome Sequencing Project, National-Heart, Lung and Blood Institute (2014) Loss-of-Function Mutations in APOC3, Triglycerides, and Coronary Disease. The New England Journal of Medicine, 371, 22-31.
http://dx.doi.org/10.1056/NEJMoa1307095 - 76. Fouchier, S.W., Sankatsing, J., Peter, J., Castillo, S., Pocovi, M., Alonos, R., Kastelein, J.J.P. and Defesche, J.C. (2005) High Frequency of APOB Gene Mutations Causing Familial Hypobetalipoproteinaemia in Patients of Dutch and Spanish Descent. Journal of Medical Genetics, 42, e23.
- 77. Chen, P., Jou, Y.-S., Fann, C.S., Chen, J.-W., Chung, C.-M., Lin, C.-Y., Wu, S.-Y., Kang, M.-J., Chen, Y.-C., Jong, Y.-S., Lo, H.-M., Kang, C.-S., Chen, C.-C., Chang, H.-C., Huangk, N.-K., Wu, Y.-L. and Pan, W.-H. (2009) Lipoprotein Lipase Variants Associated with an Endophenotype of Hypertension: Hypertension Combined with Elevated Triglycerides. Human Mutation, 30, 49-55.
http://dx.doi.org/10.1002/humu.20812 - 78. Hiramoto, M., Yoshida, H., Imaizumi, T., Yoshimizu, N. and Satoh, K. (1997) A Mutation in Plasma Platelet Activating Factor Acetylhydrolase (Val279-Phe) Is a Genetic Risk Factor for Stroke. Stroke, 28, 2417-2420.
http://dx.doi.org/10.1161/01.STR.28.12.2417 - 79. Romeo, S., Kozlitina, J., Xing, C., Pertsemlidis, A., Cox, D., Pennachio, L.A., Boerwinkle, E., Cohen, J. and Hobbs, H. (2008) Genetic Variation in PNPLA3 Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nature Genetics, 40, 1461-1465.
http://dx.doi.org/10.1038/ng.257 - 80. Cichoz-Lach, H., Partyck, J., Nesina, I., Celinski, K., Slomka, M. and Wojcierowsli, J. (2007) Alcohol Dehydrogenase and Aldehyde Dehydrogenase Gene Polymorphism in Alcohol Liver Cirrhosis and Alcohol Chronic Pancreatitis among Polish Individuals. Scandinavian Journal of Gastroenterology, 42, 493-498.
http://dx.doi.org/10.1080/00365520600965723 - 81. The ACCORD Study Groupand ACCORD Eye Study Group (2010) Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. The New England Journal of Medicine, 363, 233-244.
http://dx.doi.org/10.1056/NEJMoa1001288 - 82. Winkler, K., Abletshauser, C., Friedrich, I., Hoffmann, M.M., Wieland, H. and Marz, W. (2004) Fluvastatin Slow-Release Lowers Platelet-Activating Factor Acetylhydrolase Activity: A Placebo-Controlled Trial in Patients with Type 2 Diabetes. The Journal of Clinical Endocrinology & Metabolism, 89, 1153-1159.
http://dx.doi.org/10.1210/jc.2003-031494 - 83. Cole, P. and Rabassada, X. (2005) Enhanced Hypercholesterolemia Therapy: The Ezetimibe/Simvastatin Tablet. Drugs Today, 41, 317-327.
http://dx.doi.org/10.1358/dot.2005.41.5.893614 - 84. Weingartner, O., Bohm, M. and Lauf, U. (2009) Controversial Role of Plant Sterols Esters in the Management of Hypercholestrolemia. European Heart Journal, 40, 404-409.
- 85. Brand-Herrmann, S.M., Kuznetsova, T., Wiechert, A., Stolarz, K., Tikhonoff, V., Schmidt-Peterson, K., Telgmann, R., Casiglia, E., Wang, J.G., Thijs, L., Staessen, J.A. and Brand, E. (2005) Alcohol Intake Modulates the Genetic Association between HDL Cholesterol and the PPARγ2 Pro12Ala Polymorphism. The Journal of Lipid Research, 46, 913-919.
http://dx.doi.org/10.1194/jlr.M400405-JLR200 - 86. Davies, S.S., Pontsler, A.V., Marathe, G.K., Harrison, K.A., Murphy, R.C., Hinshaw, J.C., Prestwich, G.D., Hilaire, A.S.T., Prescott, S.M., Zimmerman, G.A. and McIntyre, T.M. (2001) Oxidized Alkyl Phospholipids Are Specific High Affinity Peroxisome Proliferator-Activated Receptor γ Ligands and Agonists. The Journal of Biological Chemistry, 276, 16015-16023.
http://dx.doi.org/10.1074/jbc.M100878200 - 87. Cepeda-Benito, A., Reynoso, J.T. and Erath, S. (2004) Meta-Analysis of the Efficacy of Nicotine Replacement Therapy for Smoking Cessation: Differences between Men and Women. Journal of Consulting and Clinical Psycho- logy, 72, 712-722.
http://dx.doi.org/10.1037/0022-006X.72.4.712 - 88. Song, W., Wang, L., Dou, L.-Y., Wang, Y., Xu, Y., Chen, L.F. and Yan, X.W. (2015) The Implication of Cigarette Smoking and Cessation on Macrophage Cholesterol Efflux in Coronary Artery Disease Patients. The Journal of Lipid Research, 56, 682-691.
http://dx.doi.org/10.1194/jlr.P055491 - 89. Bundeszentrale für gesundheitliche Aufklarung. Internet Presentation 2015. www.rauchfreiinfo.de
- 90. Vulimir, S.V., Misa, M., Hamm, J.T., Mitchell, M. and Berger, A. (2009) Effects of Mainstream Cigarette Smoke on the Global Metabolome of Human Lung Epithelial Cells. Chemical Research in Toxicology, 22, 492-503.
http://dx.doi.org/10.1021/tx8003246 - 91. Fragerstrom, K. and Aubin, H.J. (2009) Management of Smoking Cessation in Patients with Psychiatric Disorders. Current Medical Research and Opinion, 25, 511-518.
http://dx.doi.org/10.1185/03007990802707568