American Journal of Plant Sciences
Vol.4 No.1(2013), Article ID:27587,12 pages DOI:10.4236/ajps.2013.41005
Nature of Gene Action and Maternal Effects for Pod Borer, Helicoverpa armigera Resistance and Grain Yield in Chickpea, Cicer arietinum
![]()
1International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, India; 2Acharya N.G. Ranga Agricultural University, Hyderabad, India.
Email: *h.sharma@cgiar.org
Received October 12th, 2012; revised November 20th, 2012; accepted December 24th, 2012
Keywords: Chickpea; Pod Borer; Helicoverpa armigera; Gene Action; Antibiosis; Maternal Effects; Combining Ability; Inheritance of Resistance
ABSTRACT
Information on mechanisms and inheritance of resistance is critical to plan an effective strategy to breed for resistance to insect pests. Therefore, we evaluated a diverse array of chickpea genotypes (eight desi and one kabuli) with varying levels of resistance to the pod borer, Helicoverpa armigera to gain an understanding of the nature of gene action and possible maternal effects. The test genotypes were crossed in all possible combinations for a full diallel. The 72 F1s (36 direct and 36 reciprocal crosses) along with the parents were evaluated for resistance to H. armigera under field conditions, and for antibiosis mechanism of resistance (larval survival and larval weight gain) by using detached leaf assay under laboratory conditions, and grain yield under un-protected conditions in the field. Additive gene action governed the inheritance of resistance to H. armigera, while non-additive type of gene action was predominant for inheritance of antibiosis component of resistance (larval survival and larval weight) and grain yield. Greater magnitude of σ2 A (17.39 and 1.42) than σ2 D (3.93 and 1.21) indicated the preponderance of σ2 A in inheritance of resistance to pod borer, H. armigera under laboratory and field conditions, respectively. There were no maternal effects for inheritance of resistance to pod borer and grain yield. Lines with significant gca effects for pod borer damage and grain yield were identified for further use in the resistance breeding program. The implications of the inheritance pattern of pod borer resistance and grain yield are discussed in the context of strategies to enhance pod borer resistance and grain yield in chickpea.
1. Introduction
Chickpea [Cicer arietinum Linn.], also known as Bengal gram or gram, is the second most important food legume in Asia, North Africa, and Mexico [1,2]. It is grown on 10.96 million ha worldwide with an average production of 8.79 million tons. Its productivity is 790 kg·ha−1 [1-4]. India contributes a large proportion to total world area (62%) and production (75%) [1]. It is a source of high quality protein for people in many developing countries, including India. There are two types of chickpea: desi and kabuli. Desi type chickpeas accounts for 90% of world production, the remainder being kabuli type, but the area under kabuli chickpea is increasing worldwide [4]. In the recent past, chickpea, especially kabuli types, have witnessed export-driven expansion in the nontraditional areas such as Australia and Canada. In India, both types of chickpeas are grown in diverse agro-ecological niches in the post-rainy season, mainly on residual moisture left over from the monsoon rains between July to October. The current productivity of chickpea in India is nearly 870 kg·ha−1, which is far lower than its potential (up to 4 t/ha) realized in on-farm trials [4]. Over the past 50 years, the productivity of chickpea crop has not witnessed any significant increase as compared to the cereal crops, as biotic and abiotic constraints limit its production on the farmers’ fields.
Gram pod borer, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) is the most important biotic constraint, which at times causes 90 to 95% damage [5]. The yield losses due to pod borer in chickpea have been estimated at over US$328 million in the semi-arid tropics [6], and over $200 million in India [7]. Intensive use of conventional insecticides to control H. armigera has led to the development of insecticide resistant populations [8], and the resource-poor farmers in developing countries are unable to use chemical pesticides to manage this pest. Therefore, there is need for development of improved cultivars with resistance to H. armigera, which is a cost effective and environmentally benign technology to reduce yield losses due to insect pests, particularly under subsistence farming conditions in the developing countries [9]. For each $ invested in host plant resistance (HPR), farmers have realized a return of $300 [10,11]. Several chickpea genotypes have been identified with exploitable levels of resistance to H. armigera [12-17]. Identification of sources of resistance and the knowledge of mechanisms and inheritance of resistance is essential for increasing the levels and diversifying the bases of resistance to insects, and to transfer the resistance genes into high-yielding cultivars. Chickpea has abundant genetic variation, in both qualitative and quantitative traits. The extensive variability available in Cicer germplasm is important to chickpea improvement.
An understanding of inheritance of resistance is essential for a systematic and efficient approach for genetic enhancement of pod borer resistance in chickpea. Although several studies have been made to estimate combining ability and to unravel the genetics of resistance to H. armigera and grain yield in desi and kabuli chickpeas, the results vary with the genetic material involved and across locations [14,18]. The past studies were largely based on a few F1 crosses, as obtaining sufficient numbers of F1 seeds in chickpea is a limiting factor, which might lead to inappropriate estimates of genotypic components of variation. Therefore, comprehensive studies involving a large number of parents with varying levels of pod borer resistance would provide dependable estimates of genetic components of variance. A successful breeding program requires selection of large number of parents with varying levels of resistance to pod borer for hybridization, followed by selection of desirable plants from the segregating generations for developing improved cultivars. This process involves an appropriate mating design [19], and diallel analysis is one of the biometrical techniques to evaluate and characterize genetic variability in a crop species, and for selecting the progenies with greatest promise for success [20].
The objectives of the present study therefore were to understand the nature of gene action, combining ability effects of the parents and their variances to understand the genetics of resistance to H. armigera in terms of pod borer damage under field conditions, antibiosis component of resistance (larval survival and larval weight) by using detached leaf assay under laboratory conditions, and grain yield under un-protected conditions using diallel analysis [21]. Efforts were also made to study the cytoplasmic effects (maternal effects) for resistance to H. armigera and the grain yield.
2. Materials and Methods
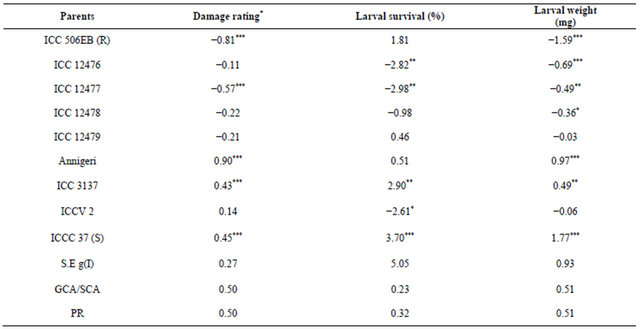
Nine diverse chickpea genotypes (involving eight desi and one kabuli genotypes, which included six pod borerresistant and three -susceptible genotypes) were selected for the studies on nature of gene action for resistance to H. armigera (Table 1). These pod borer resistant and susceptible lines were identified based on the screening of over 14,800 germplasm accessions in the field under un-protected conditions at ICRISAT, Patancheru, India [13,22]. Full-diallel crosses (including reciprocals) were made during the 2003-04 post-rainy season. The F1s along with their parents (81 entries) were evaluated during the following year, i.e. 2004-05 post-rainy seasons using a randomized complete block design (RCBD) with 3 replications at the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Andhra Pradesh, India. Each entry was raised in a two rows, 2 m long with a spacing of 60 cm between the rows and 10 cm between the plants within a row. All the recommended agronomic practices were followed for raising the crop, except crop protection measures against H. armigera. Agronomic characteristics of the chickpea genotypes used for studying the nature of gene action are presented in Table 1. Observations were recorded on five randomly tagged plants in each plot for H. armigera damage and grain yield (g plant−1). Pod borer damage was estimated as percentage of total number of pods under natural infestation. The F1s and their parents were also evaluated by using detached leaf assay under laboratory conditions. Observations were recorded on leaf damage, larval survival and larval weights (Table 2) [23].
Biometric and Genetic Analyses
The mean values of the data recorded on sampled plants for H. armigera damage and grain yield were used for statistical analysis using GENSTAT 6.0. The data on diallel crosses were analyzed following analysis of variance (ANOVA) to test the significance of differences among the parents and their F1s for pod borer damage and grain yield. Griffing [21] Method 1, model 1 was used to estimate the genetic potential of the chickpea genotypes and the genetic architecture of pod borer resistance and grain yield, as the model is based on combining ability analysis. It provided empirical summary of complex observations and a reasonable basis for assessing the breeding value of the parental lines, and predicting the performance of crosses. Being based on first degree statistics, the combining ability effects are statistically robust, but genetically neutral, as these are equally
Table 1. Characteristics of the chickpea genotypes evaluated for tolerance to pod borer, Helicoverpa armigera under natural infestation.
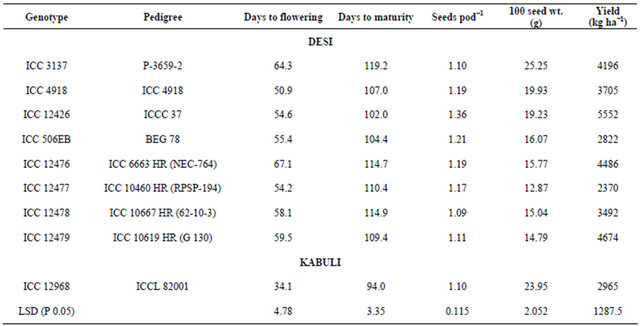
Table 2. Reaction of nine chickpea genotypes to neonate larvae of Helicoverpa armigera in detached leaf assay during the flowering stage.
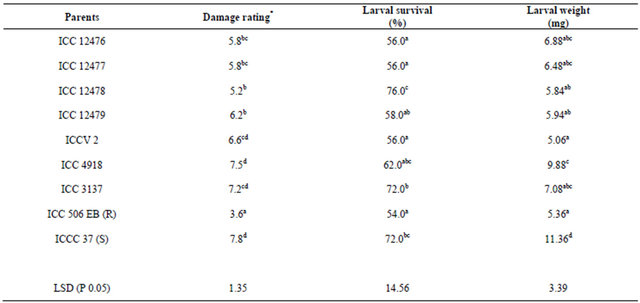
*Damage rating (1 = <10% leaf area damaged, and 9 = >80% leaf area damaged); R = Resistant check, and S = Susceptible check; The figures followed by the same letter within a column are not significantly different at P ≤ 0.05.
applicable to in-breeders and out-breeders [24]. It requires no assumptions beyond those necessary for an ANOVA [20]. Besides providing the estimates of general combining ability (gca) effects of the parents and specific combining ability (sca) effects of the crosses, the analysis provided a method for diagnosis and estimation of σ2 A (additive) and σ2 D (dominance) genetic components of the variance [25]. After confirming the significance of gca and sca effects and their variances, the additive and non-additive effects for pod borer damage and grain yield were estimated. The estimates of variances due to gca (σ2 g) and sca (σ2 s) effects provided the basis for apt diagnosis and estimates of σ2 A and σ2 D genetic components of the variance.
3. Results and Discussion
3.1. Pod Borer Resistance
There were significant differences among the parents as well as their F1s for resistance to pod borer, H. armigera, both under field and laboratory conditions (Tables 3 and 4), justifying the selection of parents for this study. In general, the mean pod borer damage was lower in F1s than the parents, but the variability in pod borer damage between the F1s was greater than the parents. There were substantial differences in gca effects of the parents (as indicated by σ2 g) for pod borer damage, which resulted in progenies (F1s) with differential abilities to resist pod borer damage (Tables 3 and 4). Both σ2 g and σ2 s were significant for pod borer damage in straight crosses. Only σ2 g was significant in reciprocal crosses, suggesting the importance of both additive and non-additive type of gene action. However, greater magnitude of σ2 A (17.39 and 1.42) than σ2 D (3.93 and 1.21) clearly indicated the preponderance of σ2 A in the inheritance of resistance to H. armigera (Tables 5 and 6). Gowda et al. [14,26] and Singh et al. [27] reported that additive and dominance genetic variances, respectively, were predominant for pod borer resistance in earlyand medium-maturity genotypes. However, additive as well as dominance components of genetic variances were equally important in the inheritance of pod borer resistance in the late-maturity genotypes. Salimath et al. [28] reported the involvement of both additive and non-additive type of gene action in the inheritance of pod borer resistance. The parental lines used in the current study mostly belonged to early-and medium-maturity groups, and the results suggested the predominance of additive type of gene action.
In the straight crosses, ICC 506EB, ICC 12477, ICC 12478, ICC 12479 and ICCV 2 were the best general combiners with significant negative gca effects and low pod borer damage (Table 7). Under laboratory conditions, ICC 506EB and ICC 12477 suffered low leaf damage rating with significant and negative gca in detached leaf assay (Table 8). Earlier studies have shown that ICC 12478, ICC 12479, ICC 14876, ICC 506EB and ICC 12477 have negative gca effects, and have a good ability to transmit additive genes to decrease pod borer damage [14,18]. These parents can be involved to generate useful variability for selecting lines with resistance to H. armigera. The hybrids ICC 506EB × ICC 3137, ICC 12476 × ICC 3137, ICC 12477 × ICC 4918, ICC 12479 × ICC 3137 and ICC 3137 × ICCV 2 showed significant and negative sca effects, and were good specific combiners for resistance to pod damage by H. armigera. In detached leaf assay, the hybrids ICC 12477 × ICCC 37, ICC 3137 × ICCV 2 and ICC 4918 × ICCV 2 showed significant and negative sca effects for antibiosis resistance to H. armigera (Table 9). It is interesting to note that these crosses involve one parent with excess of
Table 3. Estimates of mean squares and variances due to general combining ability (GCA) and specific combining ability (SCA) effects for F1 chickpea hybrids under natural conditions in the field (9 × 9 full diallel, Griffing 1956).
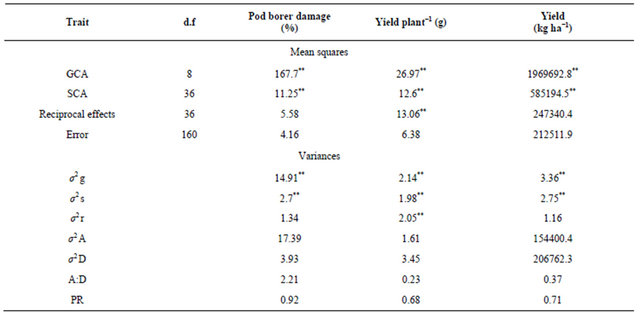
d.f. = Degrees of freedom; *,** = Significant at P = 0.05 and 0.01 respectively; σ2 g: Variance due to gca, σ2 s: Variance due to sca, and σ2 r: Variance due to reciprocal effects. σ2 A: Additive variance, σ2 D: Dominance variance, and PR: Predictability ratio.
Table 4. Estimates of mean squares and variances due to GCA and SCA for resistance to Helicoverpa armigera in chickpea (9 × 9 full diallel, Griffing 1956) (based on detached leaf assay).

d.f. = Degrees of freedom; 1Damage rating (1 = <10%, and 9 = >80% leaf/pod damage); **, *** = Significant at P = 0.01 and 0.001, respectively; σ2 g: Variance due to gca, σ2 s: Variance due to sca, σ2 r: Variance due to reciprocal effects, and σ2 e: Error variance; σ2 A: Additive variance, σ2 D: Dominance variance, and PR: Predictability ratio.
Table 5. Nature of gene action for pod damage by Helicoverpa armigera and grain yield in chickpea.
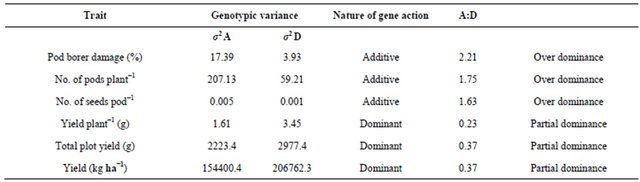
Table 6. Nature of gene action for antibiosis component of resistance to Helicoverpa armigera (based on detached leaf assay).

*Damage rating (1 = <10% leaf area damaged, and 9 = >80% leaf area damaged).
Table 7. Estimates of general combining ability (GCA) effects of nine chickpea genotypes for pod damage by Helicoverpa armigera and grain yield under natural conditions.

Table 8. Estimates of general combining ability (GCA) effects of nine chickpea parents for resistance to Helicoverpa armigera (based on detached leaf assay).
*Damage rating (1 = <10% leaf area damaged, and 9 = >80% leaf area damaged). *, **, *** = Significant at P 0.05, 0.01 and 0.001, respectively. PR: Predictability ratio.
recessive genes, and the other with equal frequency of dominant and recessive genes [14], indicating that the parents involved in the crosses are diverse for nature of genes for pod borer resistance. It is desirable to exploit the crosses with significant sca effects, involving parents contrasting for gca effects, and for reaction to pod borer damage (resistant and susceptible). Recurrent selection in the population developed by random mating of the pod borer resistant parents in high-yielding background used in a diallel would facilitate accumulation of favorable gene combinations in the homozygous and heterozygous state [14].
The sca effects for the reciprocal crosses were nonsignificant, indicating the importance of both additive and non-additive gene effects for pod borer resistance. The cross ICCV 2 × ICC 3137 showing significant and negative sca effects was good specific combiner for resistance to pod borer damage. Similar results have been
Table 9. Estimates of specific combining ability (SCA) effects of FIs for antibiosis component of resistance to Helicoverpa armigera (based on detached leaf assay).

1Damage rating (1 = <10% leaf area damaged, and 9 = >80% leaf area damaged). *, **, *** = Significant at P 0.05, 0.01 and 0.001, respectively.
reported by Singh and Paroda [29]. Better progenies have been obtained from the crosses involving diverse parents for gca effects in groundnut [30]. Langham [31] has provided the evidence for the usefulness of divergent parents for maximizing potential transgressive lines in cross derivatives in rice. Effectiveness of pedigree selection for pod borer resistance in chickpea has also been reported by Sharma et al. [32] and Dua et al. [22]. Singh et al. [33] developed pod borer resistant chickpea line, ICCV 7 using pedigree selection of the lines derived from a cross between H 208 and BEG 482. However, caution is necessary while using pedigree selection for pod borer resistance, considering the existence of tight linkage between susceptibility to fusarium wilt (Fusarium oxysporum f. sp. ciceri) and resistance to pod borer in chickpea. Biparental matings in early segregating generations of a multiple cross involving a few pod borer and fusarium wilt resistant genotypes in high yielding background would provide increased opportunity for recombination, which will facilitate disruption of tight linkage between the genes for these traits [34,35]. Germplasm lines (ICC 12478, ICC 12479 and ICC 14876) having stable resistance to H. armigera and moderate yield potential [36] could be used in enhancement of pod borer resistance in elite agronomic background.
3.2. Larval Survival
There were significant differences among the parents as well as their F1s for survival of H. armigera larvae (Table 4). In general, the mean larval survival was lower in F1s than in parents. Greater magnitude of σ2 D (0.50) than σ2 A (0.23) clearly indicated the preponderance of σ2 D in the inheritance of antibiosis component of resistance (% larval survival) to H. armigera larvae (Table 4). In the straight crosses, ICC 12476, ICC 12477 and ICCV 2 were the best general combiners with significant negative gca effects for larval survival (Table 8). The hybrids ICC 12476 × ICC 12478 and ICC 12477 × ICC 12478 showed significant and negative sca effects and were good specific combiners for resistance to survival of H. armigera larvae (Table 9).
3.3. Larval Weight Gain
There were significant differences among the parents as well as their F1s for weight of the H. armigera larvae. In general, the mean larval weights were lower in F1s than in the parents. Greater the magnitude of σ2 D (50.21) than σ2 A (38.37) clearly indicated the preponderance of σ2 D in the inheritance of antibiosis to H. armigera in terms of weight gain by the H. armigera larvae in detached leaf assay (Table 4). In the straight crosses, ICC 506EB, ICC 12476, ICC 12477 and ICC 12478 were the best general combiners with significant negative gca effects and low larval weights (Table 8). The hybrids ICC 12476 × ICC 3137, ICC 12476 × ICC 506EB, ICC 12478 × ICCC 37, ICC 12479 × ICC 506EB, ICC 3137 × ICC 506EB and ICC 4918 × ICCV 2 showed significant and negative sca effects, and were good specific combiners for antibiosis resistance (weight gain) to H. armigera (Table 9).
In detached leaf assay, resistance to leaf feeding was governed by additive gene action. These results are in accordance with field observations by Gowda et al. [14,26] and Singh et al. [27]. Antibiosis component of resistance (based on larval survival and larval weight) was governed by non-additive type of gene action (Table 6).
3.4. Grain Yield
There were significant differences among the parents and the F1 crosses (straight and reciprocal crosses) for grain yield (Table 3). The importance of both σ2 g and σ2 s was evident with predominance of the latter, which is amply reflected from a much higher magnitudes of σ2 D than σ2 A. Similar results have been reported by Deshmukh and Patil [37] and Gowda et al. [14]. The predominance of σ2 D indicated the importance of non-additive gene action. Since gca effects are the manifestation of additive properties of genes, parents selected based on gca effects will be useful for developing breeding lines with high grain yield. Parents with good general combining ability such as ICC 4918 could be used in breeding programs. Gowda et al. [14] reported that ICC 12476 possessed an excess of dominant genes, ICC 12426, ICC 12478 and ICC 4918 possessed an excess of recessive genes, while ICC 506EB possessed equal frequency of dominant and recessive genes for grain yield under un-protected conditions. Parents with significant and positive gca effects are diverse for the nature of gene action for grain yield. The combining ability variances were significant for both gca and sca. The predictability ratio of 0.23 showed that gca alone was not sufficient for predicting the performance of single cross progenies. Of the two genetic parameters, the magnitude of σ2 D was more than σ2 A, which emphasized that non-additive gene action, was involved in inheritance and expression of grain yield per plant (Table 5). These findings are in conformity with earlier reports [33-44]. However, the reports of Gowda [45], Asawa and Tewari [46], Sandhu et al. [47], and Gowda and Bahl [48] are contradictory to the present findings, which indicated the involvement of additive genetic variance. Singh et al. [49], Singh and Ocampo [50], Annigeri et al. [51], Sarode [52] and Girase [53] reported the importance of additive as well as non-additive genetic variances. The crosses ICC 12476 × ICCC 37, ICC 12477 ×
Table 10. Maternal effects for seeds per pod in chickpea.
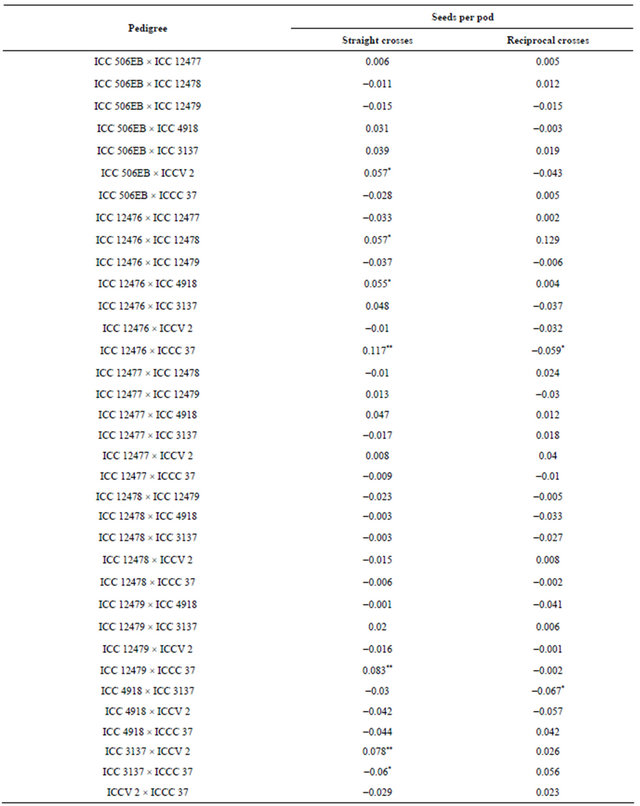
*, ** = Maternal effects significant at P 0.05 and 0.01, respectively.
ICC 4918 and ICC 12478 × ICC 12479 with highly significant and positive sca effects were good specific combiners for grain yield. Interestingly, one of the parents of these crosses possessed an excess of dominant genes, while the other parent possessed an excess of recessive genes [14], suggesting the need to have genetic diversity for increased heterosis for grain yield. Theoretical investigations substantiating the necessity of parental diversity for better performance of crosses [54,55] lend adequate support to these practical considerations. Although there was a good correspondence between pod borer damage of crosses and their sca effects, the crosses (involving parents with contrasting gca effects) with significant sca effects need to be exploited for deriving superior lines for grain yield.
The significance of both σ2 g and σ2 s indicated the importance of both σ2 A and σ2 D in the reciprocal crosses. However, the higher magnitude of σ2 D than σ2 A is a clear evidence for predominance of σ2 D in the inheritance of grain yield. Crosses ICC 12477 × ICC 506EB, ICC 3137 × ICC 506EB, ICCC 37 × ICC 506EB, ICCV 2 × ICC 12476 and ICCC 37 × ICC 3137 with highly significant and positive sca effects were good specific combiners for increased grain yield. The grain yield is predominantly under the control of non-additive gene action, irrespective of the maturity groups in desi type chickpea. Due to predominance of non-fixable genetic variation coupled with low heritability, it has not been possible to achieve breakthrough in increasing the chickpea productivity. Lack of sufficient variability (due to its strictly inbreeding behavior) is one of the reasons for limited progress in increasing chickpea productivity to a desired level [36]. The use of conventional breeding methods such as pedigree, single seed descent, and bulk methods are associated with the weakness of causing rapid homozygosity and low genetic variability, especially in the presence of linkage blocks and inverse relationships among the desirable traits [56]. Breeders need to exploit both additive and non-additive gene effects, besides disrupting undesirable associations and uncovering concealed variability. Biparental mating of segregants in a multiple crossing scheme might be useful in disrupting the undesirable linkages [34,35,57].
3.5. Maternal Effects
There was no maternal inheritance for pod borer damage and grain yield. The cross, ICC 12476 × ICCC 37 showed positive and significant sca effects for seeds per pod, but ICCC 37 × ICC 12476 showed negative and significant sca effects for number of seeds pod−1, suggestive cytoplasmic inheritance for the number of seeds per pod (Table 10).
4. Conclusion
The present studies suggested that additive genetic variation was predominant for the inheritance of resistance to H. armigera, while dominance genetic variation was predominant in governing the inheritance of antibiosis component of resistance (larval survival and larval weight) and grain yield under un-protected conditions. The studies indicated the necessity of using diverse (for gca effects) parents for producing productive crosses, from which superior breeding lines could be derived for increasing the levels of resistance to pod borer, and for increasing the grain yield potential. There was no cytoplasmic inheritance for pod borer damage and grain yield, but the hybrid, ICCC 37 × ICC 12476 showed cytoplasmic inheritance for number of seeds per pod. Studies on nature of gene action are useful in eliminating less productive crosses in F1, and to concentrate on a few, but possibly more productive crosses [58]. Further studies on mechanisms and inheritance of resistance, and use of morphological, biochemical, and molecular markers will be useful for increasing the levels and diversifying the basis of resistance to H. armigera in chickpea [59,60].
REFERENCES
- P. S. Birthal, P. P. Rao, M. C. S. Mantilan and S. Bhagavabatula, “Groundnut and Soybean Economics in Asia: Facts, Trends and Outlook,” Internationl Crops Research Institute for the Semi-Arid Tropics (CRISAT), Patancheru, 2010, p. 86.
- FAO, “FAO Bulletin of Statistics,” Food and Agricultural Organization, Rome, 2010.
- P. Parthasarthy Rao, S. N. Nigam, M. C. S. Bntilan, S. Bhagavabatula and M. C. S. Mantilan, “Chickpea and Pigeonpea Economics in Asia: Facts, Trends and Outlook,” International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, 2010.
- M. Ali and H. A. Shivkumar, “Chickpea (Cicer arietinum) Research in India: Accomplishments and Future Strategies,” Indian Journal of Agricultural Sciences, Vol. 75, No. 3, 2005, pp. 125-133.
- J. Singh, A. S. Sidhu and B. S. Kooner, “Incidence of Heliothis armigera in Relation to Phenology of Chickpeas,” Journal of Research—Punjab Agricultural University, Vol. 22, No. 2, 1985, pp. 291-297.
- ICRISAT, “The Medium Term Plan,” International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, 1992.
- J. N. Sachan and G. Katti, “Integrated Pest Management,” Souvenir: 25 Years of Research on Pulses in India, International Symposium on Pulses Research, New Delhi, 2-6 April 1994, pp. 23-26.
- W. J. Armes, D. R. Jadhav and K. R. Desuza, “A Survey of Insecticide Resistance in H. armigera in Indian Subcontinent,” Bulletin of Entomological Research, Vol. 86, No. 5, 1996, pp. 499-514. doi:10.1017/S0007485300039298
- H. C. Sharma, B. U. Singh, K. V. Hariprasad and P. J. Bramel-Cox, “Host-Plant Resistance to Insects in Integrated Pest Management for a Safer Environment,” Proceedings, Academy of Environmental Biology, Vol. 8, No. 1, 1999, pp. 113-136.
- R. A. Robinson, “Return to Resistance: Breeding Crops to Reduce Pesticide Dependence,” Ag Access, California, 1996.
- H. C. Sharma, “Heliothis/Helicoverpa Management: Emerging Trends and Strategies for Future Research,” Oxford and IBH Publishing, New Delhi, 2005.
- K. S. Chabhra, B. S. Kooner, A. K. Sharma and A. K. Saxena, “Sources of Resistance in Chickpea, Role of Biochemical Components on the Incidence of Gram Pod Borer, Helicoverpa armigera (Hubner),” Indian Journal of Entomology, Vol. 52, No. 3, 1990, pp. 423-430.
- C. A. R. Dias, S. S. Lal and C. P. Yadava, “Differences in Susceptibility of Certain Chickpea Cultivars and Local Collection to Heliothis armigera (Hubner),” Indian Journal of Agricultural Sciences, Vol. 53, No. 9, 1983, pp. 842-845.
- C. L. L. Gowda, S. Ramesh, S. Chandra and H. D. Upadhyaya, “Genetic Basis of Pod Borer (Helicoverpa armigera) Resistance and Grain Yield in Desi and Kabuli Chickpea (Cicer arietinum L.) under Un-Protected Conditions,” Euphytica, Vol. 145, No. 1-2, 2005, pp. 199- 124.
- S. S. Lateef, “Gram Pod Borer, Heliothis armigera (Hub.) Resistance in Chickpea,” Agriculture Ecosystems & Environment, Vol. 14, No. 1-2, 1985, pp. 95-102. doi:10.1016/0167-8809(85)90087-8
- S. S. Lateef and J. N. Sachan, “Host Plant Resistance of Helicoverpa armigera in Different Agro-Ecological Contexts,” Chickpea in Nineties, Proceedings of the Second International Workshop on Chickpea, Patancheru, 4-8 December 1989, pp. 181-189.
- N. J. Bhatt and R. K. Patel, “Screening of Chickpea Cultivars for Their Resistance to Gram Pod Borer, Helicoverpa armigera,” Indian Journal of Entomology, Vol. 63, No. 3, 2001, pp. 277-280.
- E. Sreelatha, C. L. L. Gowda, T. B. Gaur, H. C. Sharma, S. Ramesh and H. D. Upadhyaya, “Genetic Analysis of Pod Borer (Helicoverpa armigera) Resistance and Grain Yield in Desi and Kabuli Chickpeas (Cicer arietinum) under Unprotected Conditions,” Indian Journal of Genetics, Vol. 68, No. 4, 2007, pp. 406-413.
- V. Arunachalam, “Evaluation of Diallel Crosses by Graphical and Combining Ability Methods,” Indian Journal of Genetics Vol. 36, No. 3, 1976, pp. 358-366.
- B. R. Christie and Shattuck, “The Diallel Cross: Design, Analysis, and Use for Plant Breeders,” Plant Breeding Reviews, Vol. 9, 1992, pp. 9-36.
- B. Griffing, “Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems,” Australian Journal of Biological Sciences, Vol. 9, No. 4, 1956, pp. 463-493.
- R. P. Dua, C. L. L. Gowda, H. A. Shiv Kumar, K. B. Saxena, J. N. Govil, B. B. Singh, K. K. Singh, R. P. Singh, V. P. Singh and S. Kranthi, “Breeding for Resistance to Helicoverpa: Effectiveness and Limitations,” In: H. C. Sharma, Ed., Heliothis/Helicoverpa Management: Emerging Trends and Strategies for Future Research, Oxford & IBH Publishing, New Delhi, 2005, pp. 307-328.
- V. L. Narayanamma, H. C. Sharma, C. L. L. Gowda and M. Sriramulu, “Mechanisms of Resistance to Helicoverpa armigera and Introgression of Resistance Genes into F1 Hybrids in Chickpea,” Arthropod Plant Interactions, Vol. 1, No. 4, 2007, pp. 263-270. doi:10.1007/s11829-007-9025-0
- N. W. Simmonds, “Principles of Crop Improvement,” Longman Group Limited, London, 1979, pp. 115-116.
- O. Kempthorne, “An Introduction to Genetic Statistics,” John Wiley and Sons, New York, 1957.
- C. L. L. Gowda, S. S. Lateef, J. B. Smithson and W. Reed, “Breeding for Resistance to Heliothis armigera in Chickpea,” Proceedings of the National Seminar on Breeding Crop Plants for Resistance to Pests and Diseases, Coimbatore, 25-27 May 1983, pp. 223-242.
- O. Singh, C. L. L. Gowda, S. C. Sethi and S. S. Lateef, “Inheritance of and Breeding for Resistance to Helicoverpa armigera Pod Borer in Chickpea,” Golden Jubilee Symposium of Indian Society of Genetics and Plant Breeding, 4-8 February 1991, New Delhi.
- P. M. Salimath, S. C. Shahapur, H. G. Nijagun, S. T. Khajjidoni, R. L. Ravi Kumar and B. S. Patil, “Genetic Analysis of Pod Borer Tolerance and Malic Acid Content in Chickpea (Cicer arietinum L.),” In: R. N. Sharma, G. K. Shrivastava, A. L. Rathore, M. L. Sharma and M. A. Khan, Eds., Chickpea Research for the Millennium, Proceedings of the International Chickpea Conference, Raipur, 20-22 January 2003, pp. 81-85.
- O. Singh and R. S. Paroda, “A Comparative Analysis of Combining Ability and Heterosis in Irradiated and NonIrradiated Diallel Populations of Chickpea,” Indian Journal of Pulses Research, Vol. 2, No. 1, 1989, pp. 1-9.
- V. Arunachalam, “Plant Breeding—By Design or Default? In: Breeding Strategies for 21st Century,” Proceedings of the 1st National Plant Breeding Congress, Coimbatore, 1-3 July 1998, pp. 52-67.
- D. G. Langham, “The High-Low Method in Crop Improvement,” Crop Science, Vol. 1, No. 5, 1961, pp. 376- 378. doi:10.2135/cropsci1961.0011183X000100050026x
- H. C. Sharma, C. L. L. Gowda, K. K. Sharma, P. M. Gaur, N. Mallikarjuna, H. K. Bouhariwalla and J. H. Crouch, “Host Plant Resistance to Pod Borer, Helicoverpa armigera in Chickpea,” Research for the Millennium: Proceedings of the International Chickpea Conference, Raipur, 20-22 January 2003, pp. 118-137.
- O. Singh, S. C. Sethi, S. S. Lateef and C. L. L. Gowda, “Registration of ICCV 7 Chickpea Germplasm,” Crop Science, Vol. 37, No. 1, 1997, p. 295. doi:10.2135/cropsci1997.0011183X003700010068x
- N. Kampli, P. M. Salimath and S. T. Kajjidoni, “Genetic Variability Created through Biparental Mating in Chickpea (Cicer arietinum L.),” Indian Journal of Genetics, Vol. 62, No. 2, 2002, pp. 128-130.
- N. Kampli, P. M. Salimath, S. T. Kajjidoni and R. L. Ravikumar, “Effects of Intermating in Early Segregating Generations on Character Association in Chickpea (Cicer arietinum L.),” Indian Journal of Genetics, Vol. 62, No. 3, 2002, pp. 259-260.
- E. Sreelatha, T. B. Gour, C. L. L. Gowda, M. A. Ghaffar and H. C. Sharma, “Stability of Resistance to Helicoverpa armigera in Chickpea,” In: R. N. Sharma, G. K. Shrivastava, A. L. Rathore, M. L. Sharma and M. A. Khan, Eds., Chickpea Research for the Millennium: Proceedings of the International Chickpea Conference, Raipur, 20-22 January 2003, pp. 138-142.
- R. B. Deshmukh and V. J. Patil, “Genetic Architecture of Yield and Its Components in Chickpea,” Legume Research, Vol. 18, No. 2, 1995, pp. 85-88.
- D. D. Bhatt and D. P. Singh, “Combining Ability in Chickpea,” Indian Journal of Genetics, Vol. 40, No. 2, 1980, pp. 456-460.
- S. D. Ugale, “Incorporation of Germplasm from Kabuli to Desi Cultivars and Vice-Versa in Chickpea (Cicer arietinum L.),” Ph.D. Thesis, Indian Agricultural Research Institute (IARI), New Delhi, 1980.
- R. P. Katiyar and Solanki, “Combining Ability and Gene Action for Yield and Its Component Traits in Chickpea (Cicer arietinum L.),” Proceedings, XV International Congress of Genetics, New Delhi, 1983.
- K. P. Singh and J. S. Sidhu, “Gene Action for Harvest Index and Grain Yield in Chickpea,” Proceedings, XVth International Congress of Genetics, New Delhi, 1983.
- B. A. Kunadia, M. V. Kukadia and A. R. Pathak, “Graphical Diallel Analysis in Kabuli Gram,” Gujarat Agricultural University Research Journal, Vol. 12, No. 1, 1986, pp. 71-76.
- N. V. Shinde, “Studies on Inheritance of Resistance to Fusarium oxysporum f. sp. ciceri, Grain Yield and Its Components in Chickpea (Cicer arietinum L.),” Ph.D. Thesis, Mahatma Phule Krishi Vishva Vidalaya, Rahuri, 1988.
- M. A. K. Miah and P. N. Bahl, “Genetic Divergence and Hybrid Performance in Chickpea,” Indian Journal of Genetics, Vol. 49, No. 1, 1989, pp. 119-124.
- C. L. L. Gowda, “Genetic Analyses of Yield Components in Bengal Gram (Cicer arietinum L.),” Ph.D. Thesis, Indian Agricultural Research Institute (IARI), New Delhi, 1975.
- B. M. Asawa and A. S. Tewari, “Analysis of Genetic Architecture in Segregating Population of Gram (Cicer arietinum L.),” Zeitschrift Pflanzenzuchtung, Vol. 77, No. 3, 1976, pp. 251-256.
- T. S. Sandhu, H. S. Brar and B. S. Arora, “Combining Ability from Diallel Cross of Chickpea (Cicer arietinum L.),” Crop Improvement, Vol. 4, No. 1, 1977, pp. 11-17.
- C. L. L. Gowda and P. N. Bahl, “Combining Ability in Chickpea,” Indian Journal of Genetics, Vol. 38, No. 2, 1978, pp. 245-251.
- O. Singh, C. L. L. Gowda, S. C. Sethi, T. Dasgupta and J. B. Smithson, “Genetic Analysis of Agronomic Characters in Chickpea. I. Estimates of Genetic Variances from Diallel Mating Designs,” Theoretical and Applied Genetics, Vol. 83, No. 8, 1992, pp. 956-962.
- K. B. Singh and B. Ocampo, “Interspecific Hybridization in Annual Cicer Species,” Indian Journal of Genetics, Vol. 47, No. 3, 1993, pp. 199-204.
- B. S. Annigeri, P. M. Salimath, S. J. Patil and B. H. Venkataswamy, “Genetic Analysis to Identify Potential Parents and Crosses for Yield Improvement in Chickpea,” Indian Journal of Genetics, Vol. 56, No. 1, 1996, pp. 114-116.
- N. D. Sarode, “Diallel Analysis in Chickpea (Cicer arietinum L.),” M.Sc. (Agri.) Thesis, Mahatma Phule Krishi Vishva Vidalaya, Rahuri, 1997.
- V. S. Girase, “Genetic Architecture, Transgressive Segregation and Inheritance of Resistance to Wilt (Fusarium oxysporum f sp. ciceri),” Ph.D Thesis, Mahatma Phule Krishi Vishva Vidalaya, Rahuri, 1999.
- C. E. Cress, “Heterosis of the Hybrid Related to Gene Frequency Differences between Populations,” Genetics, Vol. 53, No. 2, 1966, pp. 269-274.
- K. Mather and J. L. Jinks, “Biometrical Genetics—The Study of Continuous Variation,”3rd Edition, Chapman and Hall, London, 1982.
- M. T. Clegg, R. W. Allard and A. L. Kahiar, “Is the Gene Unit of Selection? Evidence from Two Experimental Plant Populations,” Proceedings of the National Academy of Sciences of the United States, Vol. 69, No. 9, 1972, pp. 2474-2478. doi:10.1073/pnas.69.9.2474
- V. S. Girase, R. B. Deshmukh and J. V. Patil, “Genetic Architecture for Grain Yield and Its Components in Chickpea—A Review,” Journal of Maharashtra Agricultural Universities, Vol. 27, No. 1, 2002, pp. 22-25.
- K. B. Singh, “Exploitation of Heterosis in Pulse Crops,” Indian Journal of Genetics, Vol. 34A, 1974, pp. 752-756.
- H. C. Sharma and J. H. Crouch, “Molecular Marker Assisted Selection: A Novel Approach for Host Plant Resistance to Insects in Grain Legumes,” Pulses in New Perspective, Proceedings of the National Symposium on Crop Diversification and Natural Resource Management, Kanpur, 20-22 December 2004, pp. 147-174.
- [61] H. C. Sharma, V. Rajeev, P. M. Gaur and C. L. L. Gowda, “Potential for Using Morphological, Biochemical, and Molecular Markers for Resistance to Insect Pests in Grain Legumes,” Journal of Food Legumes, Vol. 21, No. 4, 2007, pp. 211-217.
NOTES
*Corresponding author.

