World Journal of Vaccines
Vol.04 No.02(2014), Article ID:45852,7 pages
10.4236/wjv.2014.42011
Immunostimulatory Effect of Levamisole on the Immune Response of Goats to Peste des petits ruminants (PPR) Vaccination
Undiandeye Jude Undiandeye1, Bamidele Soji Oderinde2, Abduldahiru El-Yuguda1, Saka Saheed Baba1*
1Animal Virus Research Laboratory, Department of Veterinary Microbiology and Parasitology, Faculty of Veterinary Medicine, Maiduguri, Nigeria
2Department of Medical Laboratory Science, College of Medical Sciences, University of Maiduguri, Maiduguri, Nigeria
Email: *aramidebaba@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 March 2014; revised 18 April 2014; accepted 25 April 2014
ABSTRACT
The use of Levamisole hydrochloride as an immunostimulant has been successful in the control of certain diseases such as Derzsy’s disease in chicken as well as in increasing lymphocytes and weight gain in pigs. However, its use as immunostimulant in the prevention of Peste des petits ru- minants (PPR) in goats has not been investigated. In this study, the use of Levamisole in enhancing immune response to PPR vaccination including its effects on weight gain was investigated among groups of goats (groups A, B, C and D). Virus neutralization test was used to determine the antibo- dy profile in vaccinated goats while the total and differential leucocyte counts and body mass in- dex (BMI) were determined using standard procedures. Goats in group A (Levamisole primed and PPR vaccinated) seroconverted to more than 3-folds of the initial pre-vaccination geometric mean titre (GMT) of neutralizing antibody beginning from second week post-vaccination and remained high throughout the period of the experiment. Similarly, there was significant increase (p < 0.01) in leucocyte production, particularly the lymphocytes following administration of Levamisole with the PPR vaccine. Goats in group B (vaccinated only), also seroconverted to significant GMT value as in group A but the value dropped significantly on the 4th week post vaccination. Group C that was administered Levamisole only, had significant increase in leucocyte value following treat- ment with the drug. Group D (control) had no significant change in their leucocyte. Groups C and D had no significant change in GMT throughout the period of experiment. Apart from group C, there was no significant change (p > 0.05) in BMI of animals in other groups throughout the period of the experiment. The results of this study further confirm the immunostimulatory effect of Levamisole when used in combination with a vaccine.
Keywords:
Levamisole Hydrochloride, Homologous PPR Vaccine, Immunostimulant, Goats, Maiduguri Nigeria

1. Introduction
A national livestock census has put the goat population in Nigeria at 34.5 million [1] . The keeping of goats in any community is related to the value attached to their production. Goats are mainly kept for meat [2] . Goat dung is also used as manure to improve the crop yield of local farmers. However, diseases and other factors in- cluding poor management limit the production of this group of ruminants. Chief amongst these diseases is the Peste des petits ruminants [3] [4] . Peste des petits ruminants (PPR) is a highly contagious disease of small ru- minants caused by a virus in the genus Morbillivirus, Family Paramyxoviridae. The infection is responsible for high morbidity and mortality in sheep and goats and some wild small ruminant species. Recently, severe out- break of the disease has been reported among dromedary camels in Sudan [5] . The huge number of small rumi- nants reared in the endemic areas makes PPR a serious disease threatening the livelihood especially of poor far- mers. In order to protect susceptible animals from the scourge of PPR, a homologous attenuated PPR vaccine has been developed and is commonly used for prevention and control of the disease [6] and recently the efficacy of a candidate thermostable PPR vaccine has been demonstrated in small ruminants in Nigeria [7] . In spite of rigorous vaccination exercise against PPR, outbreaks of the disease continue to occur among vaccinated and nonvaccinated animals [8] . Hence, the need to boost the immune response of goats to vaccination by employing the simultaneous administration of an immunostimulant and PPR vaccine. The use of immunostimulatory agents such as aluminium salts [9] [10] and Levamisole [11] [12] has been reported to help in stimulating the immune system, encourages and sustains the immune system and its response to a vaccine without having any specific antigenic effect in itself [10] . Levamisole hydrochloride, a synthetic phenylimidazothiazole is used primarily as a potent anthelminthic [13] . It was first introduced in 1966 [14] and later found to have immunostimulatory properties [15] . It has been used as an immunostimulator to stimulate the immune system either alone or in combination with other substances in the treatment of cancer [16] and to improve weight gain [17] . It has also been used as immunopotentiator in the treatment of lepromatous leprosy and as adjuvant [18] [19] . Due to the devastating effect of PPR virus infection and occasional failure of PPR vaccine efficacy, it has become necessary to determine the effect of Levamisole on the immune response to vaccination. This study was designed essen- tially to determine the immunostimulatory effect of Levamisole when used in combination with homologous PPR vaccine in goats.
2. Materials and Methods
2.1. Study Area
The study was carried out in Maiduguri, Borno State, located within latitude 110 551 N and longitude 130 091 E. The state has small ruminant population comprising of 3,182,537 goats [20] and is located in the northeastern part of Nigeria within the Sahel savannah vegetation. It shares boundaries with three countries; Chad, to the North; Cameroon, to the north east and Niger to the north.
2.2. Experimental Goats
Forty Sahel breed of goats (aged eight months and above) were randomly selected from amongst flocks belong- ing to small holder farmers and grouped into four of ten goats each, designated Group A, B, C and D. All expe- rimental goats were fed with ground nut hay, beans husk and supplemented with wheat bran and were given wa- ter ad libitum. Group A consisted of goats that were pre-treated with oral Levamisole and subsequently vacci- nated with PPR vaccine. Group B comprised of goats that were only vaccinated with PPR vaccine. Group C were goats that were only treated with oral Levamisole. Group D consisted of control animals that were neither pre-treated with Levamisole nor vaccinated with PPR vaccine.
2.3. Reconstitution of and Administration of Levamisole
Six hundred milligram (600 mg) of commercially available Levamisole bolus was obtained from a reputable marketer in Maiduguri, Nigeria and reconstituted in 50 ml sterile diluent to obtain the desired concentration of 12 mg/ml. The drug was administered orally to each goat at an immunostimulatory dose of 5 mg/kg every other day for four days in groups A and C as previously described by [19] .
2.4. PPR Vaccine
PPR vaccine with batch number 028D was obtained from Laboratoire National Veterinaire (LANAVET), Ca- meroon (www.lanavet.com). The vaccine is a live attenuated homologous vaccine used for active immunization of sheep and goats against PPR disease and proved to be safer and provides long immunity so that revaccination will not be necessary. In this study, the vaccine was reconstituted to a concentration of 3Log10 TCID50/ml and administered deep intra-muscularly to each goat in groups A and B two days after the last dose of Levamisole.
2.5. Determination of Viability of PPR Vaccine
To ensure the viability of the vaccine used, potency testing was carried out on the vaccine using the standard procedure for determination of vaccine potency. On the 7th day post inoculation of PPR vaccine into Vero cells, cytopathic effect was observed on the cells and the titre of the vaccine was determined.
2.6. Blood Sample Collection
Blood sample was collected from the jugular vein of individual goat on day 0 (pre-vaccination), 7, 14 and 28 post vaccination. In each case, 5 ml of blood was divided into two (2 ml in EDTA tubes for hematology, and 3 ml in plain tubes for serology).
2.7. Total Leucocyte Count
Neubauer counting chamber [21] was used for the white blood cell count. The blood was well mixed and drawn using aspirator and white blood cell pipette to 0.5 mark. The white cell count dilution fluid (Turks dilution fluid) was aspirated until the mixture reached the 11 mark. The content of the pipette was mixed for 3 minutes. The first 4 or 5 drops of the mixture were expelled onto a piece of gauze; a clean counting chamber with cover glass was filled. The filled counting chamber was allowed to stand for approximately 1 minute before counting. Using ×10 eye piece and ×40 objective, all cells contained in the 4 corner square millimeters were enumerated for WBC. The number of WBC per mm3 was calculated as shown below:
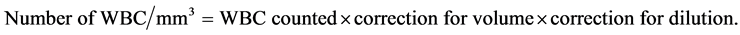
2.8. Differential Leucocyte Count (DLC)
Battlement method as described by [22] was used for DLC. A drop of immersion oil was dropped at the end of the stained smear and mounted on the microscope and counted under ×100 objective.
2.9. Virus Neutralization Test
One hundred and forty eight serum samples collected at various periods from the experimental goats were tested for presence of PPR virus specific antibody using the virus neutralization test as described by [23] . Vero cells from the Regional Polio Laboratory, Maiduguri; PPR vaccine virus from LANAVET, Garoua, Cameroon and positive sera obtained from the Animal Virus Research Laboratory, University of Maiduguri were used in the virus neutralization test. Sera were heat inactivated at 56˚C for 30 minutes. The presence of virus neutralizing antibody (VNA) in serum samples and their titres were determined according to the OIE protocol [23] . The re- ciprocal of the highest dilution of sera showing complete inhibition of cytopathic effect was taken as VNA titre.
2.10. Determination of Body Mass Index (BMI) of Experimental Goats
The weight and height of individual animal were obtained simultaneously with the blood samples to ascertain the BMI of goats on days 0, 7, 14 and 28 post-vaccination. The BMI was determined using the formula: BMI = weight in kg/height in m2.
2.11. Statistical Analysis
The differences between variables were determined by X2 or t-test as appropriate and the level of statistical sig- nificance was established at p ≤ 0.05. The Geometric mean titres (GMT) of antibodies were calculated using the formula described in “Descriptive statistics” [24] . The GMT was calculated using the formula 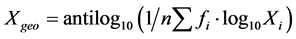 where fi = frequency and Xi = reciprocal of dilution. The GMT is the ap- propriate estimate of central tendency in dilution assay since each individual observation is represented.
where fi = frequency and Xi = reciprocal of dilution. The GMT is the ap- propriate estimate of central tendency in dilution assay since each individual observation is represented.
3. Results
Results of virus neutralization test indicated seroconversion by significant rise in PPR virus antibody titre over time among animals in groups A, and B. However, no significant change in antibody titre was observed among members of groups C and D throughout the period of the experiment. Geometric mean titre (GMT) values of the reciprocal of PPR virus neutralizing antibody titre for all the groups including the pre-vaccination antibody and weekly post vaccination antibody values are presented in Table 1. Seromonitoring of vaccinated goats revealed a considerable increase in antibody titre (≥3-folds) one week after vaccination in groups A (pre-treated and vac- cinated) and B (vaccinated only). However, at the 4th week post vaccination, there was significant (p < 0.05) drop in GMT of antibodies among the group B goats (Table 1). Group C (pre-treated only) and group D (control) recorded no significant rise in antibody titre throughout the period of seromonitoring (Table 1). Hematological analysis revealed that total leucocyte count (TLC) increased significantly overtime in groups A, B and C. How- ever, there was no significant change in TLC in the control group throughout the period of the experiment (Ta- ble 2). The weekly changes in TLC in groups A, B and C were statistically significant (p < 0.01). But weekly change in group D was not significant (p > 0.05) (Table 2). The differential leucocyte count (DLC) pattern showed that goats in group A (vaccinated and pre-treated), had no significant change in the weekly neutrophil values overtime. However, significant changes (p < 0.01) were recorded in the weekly values of lymphocytes, monocytes and eosinophils (Table 3). Generally, the post-vaccination DLC values were significantly higher than the pre-vaccination values among goats in group A (Table 3). There was a significant increase (p < 0.01) in lymphocyte values overtime among the group B goats. Generally, the postvaccination DLC values were signifi- cantly higher than the prevaccination values (Table 3). Similarly in group C (pre-treated only), the post vaccina- tion values were significantly higher than the prevaccination values (Table 3). The difference is more marked with lymphocytes and eosinophils when compared with other leucocytes. In group D (control group), the differ- ence in total leucocyte was not significant (p > 0.05) between prevaccination and postvaccination values. How- ever, occasional differences (p < 0.05) in DLC were noted at different periods (Table 3). Apart from animals in group C, where considerable increase in BMI was recorded, there was no significant change (p > 0.05) in BMI values between experimental groups as well as between prevaccination and postvaccination values throughout the period of experiment (Table 4).
Table 1. Geometric Mean Titre (GMT) values of reciprocal of neutralizing antibody titre in experimental goats at different periods.
Note: n: Sample size; PRE-VAC: Pre-vaccination; 1 WK POSTVAC: One week post vaccination; 2 WKS POSTVAC: Two week post vaccination; 4 WKS POSTVAC: Four weeks post vaccination.
Table 2. Means and standard deviation of total leucocyte count (TLC) of experimental goats per group at different periods.
Table 3. Means and standard deviation of differential leucocyte count (DLC) of the different groups at different periods.
Table 4. Means and standard deviation of BMI values of goats in different groups at various periods post vaccination.
Note: n = Sample size; PRE-VAC: Pre-vaccination; 1 WK POSTVAC: One week post vaccination; 2 WKS POSTVAC: Two week post vaccination; 4 WKS POSTVAC: Four weeks post vaccination.
4. Discussion
PPR continues to be a major socio-economically important disease of small ruminants in Nigeria. The disease is characterised by high morbidity and mortality and is among the greatest threats to a successful livestock produc- tion in many parts of the world where it exists [4] . The simplest and most logical preventive measure against in- fectious diseases is vaccination and this practice is considered by far the most humane and cost effective method of combating the spread of diseases [25] . However, in tropical areas disease outbreaks have been reported among vaccinated and non-vaccinated animals due to vaccine failure as a result of rapid vaccine deterioration and poor immune response of goats to vaccination. This necessitates the need to boost the immune response of goats to vaccination by employing the simultaneous administration of an immunostimulant and PPR vaccine. Levamisole has been used as an immunostimulant in humans and animals [26] . It has beneficial effects on host defense mechanism and restores depressed immune responses in both animals and humans [27] .
This study reveals that experimental goats in group A (pre-treated with Levamisole and vaccinated with PPR vaccine) had high levels of seroconversion following vaccination with possibility of better protective immunity over a long period of time when compared with those in group B, that received vaccine only. Significant change in GMT of the reciprocal of neutralizing antibody was observed beginning from second week post-vaccination in group A and this pattern was observed throughout the period of experiment. This observation is in agreement with the report of [28] where it was observed that Levamisole had an immunostimulating effect on the immune response of sheep to blue tongue vaccination. There was no seroconversion among members of unvaccinated but Levamisole treated group C indicating that Levamisole alone does not initiate the production of antibodies against PPR virus. The study also revealed that levamisole enhances the production of immune cells. This is demonstrated in groups A and C where lymphocytes, monocytes and eosinophil were significantly increased following administration of Levamisole. Bozic et al. [19] demonstrated that Levamisole exerts its immunosti- mulatory activities in pigs against colibaccillosis by recruitment and activation of cells that participate in cell- mediated immunity. In addition, Kumar et al. [17] observed increase in lymphocyte following administration of Levamisole possibly as a result of increase in the function of lymphoid organs such as the thymus and spleen resulting in increased proliferation of the bone marrow stem cells. The observed pattern of leucocyte change could have been as a result of increase in the function of the lymphoid organs due to Levamisole. Its effect when combined with PPR vaccine as in goats in group A, resulted in high seroconversion and significant change in leucocyte value. The lack of significant change in body mass index (BMI) among animals in all the groups could be attributed to the short duration of the study. This is in disagreement with [17] who observed that Levamisole improves weight gain in animals. It is possible that animals in groups A and C would improve in weight gain if the study is extended for more than four weeks. The study could not be extended beyond the experimental pe- riod because of its preliminary nature and limited funds. Besides, since the study was carried among small hold- er livestock, owners could not allow the extension of the study period and challenge experiments. Nonetheless this study has provided evidence for the usefulness of Levamisole in enhancing the development and sustaining humoral immune response following vaccination of goats with PPR vaccine. Levamisole appears safe when used in humans or when animal products from treated animals are consumed by humans. The drug has been used in humans for diseases related to imbalances in the regulation of immune responses or deficiencies of the immune system, including autoimmune diseases, chronic and recurrent diseases, chronic infections and cancer. It has beneficial effect on host defense mechanisms and restores depressed immune responses in animals and humans [29] .
5. Conclusion
Levamisole appeared to have an immunostimulating effect on the response of goats to PPR vaccination. The study has provided experimental evidence that Levamisole can be used as an immunostimulant in combination with PPR vaccine to prolong and maintain high antibody titre in vaccinated animals by increasing the production of cells that participate in immune protection. Further studies on cell mediated and humoral immune response of vaccinated goats over extended period, would provide evidence for the increased mobilization of immune cells and duration of immunity in animals pretreated with Levamisole before vaccination.
Acknowledgements
The authors thankfully acknowledge the technical assistance of David Bukbuk, Musa, Joshua and Monilade. The senior author was a postgraduate (MSc Microbiology) student in the Department of Veterinary Microbiology and Parasitology.
References
- Shamaki, D., Olaleye, O.D., Obi, T.U., Diallo, A., Majiyagbe, K.A. and Lombin, L.H. (2004) Peste des petits Rumi- nants (PPR) in Nigeria: Serology and Molecular Epidemiology. Vom Journal of Veterinary Science, 1, 8-27.
- Williamson, G. and Payne, W.J.A. (1978) An Introduction to Animal Husbandry in the Tropics. 3rd Edition, Longman, London, 1-755.
- Durojaiye, O.A. (1988) Control of Viral Diseases of Livestock in Africa: Problems and Prospects. In: Williams, A.O. and Masiga, W.N., Eds., Viral Diseases of Animals in Africa, Proceedings of International Symposium on Viral Dis- eases in Africa, Organization of African Unity Scientific, Technical and Research Commission, Lagos, 173-182.
- Sarkar, J., Sreenivasa, B.P., Singh, R.P., Dhar, P. and Bandyopadhyay, S.K. (2003) Comparative Efficacy of Various Chemical Stabilizers on the Thermostability of a Live Attenuated Peste des petits Ruminant (PPR) Vaccine. Vaccine, 21, 4728-4735. http://dx.doi.org/10.1016/S0264-410X(03)00512-7
- Khalafalla, A.I., Saeed, I.K., Ali, Y.N., Abdurrahman, M.B., Kwiatek, O., Libeau, G., Obeida, A.A. and Abbas, Z. (2010) An Outbreak of Peste des petits Ruminants (PPR) in Camels in the Sudan. Acta Tropica, 116, 161-165. http://dx.doi.org/10.1016/j.actatropica.2010.08.002
- Diallo, A., Minet, C., LeGoff, C., Berhe, G., Albina, E., Libeau, G. and Barrett, T. (2007) The Treat of Peste des pestits Ruminants: Progress in Vaccine Development for Disease Control. Vaccine, 25, 5591-5597. http://dx.doi.org/10.1016/j.vaccine.2007.02.013
- Baba, S.S., El-Yuguda, A.D., Egwu, G.O., Ribadu, A.Y., Ambali, A.G., Abubakar, M.B., Ibrahim, U.I. and Zoyem, N. (2007) Development and Evaluation of the Efficacy of Heat Tolerant Peste des petits ruminants (PPR) Vaccine in Ni- geria. Proceedings of International Conference on Adaptive Science & Technology (ICAST 2007), Accra, 10-12 De- cember 2007, 138-141.
- El-Yuguda, A.G., Chabiri, L.M., Adamu, F. and Baba, S.S. (2009) Peste des petits Ruminants Virus (PPRV) Infection among Small Ruminants Slaughtered at the Central Abattoir, Maiduguri, Nigeria. Sahel Journal Veterinary Sciences, 8, 93-96.
- Glenny, A.T., Pope, C.G., Waddington, H. and Wallace, U. (1926) Immunology Notes. XXIII. The Antigenic Value of Toxoid Precipitated by Potassium Alum. Journal of Pathology & Bacteriology, 29, 31-40. http://dx.doi.org/10.1002/path.1700290106
- Baylor, N.W., Egan, W. and Richman, P. (2002) Aluminum Salts in Vaccines. US Perspective. Vaccine, 20, S18-S23. http://dx.doi.org/10.1016/S0264-410X(02)00166-4
- Renoux, M. and Renoux, G. (1972) Levamisole Inhibits and Cures a Solid Malignant Tumor and Its Metastasis in Mice. Nature, 240, 1084-1088.
- Moertel, C.G., Fleming, T.R., Mac Donalds, J.S., Haller, D.G., Laurie, J.A. and Goodman, P.J. (1990) Levamisole and Fluorouracil for Adjuvant Therapy of Resected Colon Carcinoma. New England Journal of Medicine, 322, 352-358. http://dx.doi.org/10.1056/NEJM199002083220602
- Chen, L.Y., Lin, Y.L. and Chiang, B.L. (2008) Levamisole Enhances Immune Response by Affecting the Activation and Maturation of Human Monocyte-Derived Dendritic Cells. Clinical & Experimental Immunology, 151, 174-181. http://dx.doi.org/10.1111/j.1365-2249.2007.03541.x
- Thienpoint, D., Vanparijs, O.F. and Ruemaekers, A.H. (1996) Tetramisole—A New, Potent Broad Spectrum Anthel- minthic. Nature, 209, 1084-1086. http://dx.doi.org/10.1038/2091084a0
- Shari, S. (2007) Levamisole Hydrochloride: Its Application and Usage in Freshwater Aquariums. http://www.loaches.com
- Janik, J., Kopp, W.C., Smith, J.W., Longo, D.L., Alvord, W.G., Sharfman, W.H., Fenton, R.G., Sznol, M., Steis, R.G. and Creekmore, S.P. (1993) Dose-Related Immunologic Effects of Levamisole in Patients with Cancer. Journal of Clinical Oncology, 1, 125-135.
- Kumar, S., Dawey, C.E. and Friendship, R.M. (1999) Improve Weight Gain in Pigs Using Levamisole as an Immuno- modulator. Swine Health Production, 7, 103-107.
- Kayatas, M. (2002) Levamisole Treatment Enhances Protective Antibody Response to Hepatitis B Vaccination in Hemodialysis Patients. Journal of Artificial Organ, 26, 492-496. http://dx.doi.org/10.1046/j.1525-1594.2002.06928.x
- Bozic, F., Bilic, V. and Valpotic, I. (2003) Levamisole Mucosal Adjuvant Activity for a Live Attenuated Escherichia coli Oral Vaccine in Weaned Pigs. Journal of Veterinary Pharmacology and Therapeutics, 6, 225-231. http://dx.doi.org/10.1046/j.1365-2885.2003.00458.x
- RIM (1992) Nigerian National Livestock Resource Survey. 6 vols, Report by Resource Inventory and Management Limited (RIM) to FDLPCS, Abuja.
- Brown, A.B. (1976) Hematology: Principle and Procedure. 2nd Edition, Lea and Fibiger, Philadelphia, 56-81.
- Hewitt, S.G. (1984) Hematology. In: Manual of Veterinary Investigation, Laboratory Techniques, Vol. 2, Ministry of Agriculture, Fisheries and Food, London, 77-79.
- Office International des Epizootics/World Organization for Animal Health (OIE) (2004) OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 5th Edition, OIE, Paris, 1-12.
- Centre for Disease Control (CDC) (1988) Descriptive Statistics: Measure of Central Tendency and Dispersion. Centre for Disease Control and Prevention Atlanta, Georgia.
- Sen, A., Saravanan, P., Balamurugan, V., Rajak, K.K., Sudhakar, S.B., Bhanuprakash, V., Parida, S. and Singh, S.K. (2010) Vaccines against Peste des petits Ruminants Virus. Expert Review of Vaccines, 9, 785-796. http://dx.doi.org/10.1586/erv.10.74
- Wauwe, J.V. and Jassen, P.A.J. (1991) The Biochemical Mode of Action of Levamisole: An Update. International Journal of Immunopharmacology, 13, 3-9. http://dx.doi.org/10.1016/0192-0561(91)90019-4
- Amery, W.K. and Gough, D.A. (1981) Levamisole and Immunotherapy: Some Basic Consideration and Their Relevance to Human Diseases. Oncology, 38, 168-181.
- Stelletta, C., Cuteri, V., Bonizzi, L., Frangipane di Regalbono, A., Orsi, F., Nisoli, L., Lulla, D. and Morgante, M. (2004) Effect of Levamisole Administration on Bluetongue Vaccination in Sheep. Veterinaria Italiana, 40, 635-639.
- Sanford, S. (2007) Levamisole Hydrochloride. http://www.loaches.com
NOTES

*Corresponding author


