Food and Nutrition Sciences
Vol. 2 No. 3 (2011) , Article ID: 4900 , 7 pages DOI:10.4236/fns.2011.23028
L-Homoarginine Accumulation in Grass Pea (Lathyrus sativus L.) Dry Seeds. A Preliminary Survey
![]()
Istituto di Genetica Vegetale-CNR Via Amendola, Bari, Italy.
Email: angelarosa.piergiovanni@igv.cnr.it
Received February 16th, 2011; Revised February 28th, 2011; Accepted March 10th, 2011.
Keywords: capillary zone electrophoresis, ecotype, genetic variation, Lathyrus sativus, non-protein amino acids, rainfall amount
ABSTRACT
Grass pea (Lathyrus sativus L.) has great agronomic potential as grain and forage legume, and presently is considered as a model crop for sustainable agriculture. However, the development into an important food legume has been hindered by the presence of the neurotoxic amino acid β-N-Oxalyl-α, β-diaminopropionic acid (β-ODAP). Recent studies reported that homoarginine (Har) can counteract this toxic action. This research was undertaken to shed light on the variation of Har amount within grass pea. The influence of the environment and of the year-to-year variation of climatic conditions was also investigated. Seven Italian grass pea ecotypes were evaluated for two subsequent growing seasons in two locations of southern Italy. In contrast with previous studies, collected data evidenced a significant variation of Har amount among the tested ecotypes. Moreover, a significant positive correlation between Har and ODAP level was observed. The effect of year-to-year variation of temperature and rainfall quantity is also discussed.
1. Introduction
Over 300 non-protein amino acids naturally occur in plants. In large part, they are intermediates in both synthesis and catabolism of protein amino acids and usually occur in different plant tissues, free or as 4-glutamyl derivatives [1,2]. Legumes contain not only high concentrations of non-protein amino acids but also a more diverse range than any other plant species. Seeds are generally the most concentrated sources of these compounds [3]. Previous studies demonstrated that non-protein amino acids plays different roles such as intermediates in the synthesis of protein amino acids, defensive agents [4], multifunctional hormones [5] or osmoregulators [6]. Some dietary non-protein amino acids are implicated in poisonings, diseases and disorders [7] while others can have beneficial effects [8]. The toxicity of non-protein amino acids has been observed in insects, laboratory and farm animals and in humans, but there are striking differences among the species in their sensitivity to these compounds. For example, it has been demonstrated that in both mammalians and birds, the biochemical versatility for amino acid detoxification is considerably reduced [9].
The relatively high concentration of non-protein amino acids in seeds is a major factor limiting exploitation of alternative grain legumes, such as Lathyrus sativus and Vicia sativa, as protein sources for poultry and other nonruminant animals [9,10]. Grass pea (Lathyrus sativus L.) is an important annual crop for human and animal consumption because it may be the only affordable food in times of food shortage or under adverse environmental conditions. Unfortunately, consumption of large quantities or prolonged eating can give rise to the neurolathyrism [2,7]. It has been demonstrated that all Lathyrus species which synthesise β-N-Oxalyl-α, β-diaminopropionic acid (β-ODAP), the toxin responsible of neurolathyrism, also accumulate L-homoarginine (Har). β-ODAP and Har are the major free non-protein amino acids present in grass pea seeds [11]. Together they make up about 90% of ninhydrin-reacting compounds in the 70% ethanol extracts [12]. There are contrasting opinions about the impact of Har on human and animal diets. It is considered by some to be a positive factor because can be converted into lysine by the mammalian liver [13]. However, Breitner et al. [14] suggested that the presence of Har in gene activator-repressor histones may be a direct cause of most cancer types. Dawson et al. [15] reported that Har is a modulator of the biosynthesis of nitric oxide (NO) which, in turn, reduces the excitation of neuronal receptors [13]. Finally, it has been proposed that Har could modulate to some extent the toxicity of β-ODAP [16,17].
Since non-protein amino acids occur in legume seeds in unconjugated forms, their extraction with solvents or aqueous solutions is easy and quantitative. HPLC or capillary zone electrophoresis (CZE) methods have been developed to analyse the extracts. Sample preparation required by these techniques is different. HPLC methods necessitate of long derivatization times before analysis [18-23], while CZE allows selective and rapid measurements without the off-line derivatization step and brought less waste [11,24]. Although Har could play a positive effect in contrasting neurolathyrism, very little attention has been devoted to evaluate the effect of grain yield, location and growing season on its storage in seeds. The aim of this contribution was provide information on this topic by comparing different genotypes cultivated for two subsequent years in two environments of Southern Italy characterised by different soil properties and unlike climatic conditions.
2. Materials and Methods
The material utilised in the present study consisted of 6 ecotypes of grass pea (Lathyrus sativus L.), traditionally cultivated in small areas located in three regions (Apulia, Basilicata and Campania) of southern Italy, and the line n. 3151 belonging to the “Iannelli Germplasm Collection” held at University of Basilicata (Potenza, Italy) (Table 1). The field trials were conducted, for two consecutive years (2006-2007 and 2007-2008), at Battipaglia, a village situated at 7 km from the coast of Tirrenium sea (40˚36'N 14˚58'E, 65 m asl, Campania region) and at Guardia Perticara, a village placed in a hilly area along the Appenninic ridge (40˚22'N 16˚2'E, 720 m asl, Basilicata region). Intensive agricultural systems are predominant in the open plain of Battipaglia, while Guardia Perticara is located in hilly zones where traditional agriculture is still practiced. Moreover, the locations differ for soil type (Battipaglia - clay Gleyic Luvisol type, sand - 31%, silt - 29.2% and clay - 39.8%; Guardia Perticara – clay sandy soil, sand - 46.9%, silt - 19.2% and clay - 33.9%) and climatic conditions (Figure 1). The layout of the field experiments was a randomised complete block design. The ecotypes were sown in plots 4 m2 with 40 cm between rows and 4 cm within the row for a density of 62.5 seeds per square meter. Sowing and harvesting date between growing seasons and locations were: I decade November 2006 - II decade June 2007; II decade October 2007 - II decade July 2008 at Battipaglia; II decade November 2006 - II decade July 2007; II decade November 2007 - I decade July 2008 at Guardia Perticara. Seed samples were dried at 105 °C until constant weight, so to ormalize grain yield reported in Table 1 at 13% moisture.
2.1. Homoarginine Extraction
Approximately 150 g of dry seeds were taken from the bulk material harvested for each sample. Seeds were ground using a Cyclotec 1093 mill Tecator (Sweden). The non-protein amino acids were extracted from meal with a mixture ethanol-water (6:4, v/v) by tumbling the capped plastic tubes for 1h at room temperature [11]. The slurries were centrifuged at 2800 g for 20 min and the supernatant recovered. Then fresh aqueous ethanol was added to the pellet to a second extraction step. The pooled supernatants were stored at −20˚C until analysed by capillary zone electrophoresis. The extraction of each sample was repeated twice, and each extract was analysed in triplicate by CZE and the content was expressed as mean value.
2.2. Capillary Zone Electrophoresis (CZE) Analyses
A P/ACE MDQ Beckman-Coulter (USA) was used to analyse the seed extracts. The CZE analyses were performed according to Zhao et al. [11] with slight modifycations. Separations were achieved using uncoated fusedsilica capillaries 55 cm long (42 cm to detector) with 50 mm ID. The capillary temperature was kept at 30˚C and a constant voltage of 25 kV was applied for 6 min. The peak detection was performed at 200 nm. Beckman Karat 32 software was used for acquiring, storing and analyzing the electrophoregrams. The separation buffer was 18.5 mM Na2B4O7 (pH 9.2) and 10 mM Na2SO4 dissolved in 18 MΩ cm distilled and deionized water (MilliQ water system Millipore, USA). All the chemicals were of analytical reagent grade. A known amount of L-homoarginine, purchased from Fluka (St. Louis, USA), was dissolved in an ethanol-water mixture (6:4) and termed the stock standard solution. Homoarginine peak was identified by the increase of peak area after the addition to the sample extracts of a known volume of the standard solution. Various concentrations of L-homoarginine were prepared by diluting the stock standard solution to obtain the calibration plot. Statistica version 6.0 (Statsoft, Tulsa, OK, USA) was used for the statistical analyses.
3. Results and Discussion
In southern Italy there is a long lasting tradition of cultivation and consumption of grass pea for human use as
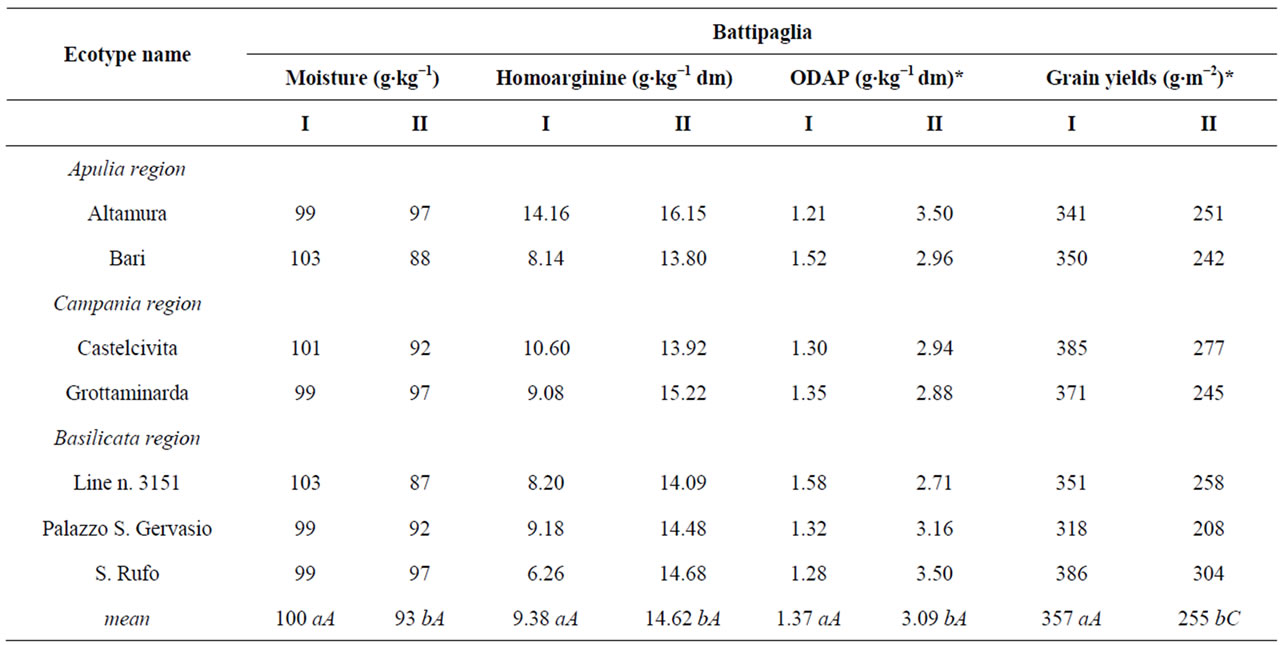
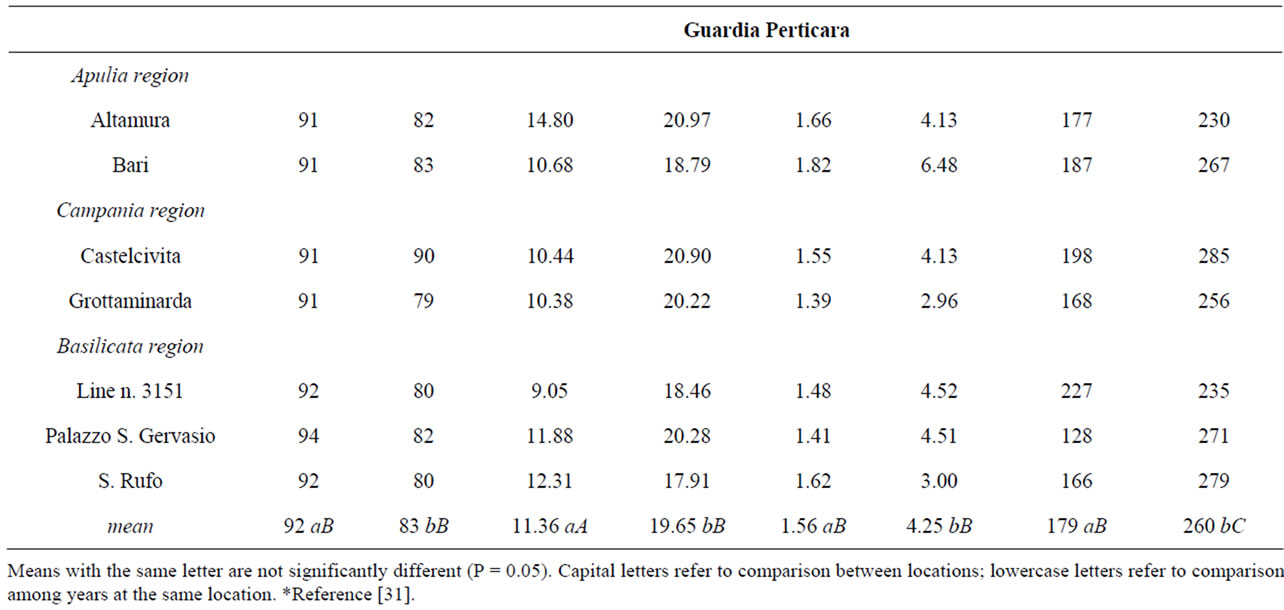
Table 1. Ecotype name, L-homoarginine and ODAP amounts, and grain yields recorded at the two locations for the tested ecotypes. The growing seasons are coded: I - 2006-2007; II - 2007-2008.
well as animal feed. In the last decade organic farmers have been rediscovering this pulse both as model crop for sustainable agriculture and traditional local product [25,26]. A few autochthonous ecotypes, still under cultivation in marginal areas of Southern Italy, were compared in this study. Field trials were carried out at Battipaglia and Guardia Perticara, two locations characterised by different agro-ecological conditions. Moreover, these locations were outside the areas where the studied ecotypes traditionally grown. This experimental design was adopted to allow the enhance-ment of the adaptation bias on the Har storage in seeds.
According to the literature [11,22], CZE analyses of alcoholic extracts of tested ecotypes revealed that, in addition to β-ODAP, Har was always the most abundant non-protein amino acid. This is a feature specific for grass pea because Har was undetectable in pea and bean seeds [27], while its presence in seeds of some Lens species has been recently reported by Rozan et al. [28,29]. Within the Lens genus the highest amounts were detected in Lens ervoides accessions (ca. 0.06 mg g−1), but the values were significantly lower than those typical of
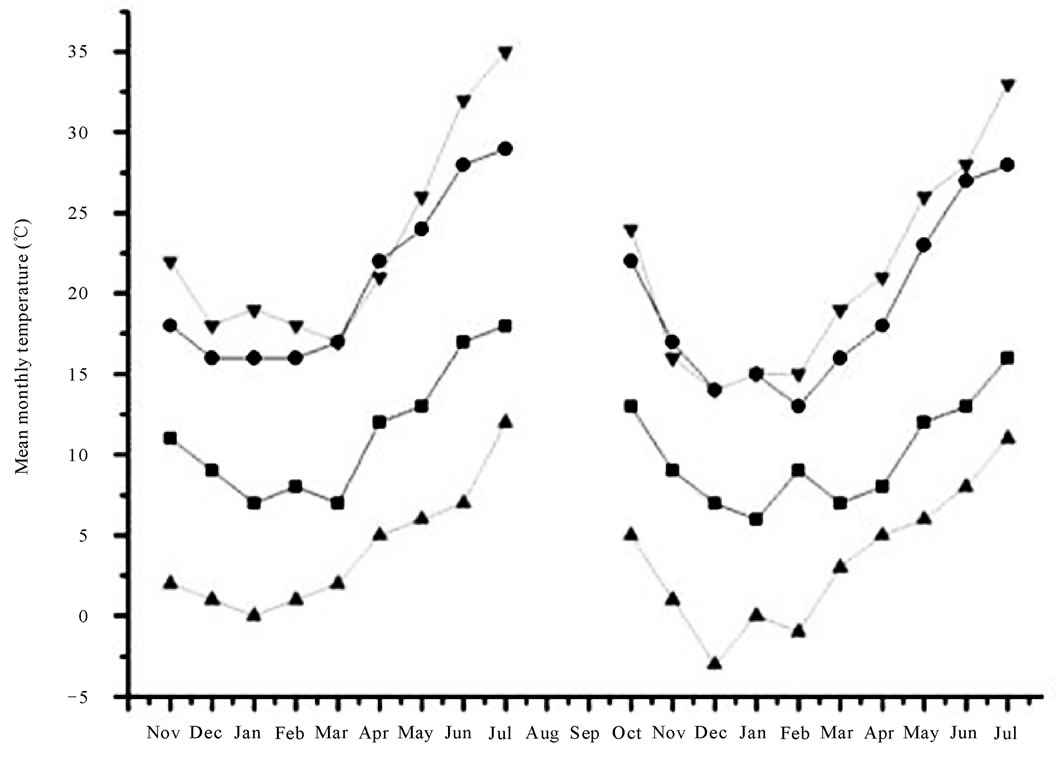
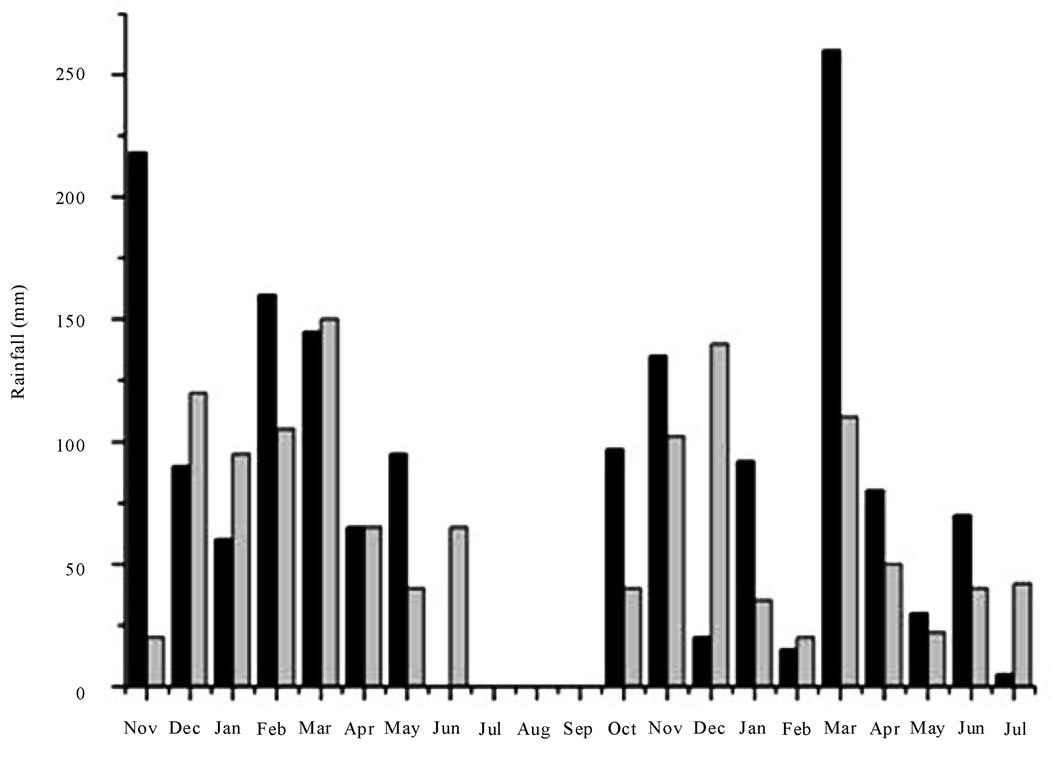
Figure 1. Top—Mean temperatures recorded from November 2006 to July 2008 at Battipaglia (■ minimum mean values; ● maximum mean values) and Guardia Perticara (▲ minimum mean values; ▼ maximum mean values). Bottom—Amount of rainfall recorded from November 2006 to July 2008 at Battipaglia and Guardia Perticara (black and grey bars, respectively).
grass pea [11,21,30].
The analysis of experimental data reported in Table 1, evidenced a threefold variation of Har amount between the extreme values (6.26 vs 20.97 g kg−1). The lowest value was recorded during the first growing season at Battipaglia for S. Rufo ecotype, while the highest one was observed in the second growing season at Guardia Perticara for Altamura ecotype. This wide variation did not agree with Fikre et al. [23], who analysing 9 genotypes with different origin, concluded that the variation of Har level within grass pea (0.68% - 0.86%) is not significant. Other episodic studies available in the literature refer to a few genotypes grown in disparate environments (i.e. Mediterranean-type climate, under rainfall conditions, high salinity of soil, etc.) generally for a single growing season. A narrow variation (5.3 - 6.7 mg g−1) was reported by Yan et al. [21], while Zhao [11] recorded a wider range (3.2 - 10.6 mg·g−1). Based on these data, the ecotypes analysed in the present study did not rank within these ranges.
In this study, statistically significant differences (P < 0.05) were observed at each location between the growing seasons (Table 1). Overall, for all the ecotypes it was observed a trend towards increasing Har values in the second season. Moreover, the Har amounts of the material grown at Battipaglia was always inferior than those recorded in the same growing season for the harvests from Guardia Perticara. However, only for the second growing season the difference between the values recorded at the two locations resulted statistically signifycant (P < 0.05). The grass pea from Altamura attracted the attention not only because it showed invariably the highest Har values, but also for a not significant yearto-year variation at Battipaglia (14.16 vs 16.15). At the opposite, the widest year-to-year variation was recorded for S. Rufo ecotype and the line n. 3151 at Battipaglia and Guardia Perticara, respectively.
Recently, it has been published a paper dealing with the evaluation of nutritional and technological quality of seeds belonging to Italian grass pea ecotypes [31]. This last study and the present one share a part of tested ecotypes, so matching the results, interesting consideration on factors affecting the Har storage can be done. A wide variation between locations as well as growing seasons was observed also for grain yield and ODAP [31]. As shown in Table 1, for each ecotype, the yield recorded in the first growing season at Battipaglia was about two times that registered at Guardia Perticara, while in the subsequent season grain yields recorded at the two locations resulted comparable. However, this year-to-year variation of grain yield did not affect the Har storage because correlation analysis did not evidence a signifycant relationship between these traits. As concerns ODAP, differences statistically significant were detected between growing season and location. If Har could really modulate to some extent the toxicity of β-ODAP, a positive correlation between the amounts of these amino acids might be highly desirable. In actual fact, correlation analysis of Har and ODAP amounts shown in Table 1, evidenced a statistically significant relationship between these amino acids (R = 0.816, P < 0.05).
Table 2 gives the results of ANOVA analysis. The effects of both growing season and location of cultivation were found important in determining the Har amount, though the significance level resulted different. Conversely, only the effect associated with the growing season was important in determining the ODAP storage. These findings indicate that, from a statistical point of view, the Har storage in seeds changed from one year to the next, and that the impact of growing location on this trait was likewise statistically significant. Although the present study cannot allow drawing unequivocal decisions on the relative importance of soil composition, yield, sowing and harvesting date, environmental conditions of growing location, genotype x environment interaction, etc. some working hypotheses can be devised. For example, grain yield should have a minor role in determining Har storage for the reason that correlation analy-
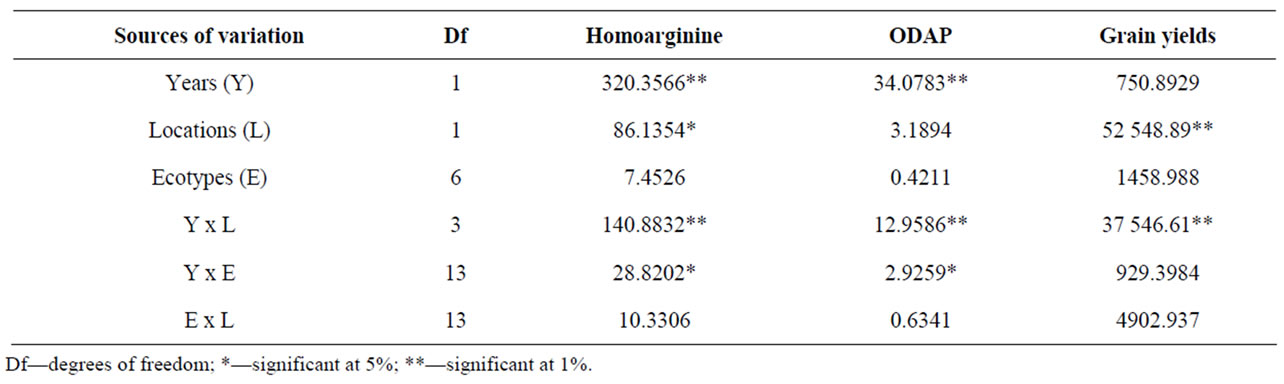
Table 2. Results of the analysis of variance for Har and ODAP amount, and grain yield. Mean square and significance are reported.
sis did not evidenced a significant relationship between these traits. Similarly, in consequence of the large overlap of sowing and harvesting periods between growing seasons at each location, it is predictable that weather conditions might have a major influence in regulating the Har accumulation. This is not surprising because it is well known that the amounts of some antinutritional and toxic factors stored in legume grains are modulated by rainfall quantity and/or average temperature during specific vegetative stages [32-34]. Graphical representations of both minimum and maximum average temperatures (calculated on monthly base) and the amount of rainfall recorded in the field trials are given in Figure 1. The plots revealed that, at each location, the variation of temperature between the growing seasons were not statistically significant, and that Battipaglia was characterrised by a lower gap between minimum and maximum mean temperatures. In regard to the precipitation value, a higher stress was suffered by plants at Guardia Perticara, being been registered at this location in the second growing season the lowest rainfall amount (489 mm) over the trial. It is interesting to underline that, for all the ecotypes, the highest Har levels were actually recorded at Guardia Perticara in the second growing season (Table 1). Based on these evidences it can be inferred that, similarly to other secondary metabolites of pulses, also Har appear to be affected by the amount of rainfall. How much the environmental conditions can affect grass pea seed quality traits has been reported in recent studies dealing with morphological and compositional seed traits [26,31].
As a consequence of the increasing interest towards Lathyrus species as alternative pulses, it has been agreed that grass pea breeding should be focused, besides to the ODAP reduction and the plant architectural ideotype, also towards the increase in seeds of the level of Har and methionine in consideration of potential beneficial effects of on human health of these amino acids [16,17,35]. The results presented in this paper give preliminary information not only on the variation of Har amount within grass pea but also on some features affecting its storage. This provides an useful starting point to program new field trials involving a higher number of genotypes and a wider range of climatic conditions.
REFERENCES
- M. Wink, “Non-Protein Amino Acids,” In: P. M. Dey and J. B. Harborne, Ed., Plant Biochemistry, Academic Press, San Diego, 1997, pp. 440-443.
- D. S. Seigler, “Non-Protein Amino Acids,” In: Plant SecOndary Metabolism, Kluwer Academic Publishers, Dordrecht, 1998, pp. 215-233.
- J. P. F. D’Mello, “Toxicity of Non-Protein Amino Acids from Plants,” In: R. M. Wallsgrave, Ed., Amino Acids and Their Derivatives in Higher Plants, Cambridge University Press, Cambridge, 1995, pp. 145-153. doi:10.1017/CBO9780511721809.011
- E. A. Bell, K. P. W. C. Perera, P. B. Nunn, M. S. J. Simmonds and W. M. Blaney, “Non-Protein Amino Acids of Lathyrus Latifolius as Feeding Deterrents and Phagostimulants in Spodoptera Littoralis,” Phytochemistry, vol. 43, No. 5, 1996, pp. 1003-1007. doi:10.1016/S0031-9422(96)00425-6
- G. Kalbin, A. B. Ohlsson, T. Berglund, J. Rydstrom and A. Strid, “UV-B Radiation Causes Changes in Intracellular Levels of Nicotinamide, Trigonelline and Glutathione in Pisum Sativum Leaves,” Phyton Annales Rei Botanicae, vol. 37, 1997, pp. 115-123.
- W. A. Tramontano and D. Jouve, “Trigonelline Accumulation in Salt-Stressed Legumes and the Role of Other Osmoregulators as Cell Cycle Control Agents,” Phytochemistry, vol. 44, No. 6, 1997, pp. 1037-1040. doi:10.1016/S0031-9422(96)00715-7
- P. S. Spencer, M. P. Ludolph, D. N. Dwivedi, J. Roy, J. Hugon and H. H. Shaumburg, “Lathyrism: Eidence for Role of the Neuroexcitatory Amino Acid BOAA,” Lancet, vol. 328, No. 8515, 1986, pp. 1066-1067. doi:10.1016/S0140-6736(86)90468-X
- Y. Sauvaire, P. Petit, C. Broca, M. Manteghetti, Y. Baissac, J. Fernandez-Alvarez, R. Gross, M. Roye, A. Leconte, R. Gomis and G. Ribes, “4-Hydroxyisoleucine: A Novel Amino Acid Potentiator of Insulin Secretion,” Diabètes, vol. 47, No. 2, 1998, pp. 206-210. doi:10.2337/diabetes.47.2.206
- C. D. Hanbury, C. L. White, B. P. Multan and K. H. M. Siddique, “A Review of the Potential of Lathyrus sativus L. and L. Cicera L. Grain as Animal Feed,” Animal Feed Science and Technology, vol. 87, No. 1, 2000, pp. 1-27. doi:10.1016/S0377-8401(00)00186-3
- R. A. Cohn and M. Streifter, “Intoxication by the Chickling Pea (Lathyrus sativus) Nervous System and Skeletal Findings,” Archives of Toxicology, No. 6 (Supplement), 1983, pp. 190-193.
- L. Zhao, X. Chen, Z. Hu, Q. Li, Q. Chen and Z. Li, “Analysis of β-N-Oxalyl-α, β-diaminopropionic Acid and Homoarginine in Lathyrus sativus by Capillary Zone Electrophoresis,” Journal of Chromatography A, vol. 857, No. 1-2, 1999, pp. 295-302. doi:10.1016/S0021-9673(99)00788-8
- F. Lambein, J. K. Khan and Y. H. Kuo, “Free Amino Acids and Toxins in Lathyrus sativus Seedlings,” Planta Medica, vol. 58, No. 4, 1992, pp. 380-381. doi:10.1055/s-2006-961491
- E. A. Bell, “Non Protein Amino Acids of Plants: Significance in Medicine, Nutrition and Agriculture,” Journal of Agricultural and Food Chemistry, vol. 51, No. 10, 2003, pp. 2854-2865. doi:10.1021/jf020880w
- T. C. Breitner, “Presence of Homoarginine in Gene Activator-Repressor Histones May Be the Direct Cause of Most Cancers,” Speculations in Science and Technology, vol. 11, 1988, pp. 328-329.
- V. L. Dawson, T. M. Dawson, E. D. London, D. S. Bredt and S. M. Snyder, “Nitric Oxide Mediates Glutamate Neurotoxicity in Primary Cortical Cultures,” Proceedings of the National Academy Sciences, vol. 88, No. 14, 1991, pp. 6368-6371. doi:10.1073/pnas.88.14.6368
- H. K. M. Yusuf, K. Hoque, A. Uddin, B. C. Roy and F. Lambein, “Homoarginine Antagonizes the Toxicity of Lathyrus Toxin in 1-Day-Chiks,” Bengal. Journal of Physiology and Pharmacology, vol. 10, No. 1, 1995, pp. 74-75.
- M. Z. Shamin, M. S. Hossain, K. Islam, H. K. M. Yusuf and F. Lambein, “Mechanism of ODAP Toxicity in OneDay-Old Chicks,” Dhaka University Journal of Biology Science, vol. 11, No. 1, 2002, pp. 1-7.
- J. K. Khan, N. Kebede, Y. H. Kuo and A. De Bruvn, “Analysis of the Neurotoxin Beta-ODAP and Its Alphaisomer by Precolumn Derivatization with Phenylisothiocyanate,” Analytical Biochemistry, vol. 208, No. 2, 1993, pp. 237-240. doi:10.1006/abio.1993.1038
- X. Chen, F. Wang, Q. Chen, X. C. Qin and Z. Li, “Analysis of Neurotoxin 3-N-Oxalyl-l-2,3-diaminopropionic Acid and Its α-isomer in Lathyrus sativus by High-Performance Liquid Chromatography with 6-Aminoquinolyl-N-hydroxysuccinimidyl Carbamate (AQC) Derivatization,” Journal of Agricultural and Food Chemistry, vol. 48, No. 8, 2000, pp. 3383-3386. doi:10.1021/jf000033y
- F. Wang, X. Chen, Q. Chen, X. Qin and Z. Li, “Determination of Neurotoxin 3-N-oxalyl-2,3-diamino Propionic Acid and Non-Protein Amino Acids in Lathyrus sativus by Precolumn Derivatization with 1-fluoro-2,4-dinitrobenzene,” Journal of Chromatography A, vol. 883, No. 1-2, 2000, pp. 113-118. doi:10.1016/S0021-9673(00)00264-8
- Z. Yan, Y. Wang, C. Jiao, F. Li, Y. Liang and Z. Li, “High Performance Liquid Chromatographic Analysis of Neurotoxin β-N-Oxalyl-α, β-diaminopropionic Acid (β- ODAP), Its Non-neurotoxic Isomer α-ODAP and Other Free Amino Acids in Lathyrus sativus,” Chromatographia, vol. 61, No. 5-6, 2005, pp. 231-236. doi:10.1365/s10337-005-0500-4
- Z. Yan, C. J. Jiao, F. Li, Y. Liang and Z. Li, “Analysis Toxin β-N-Oxalyl-α, β-diaminopropionic Acid (β-ODAP), Its Isomer α-ODAP and Other Free Amino Acids in Lathyrus sativus,” Chinese Chemical Letters, vol. 16, No. 5, 2005, pp. 627-630.
- A. Fikre, L. Korbu, Y. Kuo and F. Lambein, “The Contents of the Neuro-Excitatory Amino Acid β-ODAP (β-NOxalyl-α, β-diaminopropionic Acid) and Other Free and Protein Amino Acids in the Seeds of Different Genotypes of Grass Pea (Lathyrus sativus L.),” Food Chemistry, vol. 110, No. 2, 2008, pp. 422-427. doi:10.1016/j.foodchem.2008.02.019
- A. M. K. Arentoft and B. N. Greirson, “Analysis of 3-(N-Oxalyl)-l-2,3-diaminopropionic Acid and Its α-Isomer in Grass Pea (Lathyrus sativus) Capillary Zone Electrophoresis,” Journal of Agricultural and Food Chemistry, vol. 43, No. 4, 1995, pp. 942-945. doi:10.1021/jf00052a018
- S. Tavoletti, L. Iommarini, P. Crinò and E. Granati, “Colction and Evaluation of Grass Pea (Lathyrus sativus L.) Germplasm of Central Italy,” Plant Breeding, vol. 124, No. 4, 2005, pp. 388-391. doi:10.1111/j.1439-0523.2005.01125.x
- G. B. Polignano, V. Bisignano, V. Tomaselli, P. Uggenti, V. Alba and C. D. Gatta, “Genotype × Environment Interaction in Grass Pea (Lathyrus sativus L.) Lines,” International Journal of Agronomy, Vol. 2009, No. 1, 2009, Article ID 898396.
- Y. H. Kuo, P. Rozan, F. Lambein, J. Frias and C. VidalValverde, “Effects of Different Germination Conditions on the Content of Free Protein and Non-Protein Amino Acids of Commercial Legumes,” Food Chemistry, vol. 86, No. 4, 2004, pp. 537-545. doi:10.1016/j.foodchem.2003.09.042
- P. Rozan, Y. H. Kuo and F. Lambein, “Free Amino Acids Present in Commercially Available Seedlings Sold for Human Consumption. A Potential Hazard for Consumers,” Journal of Agricultural and Food Chemistry, vol. 48, No. 3, 2000, pp. 716-723. doi:10.1021/jf990729v
- P. Rozan, Y. H. Kuo and F. Lambein, “Amino Acids in Seeds and Seedlings of the Genus Lens,” Phytochemistry, vol. 58, No. 2, 2001, pp. 281-289. doi:10.1016/S0031-9422(01)00200-X
- F. Lambein, B. Chowdhury and Y. H. Kuo, “Biochemistry of the Lathyrus Toxins,” In: P. N. Mathur, V. Ramanatha Rao and R. K. Arora, Eds., Lathyrus Genetic Resources Network Proceeding of a IPGRI-ICARDA-ICAR Regional Working Group Meeting 8-10 December 1997, IPGRI Office for South Asia, New Delhi, 1997, pp. 60-63.
- A. R. Piergiovanni, F. Lupo and M. Zaccardelli, “Environmental Effect on Yield, Composition and Technological Seed Traits of Some Italian Ecotypes of Grass Pea (Lathyrus sativus L.),” Journal of the Science of Food and Agriculture, vol. 91, No. 1, 2011, pp. 122-129. doi:10.1002/jsfa.4161
- J. D. Berger, K. H. M. Siddique and S. P. Loss, “Cool Season Grain Legumes for Mediterranean Environments: Species × Environment Interaction in Seed Quality Traits and Anti-Nutritional Factors in the Genus Vicia,” Australian Journal of Agricultural Research, vol. 50, No. 3, 1999, pp. 389-402. doi:10.1071/A98098
- A. R. Piergiovanni and D. Pignone, “Effect of Year-toYear Variation and Genotype on Trypsin Inhibitor Level in Common Bean (Phaseolus vulgaris L.) Seeds,” Journal of the Science of Food and Agriculture, vol. 83, No. 5, 2003, pp. 473-476. doi:10.1002/jsfa.1404
- K. Urga, H. Fufa, E. Biratu and A. Husain, “Evaluation of Lathyrus sativus Cultivated in Ethiopia for Proximate Composition, Minerals, β-ODAP and Anti-Nutritional Components,” African Journal of Food Agriculture and Nutritional Development, vol. 5, No. 1, 2005, pp. 1-15.
- A. Fikre, A. Yami, Y. H. Kuo, S. Ahmed, G. Gheysen and F. Lambein, “Effect of Methionine Supplement on Physical Responses and Neurological Symptoms in Broiler Chicks Fed Grass Pea (Lathyrus sativus) Based Starter Ration,” Food and Chemical Toxicology, vol. 48, No. 1, 2010, pp. 11-17. doi:10.1016/j.fct.2009.08.020

