Agricultural Sciences
Vol.08 No.05(2017), Article ID:76518,12 pages
10.4236/as.2017.85029
Evaluation of 2,4-D-Choline Based Herbicide Systems in 2,4-D Tolerant Soybean (Glycine max L.)
Dwayne D. Joseph1, Colton H. Sanders2, Michael W. Marshall2*
1Department of Plant and Environmental Sciences, Clemson University, Clemson, SC, USA
2Edisto Research and Education Center, Clemson University, Blackville, SC, USA
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 5, 2017; Accepted: May 21, 2017; Published: May 26, 2017
ABSTRACT
Weeds are the most limiting factor in soybean production in South Carolina. With early emergence and rapid growth, weeds effectlively compete for water, nutrients, and light resources. The recent evolution of herbicide resistant weeds has made it increasingly difficult for growers to effectively control weeds in soybean. Glyphosate and ALS-resistant Palmer amaranth biotypes have spread rapidly throughout South Carolina, especially in areas where resistance management is lacking. Soybean varieties have been recently developed with tolerance to 2,4-D. Field experiments were conducted at the Clemson University Edisto Research and Education Center located near Blackville, SC in 2012 and 2013 to evaluate selected 2,4-D choline based herbicide programs for weed management in 2,4-D tolerant soybean. Overall, all herbicide treatments were effective in controlling weeds at the POST2 timing. Palmer amaranth control was excellent; however, pitted morningglory was the most difficult. The 2,4-D plus glyphosate pre-mixture provided excellent control for all three weed species with >95% control at POST2 timing. In these treatments, the rate of 2,4-D choline plus glyphosate (1.09 kg ae ha−1 or 1.64 kg ae ha−1) did not have a significant effect on weed control (P = 0.3772). There was a decrease in pitted morningglory control 3 WAP in 2012 vs 2013 in plots treated with S-metolachlor + fomesafen because of a lack of activating soil moisture in 2012. Results from this study showed that all treatments evaluated provided good to excellent control of all 3 weed species. Based on the herbicide programs evaluated in the study, herbicide resistant weeds, such as Palmer amaranth, can be effectively controlled when treated at the correct growth stage.
Keywords:
Glyphosate, Resistance, 2,4-D Choline, Weeds, Soybean

1. Introduction
Weeds demonstrate aggressive and vigorous growth habits in crop production fields and compete with crops for sunlight, water, nutrients along with other resources [1] - [8] . Management of weeds is typically accomplished using a combination of cultural, mechanical, or chemical methods [9] [10] . Weeds are controlled chemically by the use of herbicides which are toxic to the targeted weed. Herbicides are classified by their mode of action, which is the way in which the herbicide controls the susceptible plants [11] . Mode of action describes the biological process or enzyme in the plant that the herbicide interrupts, affecting normal growth and development. The mode of action may also refer to the injury symptoms seen on the susceptible plants [12] .
Herbicide resistance is defined as the inherited ability of a plant to survive and reproduce following exposure to a dose of herbicide that would normally be lethal to the wild type [13] . Resistance may occur naturally due to selection or it may be induced through such techniques as genetic engineering [13] . Currently, there are 460 unique cases (species x site of action) of herbicide resistance globally with 246 species (143 dicots and 103 monocots). Weeds have evolved resistance to 22 of the 25 known herbicide sites of action and to 157 different herbicides [14] . The sheer gravity of the problem became apparent in 1997 when South Carolina reported Palmer amaranth (Amaranthus palmeri S. Watson) with resistance to acetolactase synthase (ALS) inhibitors. In 2006, Georgia first reported the appearance of glyphosate-resistant Palmer amaranth and in 2010, Palmer amaranth was confirmed in South Carolina with multiple resistance to glyphosate and ALS-inhibiting herbicides [14] [15] .
Palmer amaranth, along with large crabgrass [Digitaria sanguinalis (L.) Scop.] and pitted morningglory [Ipomoea lacunosa (L.)], are some of the weeds that are the most difficult to manage in soybean fields in South Carolina [16] . The discovery of glyphosate and ALS-resistant Palmer amaranth biotypes in South Carolina has exacerbated the issue. Weed management should always be proactive and adaptable to remain effective and economical. Reliance on a single management tactic will lead to weeds being able to circumvent that control method [13] . Herbicide-resistant weeds have become a problem where growers rely on a single herbicide mode of action exclusively over several years; which was the problem with glyphosate use over the years. By applying two or more herbicide modes of action in conjunction, growers may be able to reduce the appearance of “super weeds” which show resistance to more than one herbicide mode of action. However, most growers do not manage resistant weeds until they become a major problem in their fields [17] [18] .
Dow AgrosciencesTM recently developed soybean that is tolerant to 2,4-D to help control troublesome, glyphosate-resistant weeds, such as Palmer amaranth. The Enlist™ Weed Control System provides tolerance to 2,4-D choline to soybean along with glyphosate and glufosinate tolerance [19] . Tolerance was achieved through the insertion of bacterial genes into soybean that allows the plant to metabolize 2,4-D.
Along with the new crop technology, Dow Agrosciences also introduced Enlist Duo™, a new herbicide technology featuring Colex-D™ Technology which is a mixture of glyphosate and 2,4-D choline. The new choline formulation has several positive attributes including ultra-low volatility, minimized potential for drift, lower odor and better handling and tank mixing characteristics than commercially available amine or ester formulations of 2,4-D currently available on the market [19] .
A new formulation of 2,4-D called 2,4-D choline was developed to minimize the off-target movement and damage potential due to volatilization and subsequent vapor drift [8] [20] [21] . Previous research has documented that up to 16% of spray solution can physically drift from the intended application area [22] . Therefore, growers will need to exercise caution when applying these new herbicide technologies to avoid injury to sensitive crops in adjacent fields. The particle drift potential of any 2,4-D formulation depends on type of nozzle used. Nozzle selection becomes an important consideration whenever “auxin-like” herbicides are being applied, the finer the droplets, the greater the ability for them to move to potentially sensitive areas. Nozzles that produce coarser size droplets will minimize 2,4-D particle drift and subsequent injury to sensitive crops in adjacent fields [23] . However, application instruction, including nozzle types, time of day of application along with other ways to mitigate drift injury are accompanied with the new herbicides technologies to help reduce misuse by growers and commercial applicators [22] .
The Enlist™ Weed Control System will allow growers to apply 2,4-D choline topically to manage troublesome and resistant weeds which impact crop yields. Glyphosate and glufosinate will continue to play a role in this new technology to control other weeds present in fields [24] [25] . A multiprong and proactive approach to weed control is critical to minimize or prevent the selection of 2,4-D resistant weed biotypes. The objective of this study was to evaluate the efficacy of 2,4-D choline based herbicide programs in 2,4-D tolerant soybean for the control of Palmer amaranth, large crabgrass and pitted morningglory.
2. Materials and Methods
Field experiments were conducted on a Dothan loamy sand (pH of 6 and organic matter of 2.1%), (fine-loamy, siliceous, thermic Plinthic Paleudults), at Clemson University Edisto Research and Education Center (33.36o N, -81.32o W) located near Blackville, SC, USA in 2012 and 2013 to evaluate 2,4-D choline based herbicide programs for weed control in 2,4-D tolerant soybean. The regulated 2,4-D-, glufosinate-, and glyphosate-tolerant soybean variety “978-HT-SOY- MR” (Dow AgroSciences; Indianapolis, IN, USA) was seeded 2.5 cm deep in rows spaced 96 cm apart on 27 June 2012 in a conventionally-tilled soil at 20 seeds m−1 using an Almaco cone plot planter (Almaco Company; Nevada, Iowa, USA). Plots dimensions were two rows wide and 9.4 m long. Due to the unavailability of 2,4-D tolerant soybean seed in 2013, a bare ground study without a soybean crop was conducted. In both years, corn (Zea mays L.) was the previous year’s crop grown at each study location.
The study was arranged in a randomized complete block design with 8 treatments and 3 replications and included a non-treated control. The treatments, timing and herbicide rates evaluated are presented in Table 1. The 2,4-D choline and glyphosate (Enlist Duo), treatment rates were selected based on the proposed use rates recommended by Dow AgroSciences and the remaining commercially available herbicide treatments rates were selected according to the standard Extension herbicide recommendations for South Carolina. Herbicides were applied in water using a CO2 pressurized back pack sprayer which delivered 140 L∙ha−1 at 235 kPa via a four nozzle boom fitted with a Turbo Teejet® 11002 Induction Flat Fan spray nozzle (Teejet, Spraying Systems Co.; Wheaton, IL, USA) at a ground speed of 5 km∙h−1.
Premergence (PRE) herbicide treatments were applied shortly after planting. Early postemergence (POST1) treatments were applied when Palmer amaranth, pitted morningglory, and large crabgrass heights ranged from 5 to 10 cm tall and mid-postemergence (POST2) treatments were applied 14 days after the POST1 application. Percent visual control weed ratings on a scale of 0 to 100 percent with 0 indicating no control and 100 indicating complete control were collected 3 weeks after PRE application (3WAP), 2 weeks after POST1 application (2WAP1) and 2 weeks after POST2 application (2WAP2). Soybean response was also evaluated at the same timing intervals as the weed ratings. Weed species population densities were estimated at the 2 WAP2 timing by randomly tossing a 0.5 m2 quadrat down the middle of the 2 treated rows and each weed species
Table 1. Herbicide treatments, application timing, and rates for 2,4-D based herbicide weed control program evaluation.
aAll POST treatments included ammonium sulfate at 2.5 % v/v. bTreatment timing: PRE, at planting; POST1, 5-10 cm weeds; POST2, 2 weeks after POST1. cActive ingredients (ai) rate used for S-metolachlor, fomesafen, sulfentrazone, cloransulum-methyl, glufosinate. Acid equivalent (ae) rate used for 2,4-D choline and glyphosate.
present was identified and counted. Weed species in the plots were visually inspected and identified based on key vegetative characteristic present on the seedling plants and compared to known visual descriptions of those weeds. The soybean crop was destroyed before the R1 reproductive stage to prevent viable seed production (a mandatory requirement by the seed provider Dow AgroSciences); therefore, yield data was not collected in 2012 and no crop (bare- ground study) was utilized in 2013.
Percent visual weed control and weed population densities were analyzed using PROC GLM procedure in SAS (SAS 9.2, SAS® Institute Inc. Cary, NC). Herbicide treatments and years were considered fixed effects in the model while replication was considered random effects. Control and species densities were combined over trial years if no significant treatment by year interaction were observed, whenever treatment by year interaction occurred the data was presented for each year. All means were separated using Fisher’s Protected LSD at P ≤ 0.05.
3. Results and Discussion
The study showed varying degrees of significance for treatment, year and treatment by year across all rating periods. Whenever a significant treatment by year interaction occurred, data were presented separately by year, if no significant treatment by year interaction occurred then the data was presented as an average of control for both trial years. In the data presented, the control parameters for untreated check treatments will not be considered on treatment significance. There was no significant soybean injury observed in 2012 (data not shown).
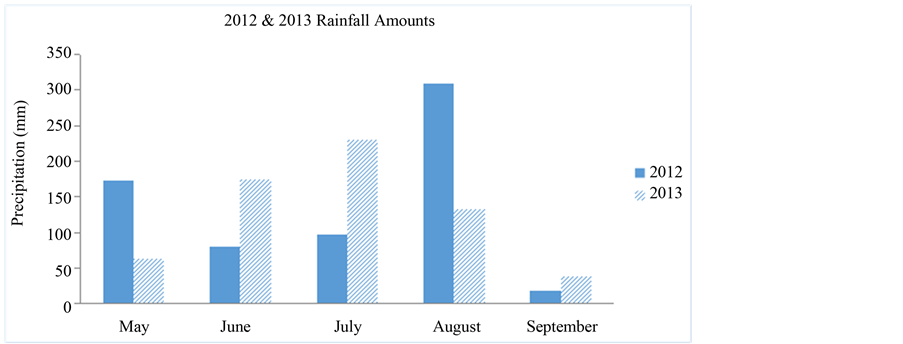
The total rainfall data at the experimental sites during 2012 and 2013 are presented in Figure 1. A total of 680 mm and 647 mm rainfall was received in 2012 and 2013, respectively at the study sites.
In 2012, rainfall received at the experimental site was lower in June and July (178 mm in 2012 versus 409 mm for 2013) compared to the rainfall received during the same period in 2011. The lower precipitation may have reduced the activity of the soil applied PRE herbicides in 2012. Overall, average air temperatures at both study sites from May through September in 2012 and 2013 were very similar in that air temperatures were highest in July (82 and 79 C in 2012 and 2013, respectively).
3.1. Palmer Amaranth Control
Palmer amaranth control across 3 rating periods (3 weeks after PRE, 3 WAP; 2 weeks after POST1, 2WAP 1; 2 weeks after POST2, 2 WAP2) varied very slightly; however, across rating times there were significant differences among treatments. Overall, there was no significant treatment by year interaction for Palmer amaranth visual control or population densities; therefore, data was combined over study years.
A PRE treatment of sulfentrazone + cloransulam-methyl followed by a POST2 application of fomesafen + glyphosate provided 100% Palmer amaranth control at all rating times (Table 3). Fomesafen + S-metolachlor were the most effective
Figure 1. Rainfall amounts for May to September 2012 and 2013 at Edisto REC, Blackville, SC.
at-plant PRE treatment providing 98% and 99% control 3 WAP. Two POST- only (no PRE) treatments of 2,4-D choline + glyphosate (0.82 + 0.82 kg ae ha−1 and 0.55 + 0.55 kg ae ha−1) premixtures at the POST1 and POST2 timings at 2 different rates. At 2 WAP1 and 2 WAP2 both treatments showed no statistical differences with 98% or better Palmer amaranth control (Table 2). Overall, Palmer amaranth control was 98% or better across all treatments.
3.2. Pitted Morningglory Control
There was greater variability observed in pitted morningglory control among treatments compared to Palmer amaranth. There was an overall significant difference in treatments across rating periods (Table 3). In addition, a treatment by year interaction was observed for pitted morningglory. Sulfentrazone + cloransulam-methyl PRE followed by fomesafen + glyphosate provided the best pitted morningglory control in both 2012 and 2013 among all rating times, with the lowest control of 98% at time of rating. In 2013, sulfentrazone + cloransulam at PRE followed by glufosinate at POST2, sulfentrazone + cloransulam at PRE followed by glyphosate + 2,4-D choline tank mix + glufosinate at POST2 and sulfentrazone + cloransulam-methyl at PRE followed by fomesafen + glyphosate at POST2 provided 100% pitted morningglory control. In 2013 2,4-D choline + glyphosate tank mix at 1.64 kg ae ha−1 and 1.09 kg ae ha−1 both showed a 5% decrease in control from 100% at 2 WAP1 to 95% at 2 WAP2 (Table 3). As was observed in Palmer amaranth, the two applied rates of 2,4-D choline + glyphosate tank mix showed no significant differences in control of pitted morningglory.
In 2012 S-metolachlor plus fomesafen PRE treatment wasn’t an effective PRE treatment with 52% control in S-metolachlor + fomesafen PRE followed by glyphosate POST2 treatment and 83% control in S-metolachlor + fomesafen PRE followed by glyphosate + 2,4-D choline tank mix POST2 treatment at 3 WAP. The control then declined to 23% and 67% respectively for each of the treatments 2 WAP1 (Table 3). However, in 2013 the same treatment combination
Table 2. Palmer amaranth (AMAPA) percent visual control and population counts as affected by herbicide treatments in 2012 & 2013.
aAll POST treatments included ammonium sulfate at 2.5 % v/v; bActive ingredients (ai) rate used for S-metolachlor (s-met.), fomesafen (fom.), sulfentrazone (sulf.), cloransulum-methyl (clorsm.), glufosinate (gluf.). Acid equivalent (ae) rate used for 2,4-D choline (24DC) and glyphosate (gly.); cMeans followed by the same letter do not differ significantly according to Fishers Protected LSD at 5%.
Table 3. Pitted morningglory (IPOLA) percent visual control and population counts as affected by herbicide treatments in 2012 & 2013.
aPOST treatments included ammonium sulfate at 2.5 % v/v; bActive ingredients (ai) rate used for S-metolachlor (s-met.), fomesafen (fom.), sulfentrazone (sulf.), cloransulum-methyl (clorsm.), glufosinate (gluf.). Acid equivalent (ae) rate used for 2,4-D choline (24DC) and glyphosate (gly.); cMeans followed by the same letter do not differ significantly according to Fishers Protected LSD at 5%.
provided 100% control 3 WAP. This difference in treatment by year may be attributed to several factors including weather conditions at time of application. Both treatments contained S-metolachlor and fomesafen which are preemergence herbicides that require soil moisture for activation and soil conditions in 2012 at the time of application were dry.
In addition, 2012 growing season was drier compared to 2013 especially during June and July (Figure 1). Differences in pitted morningglory populations between years may have contributed to the treatment by year interaction.
Fomesafen + S-metolachlor applied PRE followed by 2,4-D choline salt + glyphosate tank mix provided better pitted morningglory control than glyphosate alone. In 2012 when S-metolachlor + fomesafen was followed by a POST2 application of glyphosate, there was a 39% increase in control from 23% at 2 WAP1 to 62% at 2 WAP2 (Table 3). Within the same treatment in 2013 there wasn’t a significant difference in control.
Also, when S-metolachlor + fomesafen was followed by glyphosate + 2,4-D choline tank mix at POST2, there was an increase of 33% in control from 67%, 2 WAP1 to 100%, 2 WAP2 in 2012. Vencil [26] found that control of Ipomoea spp. by soil applied herbicides was very inconsistent, this is similar to the results with treatments applied 3 WAP in 2012 (Table 4). Elmore [27] stated that postemergence herbicides are generally more effective on Ipomoea spp. Siebert [28] also observed 2,4-D provided greater than 94% red morningglory control in sugarcane at 21 days after treatment which is similar to the results at 2 WAP2 for 2,4-D choline plus glyphosate (Table 3).
3.3. Large Crabgrass Control
Large crabgrass control was consistent across all treatments and rating timings. Overall, significant differences among treatments were observed for all rating (Table 4). Also, there was a significant treatment by year interaction 2 WAP1. On the other two rating dates (3 WAP and 2 WAP2) no treatment by year interaction was observed. Therefore, data were combined across years. All PRE treatments provided excellent crabgrass control, averaging about 98%. Levels of control did not vary among the PRE applied treatments 3 WAP; however, S- metolachlor + fomesafen was the only PRE treatment to have the same or an increase in control 2 WAP1.
At the 2 WAP1, there were control differences among treatments with the trial year showing 5 levels of significance among treatments in 2012. However, the identical rating date in 2013 showed no differences among treatments in levels of large crabgrass control. This treatment by year interaction maybe attributed to differences in soil moisture or weed pressure.
As seen with Palmer amaranth and pitted morningglory, there were no significant differences among treatments in levels of large crabgrass control when plots were treated with 2,4-D choline plus glyphosate mixture at rates of 1.64 kg ae ha−1 and 1.09 kg ae ha−1. These results are similar to the research conducted by Culpepper [29] who did not observe any significant differences in control when
Table 4. Large crabgrass (DIGSA) percent visual control and population counts as affected by herbicide treatments in 2012 & 2013.
aPOST1 and POST2 treatments included ammonium sulfate at 2.5 % v/v; bTreatment timing: PRE, at planting; POST1, 5-10 cm weeds; POST2, 2 weeks after POST1; cActive ingredients (ai) rate used for S- metolachlor, fomesafen, sulfentrazone, cloransulum-methyl, glufosinate. Acid equivalent (ae) rate used for 2,4-D choline and glyphosate; dMeans followed by the same letter do not differ significantly according to Fishers Protected LSD at 5%.
glyphosate was tank mixed with 2,4-DB.
In 2012 at 2 WAP1, the PRE application of sulfentrazone + cloransulam-me- thyl provided 83% control of large crabgrass, showing a 15% decrease from 98% at 3 WAP rating period. In 2013 at the same rating date, in the same treatment, there wasn’t a difference in control 3 WAP and 2 WAP1 (Table 4).
4. Summary
This research showed that it takes a minimum of two herbicide applications to control palmer amaranth, pitted morningglory and large crabgrass in soybean. A management regime including a PRE application followed by a POST treatment was very effective in controlling all the weed species. PRE treatments required soil moisture for activation and optimum weed control and the lack of soil moisture in 2012 may have led to some of the interactions of treatment by year observed in pitted morningglory control. In the treatments without any PRE applications, there wasn’t any control at the first rating date; however, POST1 and POST2 applications of 2,4-D choline and glyphosate provided almost complete control at subsequent rating dates. Herbicide application volume didn’t seem to have an impact on control in treatments consisting only of 2,4-D choline and glyphosate.
Palmer amaranth was the most easily controlled weed of the three weed species studied. Pitted morningglory was the hardest to control and exhibited the most variation in control as evidenced by the treatment by year interactions. 2,4-D choline, being a broadleaf herbicide would be expected to provide no control of large crabgrass; however, glyphosate as a tank mix partner with 2,4-D choline provided excellent control of large crabgrass. The Enlist Duo™ herbicide (a mixture of 2,4-D choline salt + glyphosate tank mix) is labeled for a maximum of two POST applications which need to be applied before weeds are 10 cm in height [30] . There were many weeds present at the first rating date, due to the lack of a PRE treatment before the application of 2,4-D choline + glyphosate. These weeds, although effectively controlled by the POST1 application, may have competed with the soybean and caused a reduction in growth and development. However, since this was a regulated genotype, plant destruction before flowering (R1 growth stage) was required by Dow AgroSciences. Nonetheless, Enlist Duo™ was equivalent with treatments that had a PRE followed by a POST application.
Acknowledgements
Technical Contribution No. 6550 of the Clemson University Experiment Station. This material is based upon work supported by the NIFA/USDA, under project number SC-1700384.
Disclaimer
Mention of a trade name does not imply endorsement of the product by Clemson University to the exclusion of others that might be available.
Cite this paper
Joseph, D.D., Sanders, C.H. and Marshall, M.W. (2017) Evaluation of 2,4-D-Choline Based Herbicide Systems in 2,4-D Tolerant Soybean (Glycine max L.). Agricultural Sciences, 8, 385-396. https://doi.org/10.4236/as.2017.85029
References
- 1. Rajcan, I. and Swanton, C.J. (2001) Understanding Maize-Weed Competition: Resource Competition, Light Quality and the Whole Plant. Field Crops Research, 71, 139-150.
- 2. Eaton, B.J., Russ, O.G. and Feltner, K.C. (1976) Competition of Velvetleaf, Prickly, Sida, and Venice Mallow in Soybeans. Weed Science, 24, 224-228.
http://www.jstor.org/stable/4042592 - 3. Buchanan, G.A. and Burns, E.R. (1971) Weed Competition in Cotton. I. Sicklepod and Tall Morningglory. Weed Science, 19, 576-579.
http://www.jstor.org/stable/4041705 - 4. Barrentine, W.L. (1974) Common Cocklebur Competition in Soybeans. Weed Science, 22, 600-603.
http://www.jstor.org/stable/4042480 - 5. Bensch, C.N., Horak, M.J. and Peterson, D. (2003) Interference of Redroot Pigweed (Amaranthus retroflexus), Palmer Amaranth (A. palmeri), and Common Waterhemp (A. rudis) in Soybean. Weed Science, 51, 37-43.
https://doi.org/10.1614/0043-1745(2003)051[0037:IORPAR]2.0.CO;2 - 6. Thurlow, D.L. and Buchanan, G.A. (1972) Competition of Sicklepod with Soybeans. Weed Science, 20, 379-384.
- 7. Buhler, D.D. and Gunsolus, J.L. (1996) Effect of Date of Preplant Tillage and Planting of Weed Populations and Mechanical Weed Control in Soybean (Glycine max). Weed Science, 44, 373-379.
http://www.jstor.org/stable/4045692 - 8. Norsworthy, J.K. and Oliver, L.R. (2002) Pitted Morningglory Interference in Drill-Seeded Glyphosate-Resistant Soybean. Weed Science, 50, 26-33.
https://doi.org/10.1614/0043-1745(2002)050[0026:PMIIDS]2.0.CO;2 - 9. Van Der Weide, R.Y., Bleeker, P.O., Achten, V.T.J.M., Lotz, L.A.P., Fogelberg, F. and Melander, M. (2008) Innovation in Mechanical Weed Control in Crop Rows. Weed Research, 48, 215-224.
https://doi.org/10.1111/j.1365-3180.2008.00629.x - 10. Rasmussen, I.A. (2004) The Effect of Sowing Date, Stale Seedbed, Row Width and Mechanical Control on Weeds and Yield of Organic Winter Wheat. Weed Research, 44, 12-20.
https://doi.org/10.1046/j.1365-3180.2003.00367.x - 11. Grossmann, K. (2009) Auxin Herbicides: Current Status of Mechanism and Mode of Action. Pest Management Science, 66, 113-120.
https://doi.org/10.1002/ps.1860 - 12. Jasieniuk, M., Brule-Babel, A.L. and Morrison, I.N. (1996) The Evolution and Genetics of Herbicide Resistance in Weeds. Weed Science, 44, 176-193.
http://www.jstor.org/stable/4045802 - 13. Prather, T.S., Ditomaso, J.M. and Holt, J.S. (2000) Herbicide Resistance: Definition and Management Strategies. Publication 8012, University of California, Division of Agriculture and Natural Resources, 14 p.
- 14. Heap, I.M. (2017) The International Survey of Herbicide Resistant Weeds.
http://www.weedscience.com - 15. Culpepper, A.S., Grey, T.L., Vencill, W.K., Kichler, J.M., Webster, T.M., Brown, S.M., York, A.C., Davis, J.W. and Hanna, W.W. (2006) Glyphosate Resistant Palmer Amaranth (Amaranthus palmeri) Confirmed in Georgia. Weed Science, 54, 620-626.
https://doi.org/10.1614/WS-06-001R.1 - 16. Norsworthy, J.K. (2003) Use of Soybean Production Surveys to Determine Weed Management Needs of South Carolina Farmers. Weed Technology, 17, 195-201.
https://doi.org/10.1614/0890-037X(2003)017[0195:UOSPST]2.0.CO;2 - 17. Shaner, D.L. (2000) The Impact of Glyphosate-Tolerant Crops on the Use of Other Herbicides and on Resistance Management. Pest Management Science, 56, 320-326.
https://doi.org/10.1002/(SICI)1526-4998(200004)56:4<320::AID-PS125>3.0.CO;2-B - 18. Beckie, H.J., Harker, K.N., Hall, L.M., Warwick, S.I., Legere, A., Sikkema, P.H., Clayton, G.W., Thomas, A.G., Leeson, J.Y., Seguin-Swartz, G. and Simard, M.J. (2006) A Decade of Herbicide Resistance Crops in Canada. Canadian Journal of Plant Science, 86, 1243-1264.
https://doi.org/10.4141/P05-193 - 19. Robinson, A.P., Simpson, D.M. and Johnson, W.G. (2012) Summer Annual Weed Control with 2,4-D and Glyphosate. Weed Technology, 26, 657-660.
https://doi.org/10.1614/WT-D-12-00081.1 - 20. Que Hee, S.S. and Sutherland, R.G. (1974) Volatilization of Various Esters and Salts of 2,4-D. Weed Science, 22, 313-318.
http://www.jstor.org/stable/4042312 - 21. Strachan, S.D., Casini, M.S., Heldreth, K.M., Scocas, J.A., Nissen, S.J., Bukun, B., Lindenmayer, R.B., Shaner, D.L., Westra, P. and Brunk, G. (2009) Vapor Movement of Synthetic Auxin Herbicides: Aminocyclopyrachlor, Aminocyclopyrachlor-Methyl Ester, Dicamba, and Aminopyralid. Weed Science, 58, 103-108.
https://doi.org/10.1614/WS-D-09-00011.1 - 22. Wolf, T.M., Grover, R., Wallace, K., Shewchuk, S.R. and Maybank, J. (1993) Effect of Protective Shields on Drift and Deposition Characteristics of Field Sprayers. Canadian Journal of Plant Science, 73, 1261-1273.
https://doi.org/10.4141/cjps93-165 - 23. Sciumbato, A.S., Chandler, J.M., Senseman, S.A., Bovey, R.W. and Smith, K.L. (2004) Determining Exposure to Auxin-Like Herbicides. I. Quantifying Injury to Cotton and Soybean. Weed Technology, 18, 1125-1134.
https://doi.org/10.1614/WT-03-105R1 - 24. Whitaker, J.R., York, A.C., Jordan, D.L. and Culpepper, A.S. (2010) Palmer Amaranth (Amaranthus palmeri) Control in Soybean with Glyphosate and Conventional Herbicide Systems. Weed Technology, 24, 403-410.
https://doi.org/10.1614/WT-D-09-00043.1 - 25. Hoffner, A.E., Jordan, D.L., Chandi, A., York, A.C., Dunphy, E.J. and Everman, W.J. (2012) Management of Palmer Amaranth (Amaranthus palmeri) in Glufosinate-Resistant Soybean (Glycine max) with Sequential Applications of Herbicides. ISRN Agronomy, 2012, Article ID: 131650.
- 26. Venvill, W.K., Wilcut, J.W. and Monks, C.D. (1995) Efficacy and Economy of Weed Management Systems for Sicklepod (Senna obtusifolia) and Morningglory (Ipomoea spp.) Control in Soybean (Glycine max). Weed Technology, 9, 456-461.
http://www.jstor.org/stable/3987656 - 27. Elmore, C.D., Hurst, H.R. and Austin, D.F. (1990) Biology and Control of Morningglory (Ipomoea spp.). Reviews of Weed Science, 5, 83-114.
- 28. Seibert, J.D., Griffin, J.L. and Jones, C.A. (2004) Red Morningglory (Ipomoea coccinea) Control with 2,4-D and Alternative Herbicides. Weed Technology, 18, 38-44.
https://doi.org/10.1614/WT-03-071R1 - 29. Culpepper, A.S., Gimenez, A.E., York, A.C., Batts, R.B. and Wilcut, J.W. (2001) Morningglory (Ipomoea spp.) and Large Crabgrass (Digitaria sanguinalis) Control with Glyphosate and 2,4-DB Mixtures in Glyphosate-Resistant Soybean (Glycine max). Weed Technology, 15, 56-61.
https://doi.org/10.1614/0890-037X(2001)015[0056:MISALC]2.0.CO;2 - 30. Anonymous (2017) Enlist Duo Herbicide Product Label. Dow AgroSciences Publication No. D02-407-003. Dow AgroSciences, Indianapolis, IN, 7 p.