Open Journal of Urology
Vol. 2 No. 3 (2012) , Article ID: 21973 , 8 pages DOI:10.4236/oju.2012.23021
Vitamin D3 Variation between Children and Adults with Reference to Renal Stones, Environment and Urinary Tract Infections
College of Medicine, University of Tikrit, Tikrit, Iraq
Email: *profaljebouri@yahoo.com
Received June 3, 2012; revised June 28, 2012; accepted July 10, 2012
Keywords: Vitamin D3; Children; Renal Stone; Infection; Environment
ABSTRACT
Purpose: A better understanding of urinary tract infection (UTI) and the role of host, bacterial and environmental factors have improved the ability to identify the patients at risk and prevent or minimize sequelae. Kidney stones may be a complicated subject and its etiology is related to diet, increase urinary solutes and colloids in hot weather. Hypercalcaemia produced by taking large doses of vitamin D, creates high blood pressure and calcium deposits that can produce renal and bludder stones in all age groups including children. The objective of the present study was to estimate the serum level of vitamin D among patients particularly children taking treatable vitamin D. Correlation between vitamin D renal stones and UTI was also assessed. Methods: The number of patients studied was 150 collected during 2010 and 2011 in University teaching hospital. Forty two of them were children. The patients under study should have renal stone confirmed by ultrasound examination. Urine, blood and stone samples were taken for relevant laboratory investigations including identification of bacteriuria and its causative agents. Serum ions and vitamin D were also estimated. Type of renal stone collected was chemically identified. Results: One hundred and fifty patients with urolithiasis were included in the present study whose ages ranged from 8 months to 69 years and the ratio of males to females was 1.7:1. The frequency of patients revealed UTI was 52% and 78% of the infected patients were suffered from Gram-negative bacteria particularly Escherichia coli. Renal stones of mixed chemical composition were almost 72% and 78.2% of the stones were infection type. The mean of serum calcium was 2.157 mmol/l. The serum means of vitamin D among children and adults were 50.9 and 31.4 nmol/l respectively and the peak of this vitamin was recorded during summer. Conclusion: The frequency of UTI among urolithiasis patients was greater than that of non-urolithiasis. Enterobacteriaceae was the dominant family causing UTI particularly among females. Urolithiasis was more prevalent in males (62%). Recurrence of urolithiasis was high (39%) which indicated insufficient treatment of the underlying causes. Serum ions concentrations among children and adults were variables. Vitamin D values in children were higher than those estimated among adults and the peak of its overall concentration mean was found during summer (39.7 nmol/l). There was a strong relation between vitamin D level and the incidence of urolithiasis particularly among children with dietary problems.
1. Introduction
Urinary tract infection (UTI) is a common cause of human illness and failure to diagnose and treat it properly leading to further chronic morbidity. Bacterial contamination of the urine within the urinary tract (bacteriuria) is common and can at times result in microbial invasion of tissue responsible for the formation, transport and storage of urine. UTI is one of the most common medical problems and it was estimated that 150-million patients are diagnosed with a UTI every year resulting in at least $6 billions in health care expenditure. In males, UTI is uncommon, except in the first year of life but among those who are over 60 years old, urinary tract obstruction due to prostatic hypertrophy may occur. As for women, the ascent of organisms into the bladder is easier than in men because of the relatively short urethra and absence of bactericidal prostatic secretion [1]. incidence of UTI in infants may range approximately from 0.1 to 1.0 percent in all new born infants to as high as 10 percent in lowbirth-weight infants [2]. Numerous studies have been shown that uncircumcised male infants have about 10 times as many UTIs as circumcised male infants and the infection occurring mainly during the first 3 months of life [3]. The most common organisms causing UTI in community include Escherichia coli derived from the gastrointestinal tract, in addition to Proteus, Pseudomonas species, Streptococci and Staphylococcus epidermidis. Other pathogens might also causing UTIs [1]. Nephrolithiasis is a kind of diseases associated with the presence of renal stones. Renal calculi are commonly termed kidney stones, occur in the renal pelvis, the ureter, or the bladder. In developed countries, bladder stones are now uncommon, as the causative factors of malnutrition and infection have been eliminated [4]. Urinary stones are usually divided into primary and secondary. Primary calculi are mainly of renal origin with underlying metabolic causes, while secondary stones develop around a foreign body in the urinary tract or as a result of chronic obstruction and infection. Anatomic anomalies and metabolic disorders are the most important etiological factors for childhood urolithiasis [5]. Area of high incidence of stone formation is recognized throughout the world, the etiological factors in this phenomenon are unknown. In general, stone formation is facilitated by factors that increase solutes concentration in the urine, alteration in pH, provides a nidus for precipitation, congenital abnormality ,stasis, dehydration and altered Ca2+ metabolism and metabolic disorder by hyperparathyroidism, hyperuricemia and cystinuria oxalosis, hypercalcuria secondary to neoplasm or sarcoidosis vitamin D intoxication and abnormal renal function alter the urine composition such as renal tubular acidosis and foreign body e.g. catheter [6]. Vitamin D is a group of fat-soluble secosteroids, the two major physiologically relevant forms of which are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D without a subscript refers to either D2 or D3 or both. Vitamin D3 is produced in the skin of vertebrates after exposure to ultraviolet B light from the sun or artificial sources, and occurs naturally in a small range of foods. In some countries, staple foods such as milk, flour and margarine are artificially fortified with vitamin D, and it is also available as a supplement in pill form [7]. Food sources such as fatty fish, mushrooms, eggs, and meat are rich in vitamin D and are often recommended for consumption to those suffering vitamin D deficiency [8]. Vitamin D3 results from the ultraviolet irradiation of 7-dehydrocholesterol [8]. The structural difference between vitamin D2 and vitamin D3 is in their side chains. The side chain of D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24 [9]. Vitamin D deficiency remains the main cause of rickets among young infants in most countries, because breast milk is low in vitamin D, social customs and climatic conditions can prevent adequate UVB exposure. In sunny countries such as Nigeria, South Africa, Bangladesh and Arab homeland where the disease occurs among older toddlers and children it has been attributed to low dietary calcium intakes, which are characterized by cereal-based diets with limited access to dairy products [10]. In a study done by Ibraheem in 2006 showed that healthy people in Iraq had mean levels of vitamin D about 19.21 ng/ml. Holick found that the normal range is 25 - 56 ng/ml. Vitamin D deficiency can result in lower bone mineral density and an increased risk of bone loss (osteoporosis) or bone fracture because a lack of vitamin D alters mineral metabolism in the body [11]. Vitamin D toxicity, also called hypervitaminosis D, is a potentially serious but treatable medical condition that occurs when getting too much vitamin D. The main toxicities of hypervitaminosis D are hypercalcemia and hyperphosphatemia. Hypervitaminosis D is unlikely to occur until the 25-OH(D) level, the main storage form of vitamin D, exceeds 150 ng/ml. Patients may also experience nonspecific symptoms such as nausea, vomiting, anorexia, confusion, and weakness, typically secondary to the resulting hypercalcemia. The upper limit of daily vitamin D intake required to cause toxicity is unknown; however, up to 10,000 IU daily has been considered safe in a healthy population. The recently published update recommendations for vitamin D intake, suggesting a safe upper limit of 4000 IU daily for most individuals [12].
2. Materials and Methods
2.1. Patients
2.1.1. Source of Specimens
This study was done in the Urology Department wards and out patients clinic in both Tikrit teaching and Salahadeen hospitals. The number of the patients was 150 which was recorded from March 2010 to April 2011. Patients with urolithiasis were including 93 males and 57 females. 42 of the patients were children. One hundred and one stones were collected and submitted to chemical analysis. Urine and blood samples were collected from those patients who underwent lithotomy or passed their urinary stones spontaneously and also from the 20 control subjects to evaluate their serum Ca2+, Na+, K+ and vitamin D3. Vitamin D3 was done for 78 patients only [1].
2.1.2. Samples Collection
The samples collected from the patients were urine, blood and stone if available. Urine sample was collected for microbiological examination; a midstream cleanvoided specimen was used. A mid stream urine (MSU) sample was collected (30 ml of urine) and transported separately to the laboratory within 30 minutes for microbiological study [1]. Blood was withdrawn from the upper limb of the patient after the skin was disinfected with 70% alcohol by using sterile disposable syringe and transferred into a sterile plane tube. Blood sample for calcium concentration determination was done without tourniquet and the patient in the sitting position. Five milliliters sample of blood was collected and centrifuged at 1500 rpm for 5 minutes and only unhaemolysed serum was used. The serum was placed in a plane tube and then labelled and freezed. Renal stone was collected from the patients if it was available and kept in a sterile screwcapped containers or sterile sealed bags [13].
2.2. Laboratory Methods
2.2.1. Culture of Urine Specimens
Media were prepared and sterilized according to the manufacturer’s instruction. The prepared media used for isolation, determination of the viable count and identification were carried out after being solidified [1].
2.2.2. Identification of the Bacterial Isolates
Bacterial isolates were identified by the conventional methods which include colony morphology and gram stain. Api 20E systems (bioMerieeux, France) was also utilized according to the manufacturer’s instructions.
2.2.3. Chemical Analysis of Renal Stones
The obtained renal stones were analyzed using BIOLABO kit (France) according to the manufacturer’s instructions.
2.2.4. Determination of Calcium Concentration in Serum
Calcium concentration was determined using BIOLABO kit (France) according to the manufacturer’s instructions.
2.2.5. Determination of Vitamin D3 in Serum
Vitamin D concentration was determined using (IDS, UK) according to the manufacture’s instruction.
2.3. Statistical Analysis
Statistical analyses in the present study were done by using Microsoft Office Excel 2007, SPSS version 12 (Statistical Package for Social Sciences). The programs used were F-test, T-test, least significant difference (LSD) and Chi-square [14].
3. Results
3.1. Pathogens Isolated from Urine
Urine culture was done for all the 150 patients who were included in this study. Only 78 (52%) patients have positive urine culture as shown in Figure 1" target="_self"> Figure 1. Twenty nine of them were males and 49 were females. The most common organism was E. coli which was isolated from 39 (45.9%) patients followed by Pseudomonas aeruginosa 17 (20%), Staphylococcus saprophyticus 11 (12.9%), Staphylococcus epidermidis 7 (8.2%), Enterobacter aerogenes 7 (8.2%), and lastly Proteus mirabilis 4 (4.7%). Five patients revealed mixed bacterial growth of 4 E. coli, 2 Pseudomonas aeruginosa, 3 Staphylococcus saprophyticus, 2 Staphylococcus epidermidis, and 1 Enterobacter aerogenes.
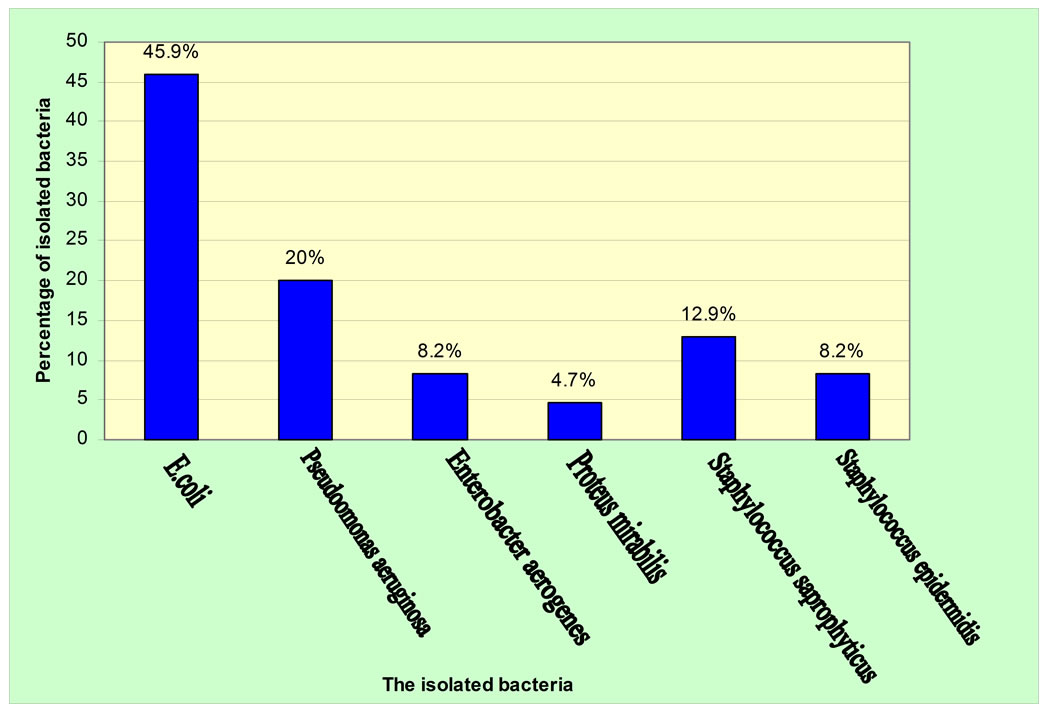
Figure 1. Percentage of isolated bacteria from urine culture of the patients.
Table 1 shows positive urine culture among patients who underwent lithotomy or passed their stones spontaneously. Seventy eight (52%) patients had positive urine culture, and the ratio of females to males was almost 1.7:1. The number of patients with infected stones who had positive urine culture was 17 (16.8%) and 10 (9.9%) patients of them were females. The number of the patients who had non infected stone with positive urine culture was 31 (30.7%), and 18 (17.8%) of patients were females. The present study showed that there were 39 (45.9%) of the isolates were urease producing organisms (4 Proteous mirabilis, 18 Staphylococcus spp. and 17 Pseudomonas aeruginosa). There were 67 (78.8%) of patients with UTI who were infected with gram negative organism while only 18 (21.1%) patients were infected with gram positive organisms.
3.2. Serum Value of Vitamin D3 in Patients
In this study vitamin D3 serum level was investigated. Table 2" target="_self"> Table 2 shows that vitamin D3 abnormalities in adults and children, with values ranged between 8.8 nmol/l and 94.7 nmol/l respectively. Statistically, there was a highly significant difference between adults and children according to T-test (P > 0.05). It was shown that the values of vitamin D3 were 42 nmol/l in overall males and in females were 33.4 nmol/l. Statistically, there was no significant
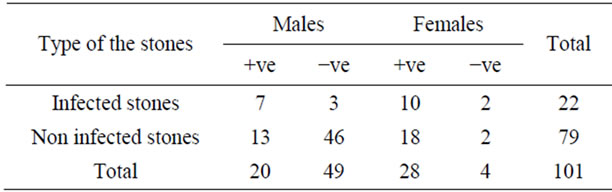
Table 1. Interrelationship between urinary tract infection and stone type.
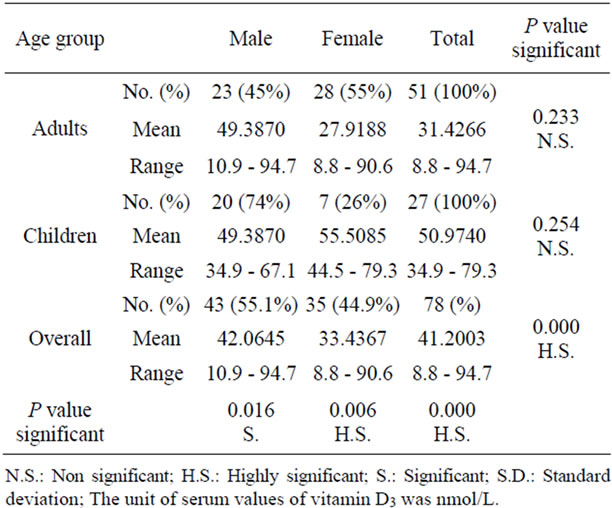
Table 2. Serum vitamin D3 levels in patients.
difference between the two values according to T-test (P = 0.084). The present study revealed that the values of vitamin D3 were 49.38 nmol/l and 55.5 nmol/l among boys and girls respectively. There were no significant difference according to T-test (P = 0.255).
Table 3 shows that there was an elevated UTI in relation to levels of vitamin D3 among adults assessed in the present study but no significant differences were concluded utilizing Chi-square test (P ≥ 0.2). An aseesssment of the same vitamin among Children revealed a very high significant differences (P = 0000) using the same statistical programing test. A high incidence with UTI was recorded among children and adults with insufficient vitamin D3. The overall comparison between the two age groups revealed a significant difference (P < 0.05).
It was found that a very high difference between the levels of vitamin D3 among adults and children and the statistical analysis using T-test showed a P less than 0.001 (Table 4). No significant differences were found concerning serum calcium of adults and children with reference to Vitamin tested (P = 0.19).
3.3. Seasonal Variation of Ions and Vitamin D3 among Patients
Table 5 shows that the comparison between adults and children according to the levels of ions and vitamin D3 with respect to seasonal variation. The overall mean of Ca2+ in spring was 2.14 mmol/l, in summer was 2.13 mmol/l, while in autumn 2.14 mmol/l. The mean of K+ in spring was 4.5 mmol/l, in summer was 4.45 mmol/l, while in autumn 4.08 mmol/l. Na+ mean in spring was 138 mmol/l, in summer was 139.7 mmol/l, while in autumn 139.4 mmol/l. Vitamin D3 mean was 36.8 nmol/l in spring, in summer was 39.7 nmol/l and in autumn 36.0 nmol/l. It was seen that vitamin D was elevated during summer among adults (36.6 nmol/l) but the highest value (49.7 nmol/l) of this vitamin was during spring among children. Potassium value (4.93 mmol/l) was elevated during spring among children while its elevation was in summer 4.54 mmol/l in adults. The highest value of S.Ca2+ among adults was 2.2 mmol/l in autumn whereas in children it was in spring with mean 2.18 mmol/l. S.Na+ was elevated in summer with mean 140.6 mmol/l in adults while in children was increased in autumn (139 mmol/l).
3.4. The Relationship between Type of Food and Renal Stone
Only 9 (6%) of the patients in the present study were eating poultry and restricted amount of tomatoes without eating red meat, whereas majority of the patients 141 (94%) had no food restriction including red meat and tomatoes as shown in Figure 2" target="_self"> Figure 2.
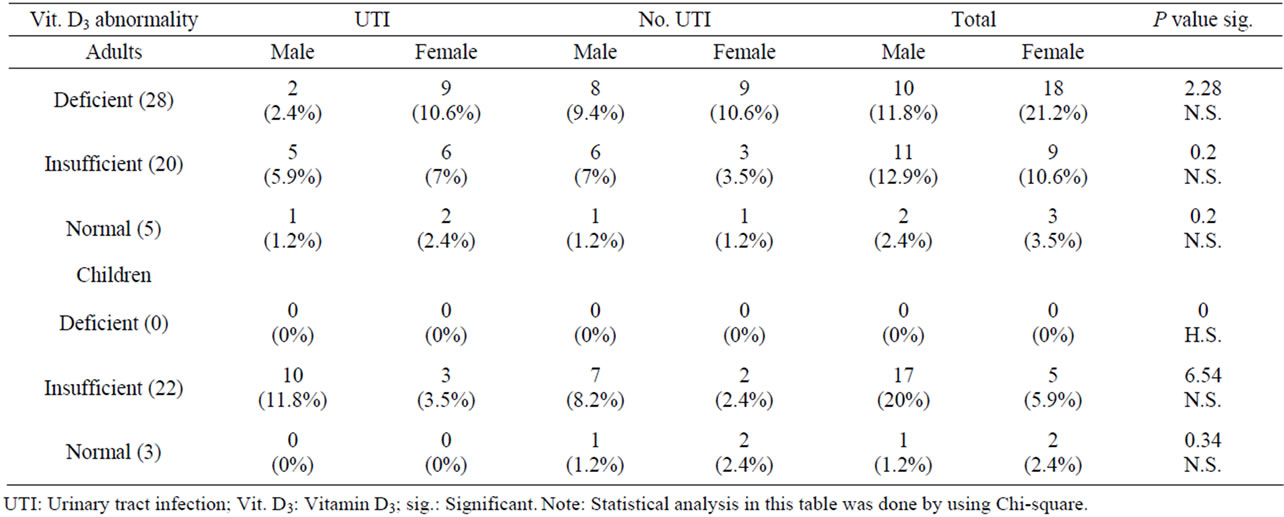
Table 3. Interrelationship between vitamin D3 abnormality and UTI in adults and children.
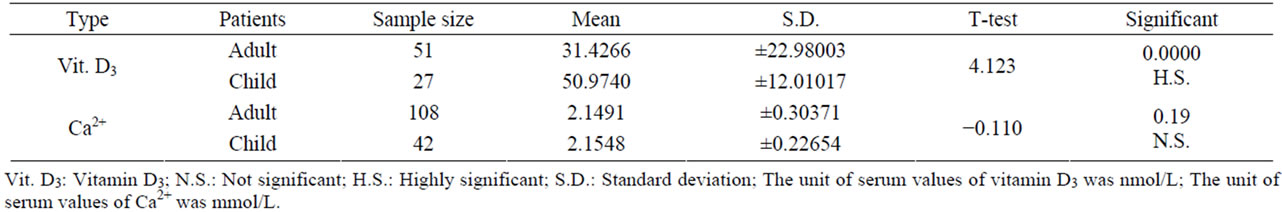
Table 4. Serum levels of vitamin D3 and calcium in the patients studied.
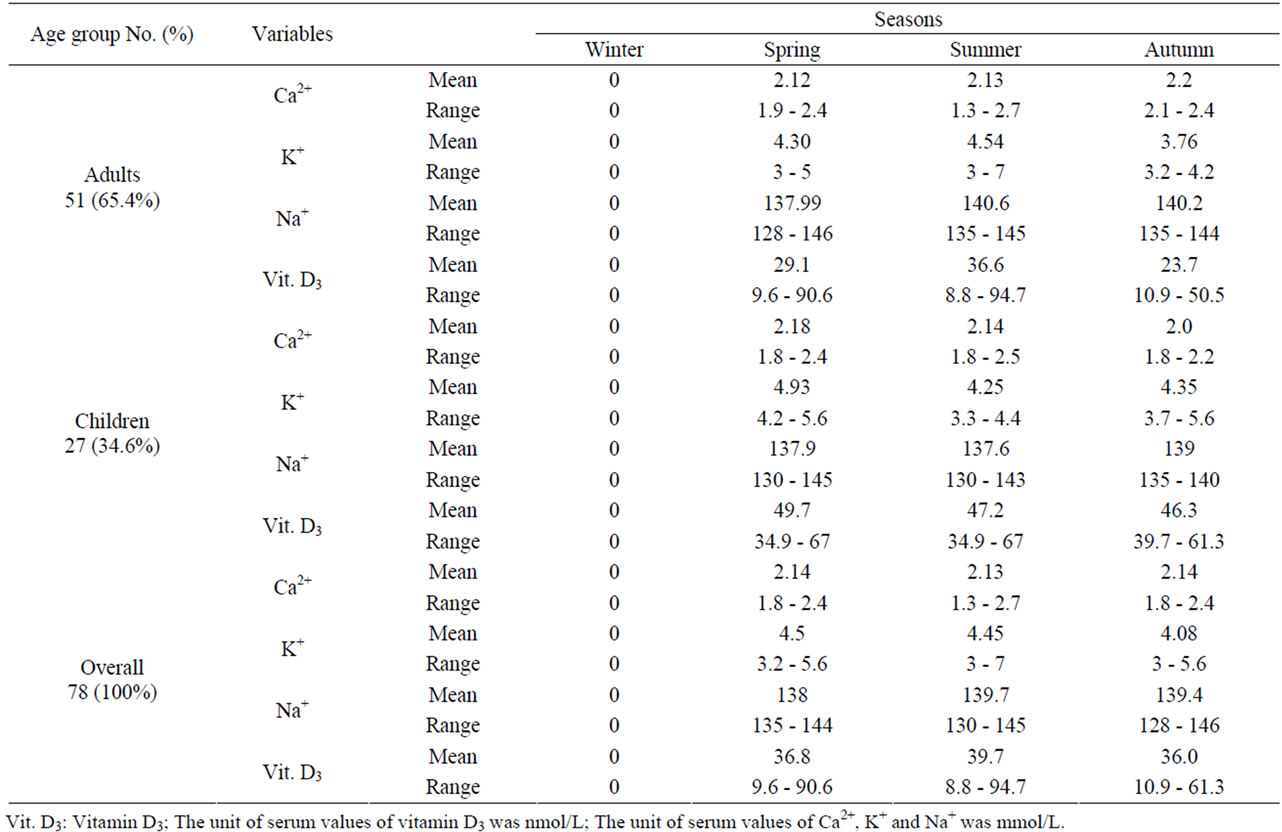
Table 5. Seasonal variation of ions and vitamin D among patients.
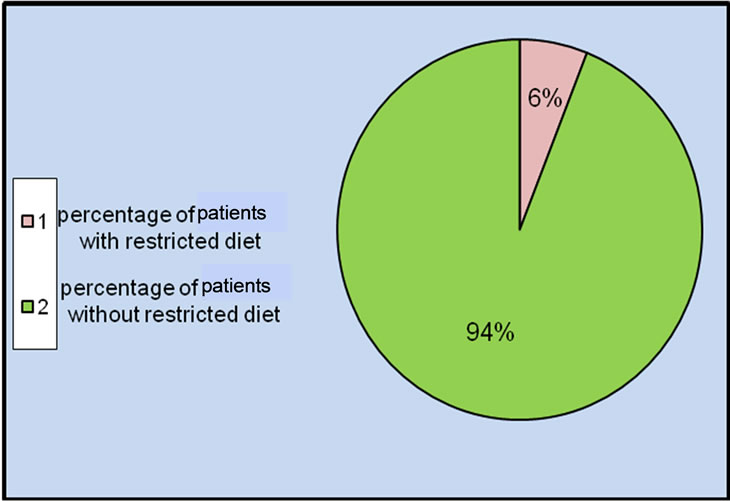
Figure 2. Distribution of patients in the study according to their nutrition.
4. Discussion
Urine culture for all 150 patients was made, it was positive for 78 patients (52%). The most organisms caused UTI in this study were belonging to gram negative bacteria isolated from 67 (78.8%) patients. These results were almost similar to those concluded by Wiesenthal et al. [15]. 17/22 (77%) of the patients with infected stones were having UTI while only 31/79 (39%) of patients with non infected stone were having UTI as was proven by urine culture. The prevalence of UTI was higher among females than male patients and the ratio was almost 1.7:1. Women are more prone to have UTI than men because in females, the urethra is much shorter and closer to the anus than in males, and they lack the bacteriostatic properties of prostatic secretions. The commonest organism isolated from the urine in this study was E. coli in a percentage of 45.9%, and it was almost similar to those carried out by Kenechukwu [16]. The prevalence of urease splitting bacteria in this study was 45.9%. Proteus mirabilis is associated with phosphate stones while E. coli are commonly associated with calcium oxalate stones.
The mean of vitamin D in children was 50.97 nmol/l with range 34.9 - 79.3 nmol/l, while in adults was 31.43 nmol/l with range 8.8 - 94.7 nmol/l as shown in Table 2" target="_self"> Table 2. Statistically, there was a highly significant difference (p < 0.001) between adults and children. This might be due to children vitamin D deficient diet associated with many parameters such as milk allergy, lactose intolerance and exclusively breast milk fed infants [17]. According to the American academy of pediatrics (AAP), infants fed human breast milk alone is provided with only 25 IU/day of vitamin D [18]. Sun light is potential source of vitamin D for infants, but the infants mostly be kept out of direct sun light and wear a protective clothing and sunscreen when exposed to sun light. AAP recommended a daily supplement of 200 IU vitamin D for breast fed infants beginning within the first 2 months of life unless they are weaned to receive at least 500 ml per day of vitamin D-fortified formula or equal to 5 µg/day [18]. Vitamin D deficiency remains the main cause of rickets among young infants in most countries, because breast milk is low in vitamin D and social customs and climatic conditions can prevent adequate UVB exposure. In sunny countries such as Nigeria, South Africa, Arab Homeland and Bangladesh where the disease occurs among older toddlers and children, it has been attributed to low dietary calcium intakes, which are characteristic of cereal-based diets with limited access to dairy products [19]. Elderly people are more prone to develop vitamin D deficiency due to the decrement of 7-dehydrocholesterol level in the skin, this may remain to the reduction in adipose tissue layer in which vitamin D is produced by photosynthesis and transported to blood stream. In addition, elderly people limited to exposure to sun light through indoor confirmation. Moreover, Holick [20] found that the normal range of serum 25-hydroxy vitamin D in human is 25 - 56 ng/ml, also he mention that the optimal 25-hydroxy vitamin D level is 45 - 50 ng/ml. The decrement in vitamin D level in Iraqi population may be attributed to the malnutrition and availability of animal sources nutrition or insufficiency of such type of diet in Iraqi people food due to the previously imposed embargo and low income of these peoples nowadays, where the diet is mainly composed of carbohydrate and fiber components, thus vegetarian diet of a major sector of Iraqi people adversely affects vitamin D status [1]. In addition to the low content of vitamin D in the whole meal in Iraqi people, also there is genetic factor which is likely to be involved in decreasing serum level of vitamin D [21]. Several studies reported that low levels of vitamin D are associated with an increase in high blood pressure and cardiovascular risk. However, High blood pressure is associated with a number of abnormalities in Ca2+ metabolism including an increase in urinary Ca2+ excretion, evidence of secondary increase in parathyroid gland activity [22]. Other study showed that low vitamin D lead to wakening of immune system [11]. Moreover, several studies showed that active vitamin D hormone increases the production of cathelicidin, an antimicrobial peptide that is produced in macrophages triggered by bacteria, viruses and fungi. Furthermore, Hoppe and Hesse [23] reported that hypervitaminosis D was due to the use of multivitamin preparations including vitamin D, or of vitamin D added to milk preparations, can induce hypercalcemia, and hypercalciuria in children.
5. Conclusion
It was concluded from the present study that the mean values of vitamin D3 estimated in children and males were more than adults and females respectively. There was no significant difference between adults and children according to the concentration of S.Ca2+ and S.K+ while there was a highly significant difference between them with reference to the concentration of S.Na+. Ca-oxalate containing stone was the most predominant which was either mixed or pure. The frequency of UTI among urolithiasis patients was greater than that of non-urolithiasis patients and UTI was more predominant in females than males. Seventy-eight percent of patients with UTI were infected with gram negative bacteria and E. coli was the most predominant microorganism isolated in this study. UTI was more common among patients with infective stones than those with non infective stones. Dietry and season parameters were highly affected the D3, serum ions concentrations, urolithiasis and UTI incidence.
REFERENCES
- M. M. A. Jebouri and N. Atala, “A Study on the Interrelationship between Renal Calculi, Hormonal Abnormalities and Urinary Tract Infections in Iraqi Patients,” Open Journal of Urology, Vol. 2, No. 1, 2012, pp. 6-10. doi:10.4236/oju.2012.21002
- J. O. Klein and S. S. Long, “Bacterial Infections of the Urinary Tract,” In: J. S. Remington and J. O. Klein, eds., Infectious Diseases of the Fetus and Newborn Infant, 4th editon, Saunders, Philadelphia, 1995, pp. 925-934.
- K. B. Roberts and O. B. Akintemi, “The Epidemiology and Clinical Presentation of Urinary Tract Infections in Children Younger than 2 Years of Age,” Pediatric Annals, Vol. 28, No. 10, 1999, pp. 644-649.
- C. T. Samuell and G. P. Kasidas, “Biochemical Investigations in Renal Stone Formers,” Annals of Clinical Biochemistry, Vol. 32, Pt. 2, 1995, pp. 112-122.
- M .Ghafoor, I. Majeed, A. Nawaz and H. A. AL-Salem, “Urolithiasis in the Pediatric Age Group,” Annals Saudia Medicine, Vol. 23, No. 3-4, 2003, pp. 201-205.
- R. C. Tiptaft and M. David, “Urology, Short Practice of Surgery,” 20th edition, Chapman and Hill, London, 1990.
- Institute of Medicine (IOM), “Dietary Reference Intakes for Calcium, phosphorus, Magnesium, Vitamin D, and Fluoride,” 1997.
- D. Joshi, J. Center and J. Eisman, “Vitamin D Deficiency in Adults,” Austarlian Prescriber, Vol. 33, No. 4, 2010, pp. 103-106.
- C. A. Burtis, E. R. Ashwood and D. E. Bruns, “Tietz Text Book of Clinical Chemistry and Molecular Diagnostics,” 4th editon, Elsevier saunders, Philadelphia, 2006.
- J. M. Pettifor, “Nutritional Rickets: Deficiency of Vitamin D, Calcium, or Both?” American Journal of Clinical Nutrition, Vol. 80, Suppl. 6, 2004, pp. 1725S-1729S.
- T. D. Bell, M. B. Demay and S. A. M. Burnett-Bowie, “The Biology and Pathology of Vitamin D Control in Bone,” Journal of Cellular Biochemistry, Vol. 111, No. 1, 2010, pp. 7-13. doi:10.1002/jcb.22661
- R. B. Jacobsen, B. W. Hronek, G. A. Schmidt and M. L. Schilling, “Hypervitaminosis D Associated with a Vitamin D Dispensing Error,” Annals of Pharmacotherapy, Vol. 45, No. 10, 2011, p. 52. doi:10.1345/aph.1Q330
- S. R. Koyyalamudi, C. H. Jeong song, K. Y. Cho and G. Pang, “Vitamin D2 Formation and Bioavailability from Agaricus Bisporus Button Mushrooms Treated with Ultraviolet Irradiation,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 8, 2009, pp. 3351-3355. doi:10.1021/jf803908q
- B. A. Forbes, D. F. Sahm, A. S. Weissfeld, L. Wilson and E. Warm, “Baily and Scott Diagnostic Microbiology,” 12th edition, Mosby Elsevier, Philadeliphia, 2007.
- I. D. Wiesenthal, D. Ghiculete, K. T. Pace, M. Ordon and R. J. Hong, “A Prospective Study Examining the Incidence of Bacteruria and Urinary Tract Infection PostShockwave Lithotripsy: The Case Against Universal Antibiotic Prophylaxis,” Canadian Urological Association Journal, Vol. 5, Suppl. 1, 2011, p. S9.
- M. Kenechukwu, O. Chinekwu, N. Davidson and U. Golibe, “Antibiotic Sensitivity Patterns in Urinary Tract Infections at a Tertiary Hospital,” Medica Journal, Vol. 5, No. 2, 2006, pp. 3331-3343.
- A. Biser-Rohrbaugh and N. Hadley-Miller, “Vitamin D Deficiency in Breast-Fed Toddlers,” Journal of Pediatrics, Vol. 21, No. 2, 2001, pp. 508-511.
- L. M. Gartner and F. R. Greer, “American Academy of Pediatrics Committee on Nutrition. Prevention Rickets and Vitamin D Deficiency: New Guidelines for Vitamin D Intake,” Pediatrics, Vol. 111, No. 4, 2003, pp. 908- 910. doi:10.1542/peds.111.4.908
- J. M. Pettifor, “Nutritional Rickets: Deficiency of Vitamin D, Calcium, or Both?” American Journal of Clinical Nutrition, Vol. 80, No. 6, 2004, pp. 1725S-1729S.
- M. F. Holick, “Calcium and Vitamin D. Diagnostics and therapeutics,” Clinical Laboratory and Medicine, Vol. 20, No. 3, 2000, pp. 569-590.
- S. A. Ibraheem, “Vitamin D Level in Iraqi Population in Health and Disease,” Master’s Thesis, University of Kufa, Najaf, 2006.
- D. A. McCarron, P. A. Pingree, R. A. Rubin, S. M. Gancher, M. Molitch and S. Krutzik, “Enhanced Parathyroid Function in Essential Hypertension: A Homeostatic Response to a Urinary Ca2+ Leak,” Hypertension, Vol. 2, No. 1, 1980, pp. 162-168. doi:10.1161/01.HYP.2.2.162
- B. Hoppe and A. Hesse, “Metabolic Disorders and Molecular Backround of Urolithiasis in Childhood,” Scanning Microscopy, Vol. 13, No. 2-3, 1999, p. 270.
NOTES
*Corresponding author.

