International Journal of Astronomy and Astrophysics
Vol.3 No.2(2013), Article ID:32463,5 pages DOI:10.4236/ijaa.2013.32012
A Molecular Hydrogen Production Model from Li and LiH in the Early Universe
Centre for Environmental Sciences, Faculty of Sciences, University of Chile, Santiago, Chile
Email: *correo@raulmorales.cl
Copyright © 2013 Raúl G. E. Morales, Mauricio R. Canales. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 14, 2013; revised March 15, 2013; accepted March 22, 2013
Keywords: H2; Li and LiH in the Dark Epoch; Li and LiH as Primitive Catalyzers; H2 Production from Li Model
ABSTRACT
Neutral isotopes and ions of H, He and Li define the chemistry of the early universe by collisional and radiative process, where under low temperature and radiation regime, only neutral species were essential in the cooling mass that gave origin to the first proto star structures. Nevertheless, up to now, in every kinetic model Li is permanently discarded from fundamental reactions due to its extremely low density. Contrarily to these previous models we have developed a novel kinetic model based on two consecutive reactions of Li and LiH with H, in order to generate a recursive process that fit well H2 production to temperatures as low as 200 K, according to the cosmological time at the end of the dark epoch. Our results show how Li and LiH merge as first catalyzers of the H to H2 chemical reaction and permit us to explain the expected abundance of H2 as the main coolant in the early universe as well as in cold regions of the cosmos.
1. Introduction
The standard Big Bang theory permits to explain how the universe had been expanding adiabatically, cooling from an initial high extreme condition of temperature and density [1]. After the first second neutrons and protons initiate the fusion reactions that set the beginning of nucleosynthesis stage at temperatures of 1010 K, where 100 seconds later this process ceases being formed mainly H and He, as well as in minor quantities Li and several cations and anions derived from these initial nuclear species under the denomination of the recombination epoch. Thus, after that free electrons take part in the atoms and ions materialization, the universe begins a new phase known as the thermal decoupling of matter and radiation while expansion and cooling continue, and the H2 molecule became one of the most fundamental coolant in the low temperature range [2].
The chemistry of the early universe is broad and diverse, whenever only few atomic species were the main reactants: H, He, Li and the isotopic forms and ions. Furthermore, the chemistry of these atomic and molecular reactants and products were mainly governed by both collisional and radiative process in gas state [3]. Some authors [4-6] have intended to represent the first proto star formations at this stage of the early universe, denominated dark epoch, by chemical reaction networks based on the neutral primordial molecules such as H2 and HD as well as H, D, He and their corresponding atomic cations and anions. However, due to the high relative abundance of H respect to the remainder atoms, H2 emerges in the universe as a primordial molecule due its high abundance and maintains a high interest in all the constitutive models of the first proto stars.
Nevertheless, in spite of the different theoretical approaches respect to generate H2, the particular role of the primordial Li as a precursor of this molecular species has not been suitably visualized up to date, particularly, in consideration to the low abundance calculated for the early universe. Effectively, Stancil et al. [7] determined the chemistry of primordial gas LiH in protoclouds through a broad set of fundamental reactions suggesting a primitive LiH abundance of 10−18 respects to the atomic hydrogen abundance as the unity. In general, this kind of primordial molecules with dipole moment has been mostly explored in order to analyze the specific role as cooling centers for radiative process immerse in atomic hydrogen big clouds, but the singular case of LiH, in different works, has been permanently discarded as an suitable species for chemical reactions due to its extremely low abundance [8].
In the early universe, H2 has been mainly conceived as product of radiative process, derived from H−, H+, H2+ and H3+ reactions [3,9]. Contrarily to these current models, we have repaired in a new role of Li and LiH species as precursors of H2, in two concerted and consecutive reactions due to Li can act as a catalyzer for neutral reactions, particularly at low densities and temperatures. Thus, the Li + H and LiH + H reactions are an effective and alternative route to the ionization processes and the same time permit us to understand in a best way the role of hydrogen at the end of the dark epoch, previous to the first proto star formations under low radiation and temperature regime [10].
Therefore, in the present work, we have introduced a chemical model that considers the H2 production from neutral atom reactions between Li and H, and the subsequent reaction between LiH and H, both cases from a collisional-radiative kinetic mechanism, under physicochemical conditions of variable low density and temperature ranges characteristics of expected primitive hydrogen cold nebulas or first generation of proto-stellar formation. Our hypothesis shows a recursive behavior of lithium reactions, which determine the onset of molecular hydrogen, even though these lithium species are found in very low abundance relative to atomic hydrogen density, in a similar behavior to catalyzer reactant.
2. Materials and Methodology
The kinetic model applied to the molecular hydrogen production comprise a collisional process scheme of two consecutive reactions between H and Li atoms in the first event and between H and LiH in the second one, all these species prevailing in the same bulk of cold gases, with temperatures lower than 200 K, such as must be expected at the end of the dark epoch in the early universe. Furthermore, according to the last calculations developed by Larena et al. [11] about H and Li in the primitive universe, we have used their density relationship of Li/H = 2.387 × 10–10. Therefore, we have assumed essentially a classical collisional-radiative kinetic model for Li and H in the first reaction and a classic collisional approach for LiH and H, in the second one, as two consecutive reactions governed by a kinetic constants k0 and k1, respectively, in a typical scheme of pseudo-first order reactions, where the relative abundance determine that 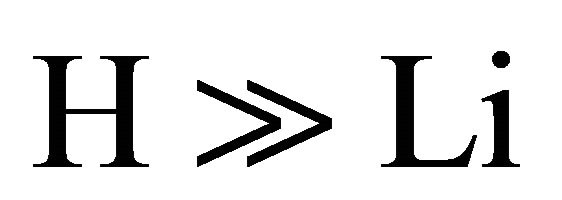 and
and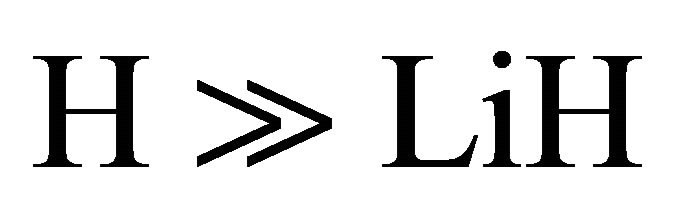 .
.
Thus, the following kinetic scheme can be described for the two main reactions and their corresponding equations in a pseudo-first order approach as:
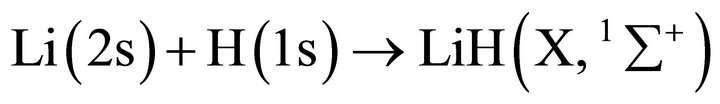
and
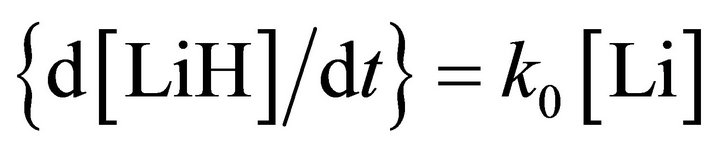 (1)
(1)
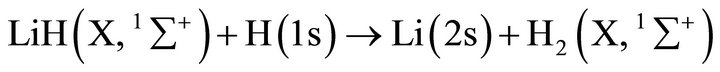
and
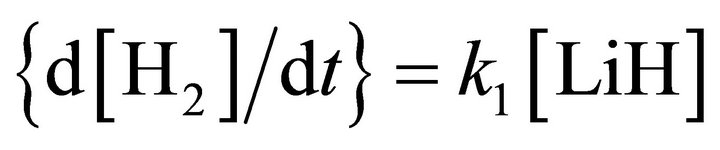 (2)
(2)
where 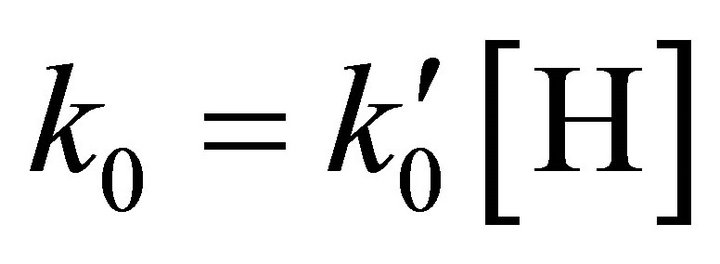 and
and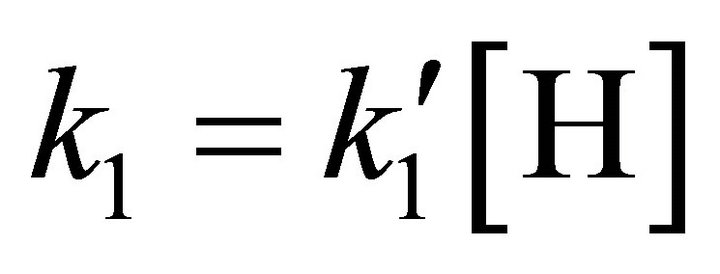 , and the final concentrations of [Li], [LiH] and [H2] are determined according to the following equations [12,13]:
, and the final concentrations of [Li], [LiH] and [H2] are determined according to the following equations [12,13]:
 (3)
(3)
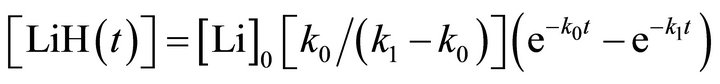 (4)
(4)
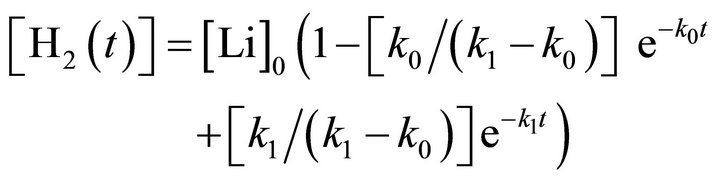 (5)
(5)
From a computational point of view the integration of the kinetic equations were done analytically according to Equations (3) to (5), which means that the abundance of species depend on the time evolution directly and no time steps are used as in case of numerical integration [14]. Consequently, the abundances of [H2] as the final product depend on the initial density of [Li]0 and the inner kinetic constants (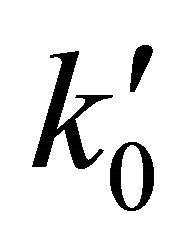 and
and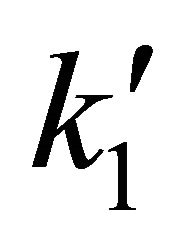 ). The computational procedure registers the abundances of [Li], [LiH] and [H2], where in this last species we have differenced two situations, by stepwise as [H2(t)] and by accretion as [H2]T.
). The computational procedure registers the abundances of [Li], [LiH] and [H2], where in this last species we have differenced two situations, by stepwise as [H2(t)] and by accretion as [H2]T.
This kinetic model was implemented in IDL 6.1 software and once established the initial code we searched its operating limits from 2.4 × 104 to 2.4 (atoms cm−3) for [H]0 and from 1.0 × 10−6 to 1.0 × 10−10 (atoms cm−3) for [Li]0. The calculations were implemented in a Power Edge T710 computer with 18 cores Intel Xeon X5670 and double precision data type of 64 bite and 48 GB of memory.
3. Results and Discussion
For hydrogen high density conditions, beyond 1.0 × 104 (atoms cm−3) and 200 K temperature, a three body collisional approach determines the normal course of the classical gaseous chemical process, where the reaction rate coefficients of the equations 1 and 2 are 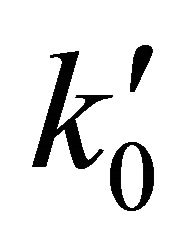 = 4.64 × 10−10 cm3·s−1 and
= 4.64 × 10−10 cm3·s−1 and 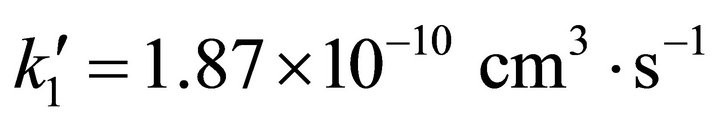 for the first and the second reactions [15], respectively. In this case, the
for the first and the second reactions [15], respectively. In this case, the 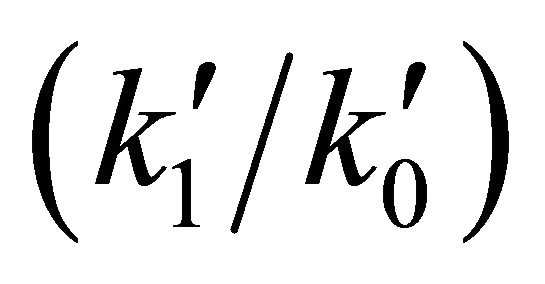 ratio reaches 0.403 and, therefore, the system follows a classical kinetic approach for a consecutive two steps reaction [12,13]. This kind of chemical reactions can typically be expected when the system moves to the Jeans’ condition, previous to the final step of each primitive proto-star hydrogen cold nebula formation. In Figure 1, we show the classical chemical dynamic of Li, LiH and H2, for a singular case of hydrogen atomic density nebula of 104 (atoms cm–3) according to our model.
ratio reaches 0.403 and, therefore, the system follows a classical kinetic approach for a consecutive two steps reaction [12,13]. This kind of chemical reactions can typically be expected when the system moves to the Jeans’ condition, previous to the final step of each primitive proto-star hydrogen cold nebula formation. In Figure 1, we show the classical chemical dynamic of Li, LiH and H2, for a singular case of hydrogen atomic density nebula of 104 (atoms cm–3) according to our model.
In Figure 1 the maximum of Li consumption coincides with the maximum of LiH formation and the H2 product obtained from every reaction step describes a sigmoidal curve up to reach the Li recuperation. However the total H2 obtained by accumulation of every cycle, due to the catalyst effect of the LiH after equation 2, begin an exponential progression. According to this approach, the total molecular hydrogen density formation reaches 1.0 × 10–5 (molecules cm–3) after three centuries. This typical density can be observed in different studies as a critical value of H2 in order to reach a significant role as molecular coolant at low temperatures near to 200 K [4].
However, in order to analyze tenuous and cold atomic hydrogen nebulas, the three body classical kinetic approach could not be used due to the low collisional probabilities of the reactants. Thus, the chemical reactions only can occur after spontaneous radiative association. Therefore, we have made use of the spontaneous radiative rate coefficient for the reaction: Li(2s) + H(1s) determined by Bennet et al. [16], which reaches 3.75 × 10–20 (cm3·s–1); while for the second reaction rate coefficient between LiH (X, 1∑+) and H(1s) we have made use of the quasi-classical trajectories reaction determined by Dunne et al. [17], which reaches a rate coefficient of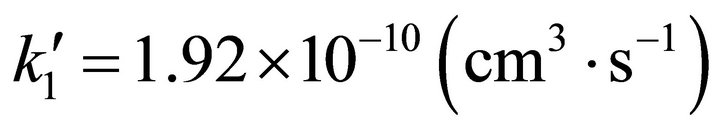 , both constants calculated at 200 K. This situation modifies strongly the current conditions, due to the
, both constants calculated at 200 K. This situation modifies strongly the current conditions, due to the 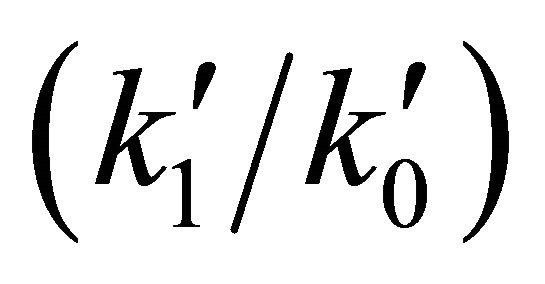 rate coefficient change from 0.403 to 5.12 × 109.
rate coefficient change from 0.403 to 5.12 × 109.
In Figure 2 we show the expected kinetic behavior for these two consecutive reactions (1 and 2) with a spontaneous radiative association between H and Li as a singular case which can be extrapolated to low atomic hydrogen density nebulas of 1 (atom cm–3) where Li can reach typical densities of 10–10 (atoms cm–3). The extremely low and slow variations of LiH under these new kinetics conditions determine only small changes in Li abundance where after five centuries reaches a constant value, following a typical catalyzer behavior. After that point, H2 by steps follows up to reach the Li density, while the total H2 by accumulation growth in an exponential progression. Finally, this last species reaches a density of 1.0 × 10–5 (molecules cm3) only after 400 million years, which compare very well to the time ranges that determine the birth lifetime of primitive stars.
Stancil et al. [7] have rigorously discussed the Lithium Chemistry in the post-recombination epoch in order to reach a more comprehensive known of this period. However, the LiH abundance has been qualified as insignificant because the low rate coefficient for the radiative association between lithium and hydrogen [7]. This result determined that LiH was disesteemed with an insignificant role in erasing primary anisotropies in the cosmic background radiation in spite of its particular big dipolar moment [4]. On the other hand, LiH as potential molecular cooling on a primordial element of gas, was discarded too, due to this species would need an abundance at least ten orders of magnitude higher to be an efficient cooler, so LiH was ruled out as an important cooler in primordial gas [8,18,19].
Under these considerations LiH was understood as negligible molecular system in the early universe and therefore unable to induce noteworthy changes in the star evolution. Therefore, from different analysis of the chemical
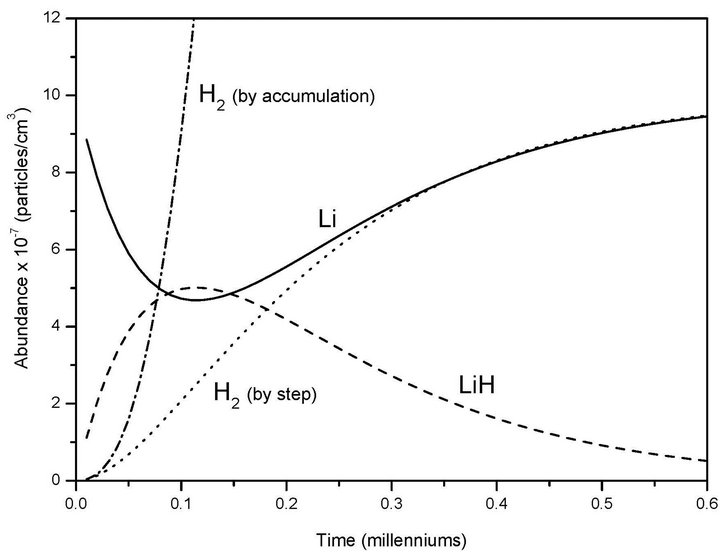
Figure 1. Molecular productions of H2 and LiH from chemical reactions derived from the present model due to classical collisional association between Li + H, as well as LiH + H, for high atomic hydrogen density nebula and Li density of 1.0 × 10–6 (atoms cm–3) at 200 K.
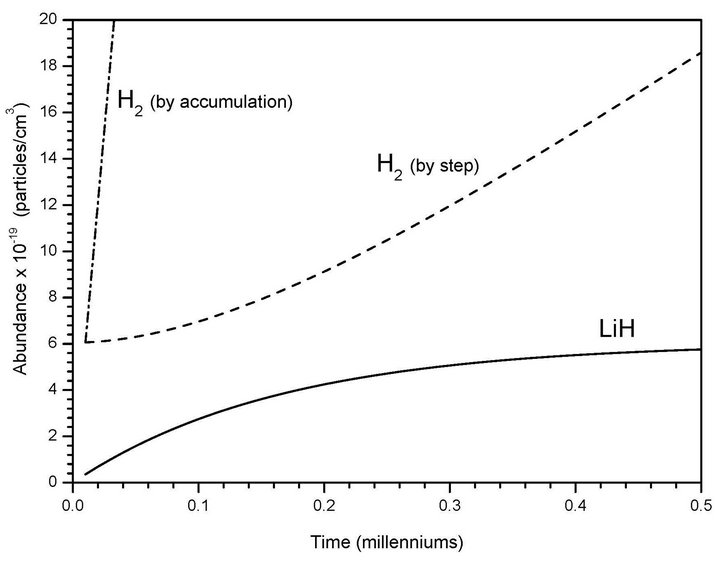
Figure 2. Molecular productions of H2 and LiH from chemical reactions derived from the present model due to collisional and spontaneous radiative association between Li + H, as well as LiH + H, for low atomic hydrogen density nebula and Li density of 1.0 × 10–10 (atoms cm–3) at 200 K.
reactions in the early universe, Li has not been suitably included in reaction networks of H2 formation [20]. However, the catalyzer role of Li emerges as an interesting and new aspect to be considered in the prestellar molecular hydrogen under low temperature and photoionization regime, as well as in the evolution of proto-star hydrogen nebulae. As can be seen, our model follows well the expected trends of H2 formation under 200 K, where the species are projected to be mainly in ground state due to the low UV radiation. By following, the well-known ionization reactions of H–, H+, 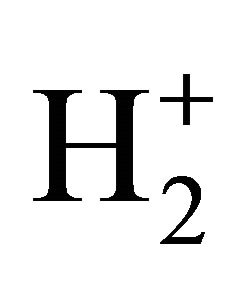 and
and 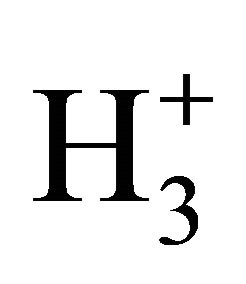 for molecular hydrogen production could not efficiently apply due to only fluxes greater than 5 × 10−15 erg cm−2·s−1·Hz−1·sr−1 converts UV pumping effective in order to increase populations of excited molecules [20]. Thus, all these atomic species are predominantly expected to be in ground state, and the H2 production, contrarily to other hypothesis, can be simply explained by means of our Li/LiH catalyzer model [21].
for molecular hydrogen production could not efficiently apply due to only fluxes greater than 5 × 10−15 erg cm−2·s−1·Hz−1·sr−1 converts UV pumping effective in order to increase populations of excited molecules [20]. Thus, all these atomic species are predominantly expected to be in ground state, and the H2 production, contrarily to other hypothesis, can be simply explained by means of our Li/LiH catalyzer model [21].
Finally, our model based in consecutive reactions of Li and LiH, for the molecular hydrogen production at low temperatures and radiation regime permits us to reinforce the coolant effect of H2 under 200 K. In addition, our model involving these two Li consecutive pseudo-first order reactions at low levels of H density could satisfactory explain the yield of H2 in early steps previous to the H gravity collapse during the first star formation, in which so far only ions-radiative processes involving H has been postulated up to date [20,22]. Furthermore, our model can be extended to other cosmic cold H-environment where the presence of high densities levels of H2 represents a challenge to our known.
4. Conclusion
This work establishes for the first time a new role for Li and LiH as two main catalyzer species of the H2 production in the early universe. Furthermore, the H2 molecular production at low temperatures and radiation regime can be well explained by means of two chemical reaction of neutral species, where both consecutive reactions of Li and LiH with H, determine a continue production of H2 reaching observed densities in good agreement to typical scale times for proto star evolution as well as cold regions of the cosmos.
5. Acknowledgements
The authors’ acknowledgement to the Centre for Environmental Sciences of the University of Chile for financial support.
REFERENCES
- P. J. E. Peebles, “Principles of Physical Cosmology,” Princeton University Press, Princeton, 1993.
- S. Weinberg, “The First Three Minutes: A Modern View of the Origin of the Universe,” Published by Basic Books, Perseus Books Group, New York, 1993.
- A. Dalgarno, “The Growth of Molecular Complexity in the Universe,” Faraday Discussions, Vol. 133, 2006, pp. 9- 25. doi:10.1039/b605715b
- D. Galli and F. Palla, “The Chemistry of the Early Universe,” Astronomy & Astrophysics, Vol. 335, 1998, pp. 403-423.
- S. Bobino, M. Tacconi, F. A. Gianturco, D. Galli and F. Palla, “On the Relative Abundance of LiH and LiH+ Molecules in the Early Universe: New Results from Quantum Reactions,” Astrophysical Journal, Vol. 731, 2011, p. 107. doi:10.1088/0004-637X/731/2/107
- M. Tegmark, J. Silk, M. J. Rees, A. Blanchard, T. Abel and F. Palla, “How Small Were the First Cosmological Objects?” Astrophysical Journal, Vol. 474, No. 1, 1997, pp. 1-12. doi:10.1086/303434
- P. C. Stancil, S. Lepp and A. Dalgarno, “The Lithium Chemistry of the Early Universe,” Astrophysical Journal, Vol. 458, No. 401, 1996, pp. 401-406. doi:10.1086/176824
- P. C. Stancil and A. Dalgarno, “Stimulated Radiative Association of Li and H in the Early Universe,” Astrophysical Journal, Vol. 479, No. 2, 1997, pp. 543-546. doi:10.1086/303920
- T. de Jong, “The Density of H2 Molecules in Dark Interstellar Clouds,” Astronomy & Astrophysics, Vol. 20, 1972, pp. 263-274.
- E. Bodo, F. A. Gianturco and R. Martinazzo, “The GasPhase Lithium Chemistry in the Early Universe: Elementary Processes, Interaction Forces and Quantum Dynamics,” Physics Reports, Vol. 384, No. 3, 2003, pp. 85-119. doi:10.1016/S0370-1573(03)00243-6
- J. Larena, J. M. Alimi and A. Serna, “Big Bang Nucleosynthesis in Scalar Tensor Gravity: The Key Problem of the Primordial 7Li Abundance,” Astrophysical Journal, Vol. 658, No. 1, 2007, pp. 1-10. doi:10.1086/511028
- I. Levine, “Physical Chemistry,” 5th Edition, McGraw Hill, New York, 2005.
- P. Atkins and J. De Paula, “Physical Chemistry,” 8th Edition, Oxford University Press, New York, 2006.
- H. Margenau and G. M. Murphy, “The Mathematics of Physics and Chemistry,” Editorial Van Nostrand, 1956.
- S. Lepp, P. C. Stancil and A. Dalgarno, “Atomic and Molecular Processes in the Early Universe,” Journal of Physics B: Atomic, Molecular and Optical Physics, Vol. 35, No. 10, 2002, pp. R1-R24. doi:10.1088/0953-4075/35/10/201
- O. J. Bennet, A. S. Dickinson, T. Leininger and F. X. Gadea, “Radiative Association in Li+H Revisited: The Role of Quasi-Bound States,” Monthly Notices of the Royal Astronomical Society, Vol. 341, No. 1, 2003, pp. 361-368. doi:10.1046/j.1365-8711.2003.06422.x
- L. J. Dunne, J. N. Murrell and P. Jemmer, “Analytical Potential Energy Surface and Quasi-Classical Dynamics for the Reaction LiH(X,1Ʃ+)+H(2S) > Li(2S)+H2(X,1Ʃ g+),” Chemical Physics Letters, Vol. 336, No. 1-2, 2001, pp. 1-6. doi:10.1016/S0009-2614(01)00102-6
- H. Mizusawa, K. Omukai and R. Nishi, “Primordial Molecular Emission in Population III Galaxies,” Publication of the Astronomy Society of Japan, Vol. 57, 2005, pp. 951-967.
- J. P. Prieto, P. Padoan, R. Jimenez and L. Infante, “Population III Stars from Turbulent Fragmentation at Redshift 11,” Astrophysics Journal, Vol. 731, 2011, p. L38. doi:10.1088/2041-8205/731/2/L38
- T. Abel, P. Anninos, Y. Zhang and M. L. Norman, “Modeling Primordial Gas in Numerical Cosmology,” New Astronomy, Vol. 2, No. 3, 1997, pp. 181-207. doi:10.1016/S1384-1076(97)00010-9
- M. Canales and R. G. E. Morales, “A Kinetic Model for H2 Formation from Li in Pre-stellar Atmospheres,” Proceedings of the 7th Congress of Chilean Environmental Chemistry and Physics—Atmospheric Section, Concepción, 20-22 October 2011, p. 2.
- V. Bromm, P. S. Coppi and R. B. Larson, “The Formation of the First Stars. I. The Primordial Star-forming Cloud,” Astrophysical Journal, Vol. 564, No. 1, 2002, pp. 23-51. doi:10.1086/323947
NOTES
*Corresponding author.

