World Journal of Vaccines
Vol.2 No.3(2012), Article ID:21522,4 pages DOI:10.4236/wjv.2012.23019
Ubiquitin Reduces Expression of Intercellular Adhesion Molecules and Tumor Necrosis Factor-α in Lung Tissue of Experimental Acute Lung Injury
![]()
Department of Critical Care Medicine, Hainan Provincial Hospital, Haikou, China.
Email: *hezy118@yahoo.com.cn
Received February 20th, 2012; revised March 25th, 2012; accepted April 23rd, 2012
Keywords: Ubiquitin; Acute Lung Injury; Intercellular Cell Adhesion Molecule-1; Vascular Cell Adhesion Molecule-1; Tumor Necrosis Factor-α
ABSTRACT
Background: Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are two important cytokines in inflammatory response, which may induce rolling and adhesion of both leukocytes and lymphocytes, while modulating vascular permeability at the same time. These adhesion molecules usually serve as surrogate markers of activation and injury of vascular endothelial cells. Tumor necrosis factor-α (TNF-α) is a key factor to induce the expression and production of the above cell adhesion molecules. However, it remains to be elucidated whether exogenous ubiquitin exerts any effect on the cytokines in sepsis-induced ALI. Methods: Sixty mice were divided randomly into five groups with twelve mice in each group, i.e. CLP group, SHAM group, UB1 group (10 mg/kg), UB2 group (5 mg/kg) and UB3 group (1 mg/kg). Mice of SHAM group underwent sham operation, and other four groups underwent CLP. Six hours after surgery, mice of three UB groups received ubiquitin by caudal vein injection while CLP and SHAM group received vehicle. Seven hours after surgery, blood and lungs of all mice were collected. ICAM-1, VCAM-1 and TNF-α level of 9% lung homogenate and serum TNF-α level were measured by ELISA. Results: Pulmonary ICAM-1, VCAM-1 and TNF-α level of three UB groups were lower than CLP and SHAM group, and there were several comparisons with a statistically significant difference. Serum TNF-α level of three UB groups were slightly lower than CLP group, but far higher than SHAM group. Pulmonary ICAM-1 level, VCAM-1 level and serum TNF-α level of UB3 group were lower than UB1 and UB2 group, and there was a significant difference in VCAM-1 between UB3 and UB1 group. Pulmonary TNF-α level of UB3 group was slightly higher than UB1 and UB2 group.
1. Introduction
Ubiquitin is a peptide of 76 amino acids found in all eukaryotic cells. Because of its participation in one selective protein degeneration pathway, the intracellular and extracellular role of ubiquitin has been the subject of laboratory investigations for decades. Recent studies have demonstrated that extracellular ubiquitin may modulate inflammatory response [1]. Furthermore, exogenous ubiquitin also exerts both anti-inflammatory effects by inhibiting septic or non-septic inflammation and immunodepression by inhibiting immune responses as well as prolonging survival of grafts [1-3].
Experiments with swine models of septic or hemorrhagic shock demonstrate that exogenous ubiquitin significantly decrease the fluid requirement for volume resuscitation, and improve ventilation and oxygenation [1]. In our preliminary study in septic mice model induced by cecal ligation and perforation (CLP), exogenous ubiquitin prevents sepsis-induced acute lung injury (ALI), by inhibiting formation of pulmonary edema and inflammatory infiltrates [2].
Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are two important cytokines in inflammatory response, which may induce rolling and adhesion of both leukocytes and lymphocytes, while modulating vascular permeability at the same time. These adhesion molecules usually serve as surrogate markers of activation and injury of vascular endothelial cells. However, it remains to be elucidated whether exogenous ubiquitin exerts any effect on the adhesion molecules in sepsis-induced ALI.
The objective of our study is to demonstrate the effect of exogenous ubiquitin on pulmonary cell adhesion molecules in mice model of CLP-induced ALI. Moreover, pulmonary and serum levels of tumor necrosis factor-α (TNF-α) are measured, due to the fact that TNF-α is a key factor to induce the expression and production of the above cell adhesion molecules [4,5]. Potential dose-effect relationship of ubiquitin is also investigated.
2. Materials and Methods
2.1. Animal Protocol
All procedures were performed in accordance with the National Institutes of Health Guidelines for Use of Laboratory Animals and approved by the Institutional Ethics Committee.
Sixty male Kunming mice, weighing 18 to 23 g, age 8 weeks, were allocated randomly into 5 groups (n = 12 in each group). In CLP group, mice were subjected to CLP (14-gauge needle) as previously described [2]. A sham operation (laparotomy and cecal exposure without any more manipulation) was performed in SHAM group as negative control.
Six hours after CLP in three intervention groups, mice received a normal saline solution containing ubiquitin (LSA14199, Bioworld, USA) at the dose of 10 mg/kg body weight (UB1 group), 5 mg/kg body weight (UB2 group), and 1 mg/kg body weight (UB3 group), respectively, through caudal vein, while mice in CLP and SHAM groups received vehicle injection of the same volume.
Seven hours after the operation, a midline incision from the pubis to the neck was performed to gain access to the heart-lung block. A blood sample was drawn from the left ventricle for determination of serum level of TNF-a. Subsequently, the mice were sacrificed by exsanguination. The heart-lung block was rapidly excised, and the pulmonary circulation was flushed via the right ventricle with 20 ml of normal saline. The serum and separated lung specimens were stored at –80˚C until further analysis.
2.2. Preparation of Lung Homogenates
Immediately before analysis, tissues were homogenized 1:5 by volume in ice-cold 10 mmol/L Tris/HCl pH 7.4, 100 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L NaF, 0.1% sodium dodecyl sulphate, 1% trition, 10% glycerol, 0.5% deoxycholate, 1 mmol/L dithiothreitol, NaVO4, NaPO3, 0.2 mg/mL phenylmethylsulphonyl fluoride, and aprotinin using a BrinkmannPolytron homogenizer (Best-Lab-Deals, Raleigh, NC). Homogenates were centrifuged (20,000 g, 5˚C, 30 mins), and supernatants (or homogenates) were aliquoted.
2.3. Cytokine Assays
The concentrations of ICAM-1, VCAM-1, and TNF-α in lung homogenates and TNF-α in serum were measured by ELISA (EK0538, EK0371, EK0527, Boster, China).
2.4. Statistical Analysis
All data are presented as mean ± standard deviation (SD). Data were compared by one-way analysis of variance with either LSD test or Tamhane’s test (α = 0.05). Correlation between cell adhesion molecules and TNF-α was analyzed with Pearson correlation efficient. A p value of <0.05 was considered to be statistically signifycant.
3. Results
Compared with SHAM group, serum TNF-α level significantly increased in CLP group (6803 ± 1375 pg/ml vs. 5596 ± 1257 pg/ml, p = 0.012), while ICAM-1, VCAM-1, and TNF-α levels in lung homogenates did not differ significantly (Table 1).
Ubiquitin treatment at 6 hours after CLP procedure significantly decreased VCAM-1 level in lung homogenates, when compared with those of SHAM group and CLP group. Moreover, VCAM-1 level was lower in UB3 group than UB1 group (p = 0.031). ICAM-1 levels in lung homogenates demonstrated a similar profile, although statistically insignificant (Table 1).
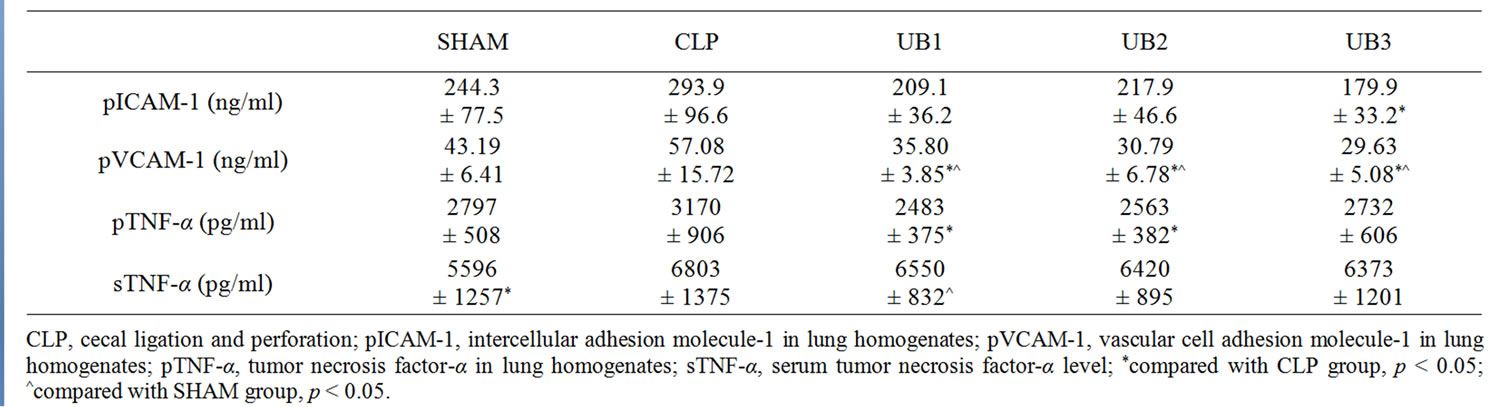
Table 1. Effect of CLP and ubiquitin treatment on pICAM-1, pVCAM-1, pTNF-α and sTNF-α level of each group.
Treatment of ubiquitin also decreased TNF-α level in lung homogenates, while the difference only reached statistical significance with higher doses of ubiquitin, i.e. 5 and 10 mg/kg body weight (Table 1). In contrast, ubiquitin did not exert any effect on the CLP-induced increase in serum TNF-α level.
Correlation between pulmonary cell adhesion molecules and TNF-α was shown in Table 2.
4. Discussion
ICAM-1 and VCAM-1 are two members of immunoglobin supergene family. Normally, ICAM-1 is only expressed at a low level in endothelial cells and epithelial cells, or constitutively on the surface of alveolar cells [4]; while VCAM-1 is constitutively expressed on a number of cell types other than vascular cells, such as chondrocytes and tissue macrophages, and is not appreciably expressed on resting vascular endothelium [5]. High-level expression of ICAM-1 and VCAM-1 could be induced by TNF-α, IL-1 and IL-6, which probably is due to activation of the transcription factors such as nuclear factor kappa B (NF-kappaB) [4,5]. Unlike VCAM-1, expression of ICAM-1 is also increased on bronchial and alveolar epithelial cells under such circumstances [6,7].
In our study, ICAM-1 and VCAM-1 level in murine lung homogenates significantly increased at seven hours after CLP, which was inhibited by intravenous ubiquitin, to a level similar to or even lower than that of SHAM group. This suggests that exogenous ubiquitin could prevent ALI secondary to not only sepsis but also other non-septic etiologies, including surgery and trauma. Moreover, ubiquitin exerts dose-dependent inhibition of ICAM- 1 and VCAM-1, with the most significant inhibittion at the dose of 1 mg/kg. This probably indicates that ubiquitin at the dose higher than 1 mg/kg might provide no further benefits.
TNF-α, produced mainly by mononuclear cells in vivo, is the core of interactive regulation of cytokine network. TNF-α regulates the release and activation of different cytokines within inflammatory cascade. In our study,
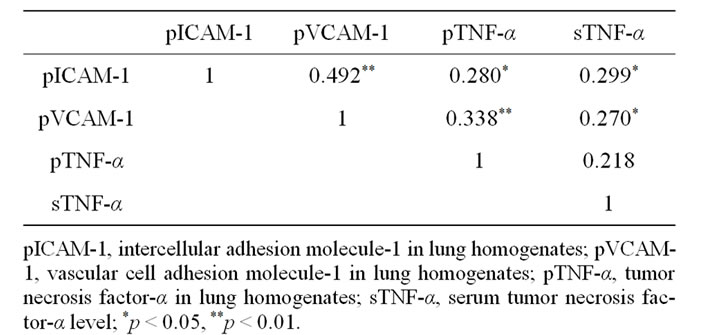
Table 2. Correlation between ICAM-1, VCAM-1, TNF-α in lung homogenates, and serum TNF-α level.
ubiquitin inhibited the sepsis-induced production of TNF- α in lung homogenates, which might in turn lead to decreased pulmonary ICAM-1 and VCAM-1 levels. The possible mechanism is that exogenous ubiquitin, when transferred into cells, may inhibit the activation of NFkappa B which reduces subsequent production of ICAM- 1, VCAM-1 and TNF-α. Similar to ICAM-1 and VCAM- 1, pulmonary TNF-α level is also affected by ubiquitin. However, unlike ICAM-1 or VCAM-1, the production of TNF-α is more significantly inhibited with higher dose of ubiquitin. The non-parallel changes of TNF-α, ICAM-1 and VCAM-1 in lung homogenates suggest that TNF-α is not the major modulator of expression of cell adhesion molecules.
Similar to the study by Majetschak and his colleagues in lethal sepsis model of swine [8], we found that exogenous ubiquitin did not affect serum TNF-α level significantly. It seems that ubiquitin does not show any inhibition of TNF-α overproduction in sepsis, therefore its effect can not be predicted by serum TNF-α level.
Overwhelming systemic inflammatory response in sepsis leads to tissue and organ damages. ALI, characterized by pulmonary edema and parenchymal infiltrates, represents pulmonary manifestation of the above inflammatory response [9]. Therefore, functional changes of endothelial cells in sepsis and ALI are extensively investigated. Although pulmonary endothelial cell is only one source of ICAM-1 production, our results suggest that exogenous ubiquitin play a protective role in sepsis-induced ALI, which might be supported if function of endothelial cells is tested with in-situ technology, or with circulating endothelial cells. At last, we believe that further investigation on dose-effect relationship is needed since we only observe a significant difference of pulmonary VCAM-1 levels between ubiquitin 1 mg/kg and 10 mg/kg group.
5. Conclusion
Exogenous ubiquitin not only significantly inhibits the increases of pulmonary ICAM-1, VCAM-1 and TNF-α level in acute lung injury of mice induced by CLP, but also lowers the increases elicited by operation and stress. But ubiquitin doesn’t effect serum TNF-α obviously. With a decline in doses, ubiquitin’s inhibition of pulmonary ICAM-1 and VCAM-1 level gradually increases while inhibition of pulmonary TNF-α level decreases.
REFERENCES
- X. D. Guan and J. Xing, “Ubiquitin in Critical Care Medicine,” Zhonghua Yixue Zazhi, Vol. 87, No. 3, 2007, pp. 273-276.
- J. Xing, X. D. Guan and B. Ouyang, “Effects of Exogenous Ubiquitin on Lung Injury and Serum Nitrite/Nitrate in Mice at the Early Stage of Sepsis after Cecal Ligation and Perforation,” Chinese Journal of Respiratory and Critical Care Medicine, Vol. 8, No. 5, 2009, pp. 432-435.
- S. A. Earle, A. El-Haddad, M. B. Patel, et al., “Prolongation of Skin Graft Survival by Exogenous Ubiquitin,” Transplantation, Vol. 82, No. 11, 2006, pp. 1554-1546.
- X. P. Zhang, D. J. Wu and X. G. Jiang, “ICAM-1 and Acute Pancreatitis Complicated by Acute Lung Injury,” Journal of the Pancreas, Vol. 10, No. 1, 2009, pp. 8-14.
- R. A. Carter and I. P. Wicks, “Vascular Cell Adhesion Molecule 1(CD106): A Multifaceted Regulator of Joint Inflammation,” Arthritis & Rheumatism, Vol. 44, No. 5, 2001, pp. 985-994. doi:10.1002/1529-0131(200105)44:5<985::AID-ANR176>3.0.CO;2-P
- M. C. Subauste, D. C. Choi and D. Proud, “Transient Exposure of Human Bronchial Epithelial Cells to Cytokines Leads to Persistent Increased Expression of ICAM-1,” Inflammation, Vol. 25, No. 6, 2001, pp. 373-380. doi:10.1023/A:1012850630351
- C. Madjdpour, B. Oertli, U. Ziegler, et al., “Lipopolysaccharide Induces Functional ICAM-1 Expression in Rat Alveolar Epithelial Cells in Vitro,” American Journal of Physiology—Lung Cellular and Molecular, Vol. 278, No. 3, 2000, pp. L572-L579.
- M. Majetschak, S. M. Cohn, J. A. Nelson, et al., “Effects of Exogenous Ubiquitin in Lethal Endotoxemia,” Surgery, Vol. 135, No. 5, 2004, pp. 536-543. doi:10.1016/j.surg.2003.09.006
- J. R. Jacobson and J. G. Garcia, “Novel Therapies for Microvascular Permeability in Sepsis,” Current Drug Targets, Vol. 8, No. 4, 2007, pp. 509-514. doi:10.2174/138945007780362719
NOTES
*Corresponding author.

