American Journal of Plant Sciences
Vol.3 No.8(2012), Article ID:22175,4 pages DOI:10.4236/ajps.2012.38128
Comparison of Flavonoid Profiles between Leaves and Stems of Calystegia soldanella and Calystegia japonica
![]()
1Department of Biological Sciences, Dankook University, Cheonan, Korea; 2Institute of Basic Science, Dankook University, Cheonan, Korea; 3NAVIBIOTECH INC., Chungnam Techno Park, Cheonan, Korea.
Email: hccha@dankook.ac.kr
Received June 4th, 2012; revised July 1st, 2012; accepted July 10th, 2012
Keywords: Flavonoid; Calystegia soldanella; Calystegia japonica; Convolvulaceae; HPLC
ABSTRACT
Two species of Calystegia, C. soldanella and C. japonica which both are vine perennials belong to Convolvulaceae habituated in extremely different environment. In order to elucidate the effect of flavonoids to tolerate environmental stress we chose a sand-dune plant C. soldanella, under unfavorable conditions such as nutrients deficiency, strong irradiance, and broad differences of temperature during day and night and compared this plant with C. japonica habituated under mild environment. Several kinds of flavonoids were found in both plants. Especially, quercetin glycosides and kaempferol glycosides were found as major flavonols in both plants. In C. soldanella, kaempferol 3-O-galactosidee-7-O-glucoside, kaempferol 3-O-digalactoside-7-O-glucoside, kaempferol 3-O-galactoside-7-O-galactoside and 4’-hydroxyflavone 7-O-glucoside were found. While in C. japonica, quercetin 3-O-galactoside 7-O-glucuronide, quercetin 3,7-O-digalactoside and kaempferol 3-O-galactoside 7-O-glucoside were found. Also, kaempferol glycoside was a major flavonol in C. soldanella, in contrast to quercetin glycoside as major flavonol in C. japonica. The content of total phenolic compounds was similar in two species but the content of total flavonoid compounds was higher by 1.5 times in C. japonica than that of C. soldanella.
1. Introduction
The genus Calystegia (Convolvulaceae) contains a large number of species widely distributed in the world. Among them, we selected two species; C. soldanella (sea bells) and C. japonica (California rose) which are very related but have different ecological niche. C. soldanella is endemic plants living in the coastal sand dunes [1]. On the other hand, C. japonica has cosmopolitan distribution in temperate and subtropical regions. This plant was used as sources of the pharmacopoeial activities such as antiseptic, antioxidant, antipyretic, diuretic, cholagogic and antihaemorrhagic activity, has been found to contain some flavonoids [2,3].
Flavonoids are widely distributed in plants fulfilling many functions. Those are the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants. The basic flavonoid structure is the flavan nucleus, which consist of 15 carbon atoms arranged in three rings (C6-C3-C6). They are antioxidants, phytoalexins and UV protectors [4-7]. The range and structural complexity of flavonoids have led to their sub classification as flavones, flavonols, flavanones, isoflavones and anthocyanins [8]. While flavones and flavonols in many plant families and anthocyanins are almost absent in flavanon-rich plants [9,10]. The flavonols quercetin, kaempferol, and their glycosides are constituents of the beverages green and black teas and red wine [10].
Flavonoids are a particularly interesting group of phenolic substances in connection with the investigation of the high-sunlight response. However, the UV-protective efficiency of phenolic “sunscreens” induced in the course of adaptation to high sunlight under natural conditions is still uncertain in many cases [11].
In this study, we extracted flavonoids from leaves and stems, and compared their profiles between C. soldanella and C. japonica. Also we measured the contents of total phenolic compounds and total flavonoid compounds, to see correlation between environmental status and accumulation of these compounds.
2. Materials and Methods
2.1. Plant Material
Mature leaves and stems of C. soldanella was harvested in July to September 2007 and 2008 in the coast of Sinduri in Taean, and of C. japonica was collected in same months and years in the Dankook University of Cheonan, Korea. And all samples were immediately freeze-dried and stored at –70˚C until to use.
2.2. Chemicals
Rutin and quercetin were purchased from Sigma Chemical Co. All solvents used for extraction and high performance liquid chromatography (HPLC) analysis were obtained from Duksan pure chemicals (Ansansi, Kyungkido, Korea) and Merck (Darmstadt, Germany). HPLCgrade water was prepared by redistillation.
2.3. Preparation of Crude Flavonoid Extracts
Freeze-dried leaves and stems (20 g of samples) were ground at high speed in a blender and extracted with 85% aqueous methanol at room temperature overnight, two times. Afterward, the slurry was filtered through Whatman No. 1 filter paper in a Buchner funnel. And the residue was re-extracted with 50% aqueous methanol at room temperature overnight. The methanol extract was evaporated under reduced pressure, then the crude extract was separated with chloroform into chlorophyll and flavonoid portions, and the residual supernatant was washed with ethyl acetate. The extracts were re-evaporated and stored at –4˚C.
2.4. Apparatus
For the HPLC analysis, we used the Shimazu Model (Class-VP) UV dual pump system (Japan) and a 7.8 mm × 30 cm Waters semi-preparative N-Bondapak C-18 Column (USA). The detection wavelength was set at 254 nm. Absolute retention times were measured. Rutin was used as an internal standard.
2.5. Separation and Analysis of Flavonoids
HPLC method was one proposed by Moon and Park [12]. Condition for the phase included the following: pump A, acetonitrile; pump B, 2% acetic acid; with a gradient elution for 27 min per cycle. A 20 ul sample of each flavonoid solution from the one-dimensional PC was injected into a column. All solvent were HPLC grade and filtered and degassed before use. The preliminary analysis of the flavonoid extracts employed two-dimensional TLC (thin layer chromatography). The extracts of each species were spotted on cellulose TLC plate (Merck, 20 cm × 20 cm), and the chromatograms were developed in TBA (tertiary-buthanol: acetic acid: water = 3:1:1, v/v/v) and 15% aqueous acetic acid. The flavonoid profiles were viewed under the UV light, and Rf values were recorded. The purified flavonoids were identified through a combination of UV spectrophotometer, HPLC and TLC, as described by Markham [13] and Harborne [14].
2.6. Determination of Total Phenolic Contents
Samples were analyzed spectrophotometrically for the contents of total phenolic using a modified Folin-Ciocalteu colorimetric method [15,16]. One g of freeze-dried leaves and stems of two species were extracted with 80% methanol overnight at room temperature. They were centrifuged at 5000 rpm for 60 minutes, and then discarded the supernatants. That pellet was incubated with methanol. 100 μl of methanol extracts was mixed with 100 ul of 50% Folin-Ciocalteu, 200 μl of 10% Na2CO3 and 800 ul of D.W. The mixtures were measured at 760 nm after centrifuge at 12,000 rpm for 10 minutes. Tannic acid was used as a standard and total phenolics were expressed as ug/g tannic acid equivalent.
2.7. Determination of Total Flavonoid Contents
One g of freeze-dried leaves and stems of two species were extracted with 80% methanol overnight at room temperature. They were centrifuged at 5000 rpm for 60 minutes, and then discarded the supernatants. That pellet was incubated with methanol. 100 μl of methanol extracts was mixed with 100 μl of 10% Al(NO3)3 and 100 ul of 1 M potassium acetate. 80% ethanol was added to adjust the final volume to 5 ml. The mixtures were allowed to stand for 40 min at room temperature before measurement at 415 nm. Quercetin was used as a standard and total flavonoids were expressed as μg/g quercetin equivalent.
3. Result and Discussion
We have identified three kinds of kaempferol glycoside from the leaves, three kinds of flavonoid compound from the stems of C. soldanella, two kinds of flavonol compounds from leaves and one flavonol compound from stems of C. japonica. In C. soldanella, only kaempferol was detected in leaves, while quercetin and kaempferol which belong to flavonol, and 4’-hydroxyflavone which belong to flavone, were isolated in stems. In C. japonica, we analyzed that the quercetin was common flavonoid from leaves and stems, but in the stems, kaempferol was additionally detected (Table 1). Thus, we could know that kaempferol and quercetin is major flavonoids in two species. Figure 1 is the structure of quercetin and kaempferol, which belong to flavonols. Few reports have been made about flavonoid profiles in members of Convolvulaceae. Menemen et al., [17] found that the flavonol glycoside kaempferol and quercetin in C. arvensis, C. sabatius subsp. sabatius, C. sabatius subsp. mauritanicus and C. siculus subsp. elongates, and isohamnetin in C. mazicum, C. trabutianus, C. tricolor subsp. tricolor, C. glaouorum and C. valentinus, that the flavones glycoside luteolin in C. mazicum. The reports about two species, The flavonids Except for that many reports briefed that
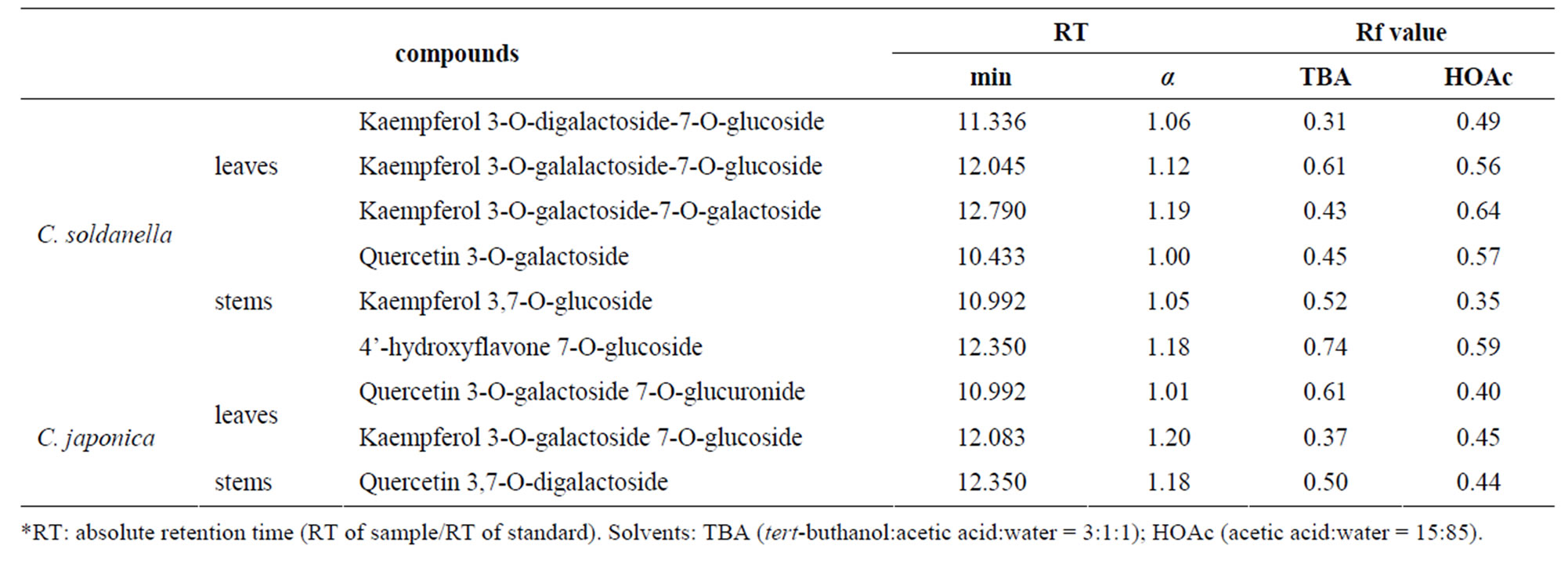
Table 1. Chromatographic properties of flavonoids identified from leaves and stems in C. soldanella and C. japonica.
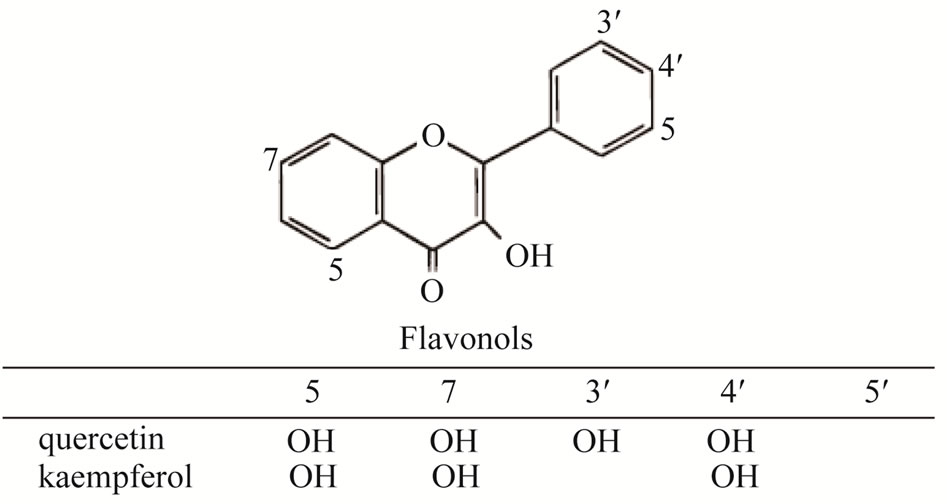
Figure 1. Structure of flavonol, quercetin and kaempferol.
cyanidin 3-glucoside, cyanidin 3-rutinoside and cyanidin 3-[6-(malonyl)-glucoside] were analyzed in the corolla mixture of C. soldanella and C. japonica [18]. Through those reports, we could confirm that convolvulacea plants have, mostly, flavonols among the flavonoids. In the present study, we found only kaempferol, quercetin and hydroxyflavone, but not isohamnetin, cyanidin and luteolin, in the leaves and stems of C. soldanella and C. japonica. The sugars we found in the flavonol-O-glycoside from C. soldanella were galalactoside, glucoside, digalactoside and galactoside, and from C. japonica were galactoside, digalactoside, glucoside and glucuronide (Table 1). Galactoside was the most common glycosidic moiety of flavonoid in two species.
The total phenolic compounds in leaves and stems of two species investigated in this study varied from 306.53 ug/g in stems of C. soldanella to 702.19 μg/g in leaves of C. japonica using the standard curve of tannic acid. This study showed that total phenolic contents in the selected species as: leaves of C. japonica (702.19 ± 0.04 μg/g) > of C. soldanella (693.22 ± 0.04 μg/g) > stems of C. japonica (395.33 ± 0.02 μg/g) > of C. soldanella (306.53 ± 0.02 μg/g). Using the standard curve generated by quercetin, total flavonoid compounds were distributed from 62.29 ± 0.01 μg/g in stems of C. soldanella to

Table 2. Contents of total phenolic, total flavonoid compounds in leaves and stems of C. soldanella and C. japonica.
634.04 ± 0.018 μg/g in leaves of C. japonica of dry product. This study showed that total flavonoid compounds in the two species as: leaves of C. japonica (634.04 ± 0.02 μg/g) > of C. soldanella (384.27 ± 0.01 μg/g) > stems of C. japonica (95.15 ± 0.01 μg/g) > of C. soldanella (62.29 ± 0.01 μg/g). The Folin-Ciocalteu reagent detects all phenolic groups found in extracts [19]. The content of total phenolic compounds was similar in two species but the content of total flavonoid compounds was higher by 1.5 times in C. japonica than that of C. soldanella (Table 2).
In the further study, we will measure the antioxidant activity of two species, and analyze the relationship between antioxidant activity and total phenolic and total flavonoid compounds.
4. Acknowledgements
The present research was conducted by the research fund of Dankook University in 2008.
REFERENCES
- J.-M. Ko, N.-R. An and H.-C. Cha, “Plant Regeneration of Calystegia soldanella by Tissue Culture of Nods,” Horticulture Environment and Biotechnology, Vol. 49, No. 5, 2008, pp. 343-346.
- E. M. M. Gaspar, “New Pentasaccharide Macrolactone from the European Convolvulaceae Calytegia soldanella,” Tetrahedron Letters, Vol. 40, No. 37, 1999, pp. 6861-6864.
- M. Tori, Y. Ohara, K. Nakashima and M. Sono, “Caffeic and Coumaric Acid Esters from Calystegia soldanella,” Fitoterapia, Vol. 71, No. 4, 2000, pp. 353-359.
- J. Li, T. M. Ou-Lee, R. Raba, R. G. Amundson and R. L. Last, “Arabidopsis Flavonoid Mutants Are Hypersensitive to UV-B Irradiation,” Plant Cell, Vol. 5, 1993, pp. 171-179.
- J. B. Harborne and C. A. Williams, “Advances in Flavonoid Research since 1992,” Phytochemistry, Vol. 55, No. 6, 2000, pp. 481-504. Hdoi:10.1016/S0031-9422(00)00235-1
- P.-G. Pietta, “Flavonoids as Antioxidants,” Journal of National Products, Vol. 63, No. 7, 2000, pp. 1035-1042. Hdoi:10.1021/np9904509
- K. Chaudhuri, S. Das, M. Bandyopadhyay, A. Zalar, A. Kollmann, S. Jha and D. Tepfer, “Transgenic Mimicry of Pathogen Attack Stimulates Growth and Secondary Metabolite Accumulation,” Transgenic Research, Vol. 18, No. 1, 2009, pp. 121-134. Hdoi:10.1007/s11248-008-9201-8
- L. Hooper, P. A. Kroon, E. B. Rimm, J. S. Cohn, I. Harvey, K. A. L. Cornu, J. J. Ryder, W. L. Hall and A. Cassidy, “Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials,” The American Journal of Clinical Nutrition, Vol. 88, No. 1, 2008, pp. 38-50.
- J. B. Harborne, “Comparative Biochemistry of the Flavonoids,” Academic Press, London, 1967.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, “Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids,” Free Radical Biology and Medicine, Vol. 20, No. 7, 1996, pp. 933-956. Hdoi:10.1016/0891-5849(95)02227-9
- A. Solovchenko and M. Schmitz-Eiberger, “Signification of Skin Flavonoids for UV-B-Protection in Apple Fruits,” Journal of Experimental Botany, Vol. 54, No. 389, 2003, pp. 1977-1984. Hdoi:10.1093/jxb/erg199
- J.-H. Mun and C.-W. Park, “Flavonoid Chemistry of Polygonum sect. Tovara (Polygonaceae): A Systematic Survey,” Plant Systematics and Evolution, Vol. 196, No. 3-4, 1995, pp. 153-159. Hdoi:10.1007/BF00982956
- K. R. Markham, “Techniques of Flavonoid Identification,” Academic Press, New York, 1982.
- J. B. Harborne and C. A. Williams, “Anthocyanins and Other Flavonoids,” Natural Product Reports, Vol. 18, No. 3, 2001, pp. 310-333. Hdoi:10.1039/b006257j
- V. L. Singleton, R. Orthofer and R. M. LamuelaRaventós, “Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of FolinCiocalteu Reagent,” Methods in Enzymology, Vol. 299, 1999, pp. 152-178. Hdoi:10.1016/S0076-6879(99)99017-1
- M. V. Eberhardt, C. Y. Lee and R. H. Liu, “Antioxidant Activity of Fresh Apples,” Nature, Vol. 405, No. 6789, 2000, pp. 903-904.
- Y. Menemen, C. A. Williams and S. L. Jury, “Flavonoid Patterns in Convolvulus L., (Convolvulaveae) Species from Morocco,” Pakistan Journal of Botany, Vol. 34, No. 3, 2002, pp. 291-295.
- F. Tatsuzawa, Y. Mikanagi and N. Saito, “Flower Anthocyanins of Calystegia in Japan,” Biochemical Systematics and Ecology, Vol. 32, No. 12, 2004, pp. 1235- 1238. Hdoi:10.1016/j.bse.2004.05.008
- F. Shahidi and M. Naczk, “Methods of Analysis and Quantification of Phenolic Compounds,” Technomic Publishing Company, Lancaster, 1995, pp. 287-293.

