American Journal of Plant Sciences
Vol.3 No.5(2012), Article ID:19478,4 pages DOI:10.4236/ajps.2012.35071
Induction of Defensive Responses in Eucalyptus globulus (Labill) Plants, against Ctenarytaina eucalypti (Maskell) (Hemiptera: Psyllidae)
![]()
1Facultad de Ciencias Forestales, Universidad de Concepcion, Concepcion, Chile; 2Departamento de Botanica, Facultad de Ciencias Naturales y Oceanograficas, Universidad de Concepcion, Concepcion, Chile; 3Centro de Biotecnologia, Laboratorio de Cultivo de Tejidos Vegetales, Universidad de Concepcion, Concepcion, Chile; 4Departamento de Quimica, Facultad de Ciencias, Universidad de Chile, Santiago, Chile.
Email: *christroncoso@udec.cl
Received August 31st, 2011; revised September 14th, 2011; accepted October 11th, 2011
Keywords: Chemical Defense; E. globules; Secondary Metabolites; Terpenes
ABSTRACT
This study evaluated the expression of defense compounds from the secondary metabolism of Eucalyptus globulus plants, subjected to direct and indirect stimuli by the insect Ctenarytaina eucalypti (blue gum Psyllid). Results showed that defense responses were activated in plants in all tested cases. Were detected and identified thirty-two compounds in the leaves of treated plants, of which five compounds differed with the control, and all are part of the chemical defenses from the plants, three of them were oxygenated monoterpenes (borneol, exo-2-hydroxy cineole and thymol), a aromatic carboxylic acid (benzoic acid) and a quinone (6-acethyl-flaviolin). The plants induced by volatile compounds and by indirect entomological manner, showed its capability to synthesize defensive compounds without a wound that promotes these responses. Were also found some constitutive secondary metabolites over expressed in the different inductions compared with the control.
1. Introduction
A set of signals are triggered when a plant has any damage caused by a biotic or abiotic agent. These signals provide complex responses in accordance to the nature of the causative agent [1].
These signals are transmitted in a systematic way to the whole plant [2]. They may be chemical (oligosaccharides, abscisic acid or sistemin) or physical (electrical signs) [3,4]. In response to these signals, plants are able to synthesize toxic or repellent metabolites with antimicrobial, antifungal and insecticidal activity [5-7].
Secondary metabolites like terpenes, alkaloids, phenols [8] and volatile compounds are involved in direct or indirect defense of plants against phytopathogen actions are among the highlights synthesized metabolites.
Has been found that plants are able to differentiate the wounds caused by biotic or abiotic agents what implies the recognition and defense response in each case [9]. This fact was confirmed by different researchers in studies performed to Arabidopsis thaliana, Nicotiana attenuata and Populus trichocarpa × deltoides, where they found out that plant were able to differentiate between a mechanical abiotic damage and one caused by insects. This ability of discrimination, prevent unnecessary energy consumption in the biosynthesis of defense compounds against herbivores [10-12].
It has also been found that plants have an alternative defense system known as “indirect defense”. When a plant is attacked by a phytopathogen it releases volatile terpenes phenolics type compounds or/and derived from phenyl propane which act as signals that attract pathogen’s parasitoids and predator [13,14].
Parasitoids attraction was experimentally proved in laboratory by Bukovinszky et al., [15] and Lou et al., [16]. It was also proved in natural conditions through studies performed by Kessler and Baldwin [17]. They quantified the mix of three compounds (cis-3-hexen-1-ol, linalool and cis-α-bergamotene) in Nicotinia attenuata, the results showed that this compounds increase the predation level of Manduca sexta eggs (Lepidoptera: Sphingidae), by Geocoris pallens (Hemiptera: Lygaeidae), a general predator.
Among the stimuli that induce the release of volatile compounds are the biotic external factors. It has been tested that volatiles from flowers and leaves of undamaged plants show different emission pattern of those which have been attacked by herbivores [18,19].
Thus, plants continuously release small amounts of volatile compounds, but this amount will increase when herbivorous insects attack and damage the plants. Studies with several plant-insect combinations have shown that insect feeding or application of oral secretions of them in spots where it has caused an injury; induce a different or more intense response of volatile than attributable solely to mechanical damages [19].
Other studies such as those performed with larvae of Helicoverpa zea, have shown that salivary secretions of insects qualitatively affect the defense response in plants [20]. However, experiments performed by Mithöfer et al., [21] which used mechanical devices that mimic the damage caused by Spodoptera littoralis on the leaves of Phaseolus lunatus showed that wounds induced the emission of volatile compounds similar to those emissions from the damage caused by the insect.
In ours atudy, we selected to Ctenarytaina Eucalypti (blue gum Psyllid), specie present in Chile since 1999 [22], one of the best known of the genus Ctenarytaina, due to the economic importance of some of their hosts, showing a marked preference for species of the genus Eucalyptus, within which is E. globulus [23,24]. E. globulus is one of the most abundant species introduced to Chile by the forestry sector, with more than 458,611 hectares planted.
However in Chile there are not studies, focused on the knowledge about of inducible defense mechanisms, of E. globulus specie against the C. eucalypti action. The species of Eucalyptus genus accumulate essential oils in the glands of those leaves that have shown antifungal, antimicrobial and insecticidal activity [6,25,26], which facilitates a study of this type.
The main objective of this work was to clarify and evaluate E. globulus capability to express secondary metabolic defense responses against stimuli caused direct and indirectly by C. eucalypti.
2. Materials and Methods
2.1. Plant Collection
Thirty-six plants of E. globulus were produced from seeds to develop the project. Seeds were put in water for 24 hours, later they were sown in a plant tray with pine bark substrate which was sterilized previously at 80˚C in an oven for 24 hours. The system was watered twice a day with a manual sprayer. Seeds emerge 6 days later and the fertilization began when they had the first real two leaves (N: 9.75 gr., P: 3.45 ml. y K: 2.5 gr. per liter of water). Fertilization was performed for two weeks using 2 ml of the solution. Irrigation was held three times a day (40 ml per plant).
2.2. Field Collection
Insects were collected from the Malven farm every day from a E. globulus plantation which had a high level of infestation. The farm is located 12 Km west of the city of Mulchén (Latitude 37˚42'6.49''S, Longitude 72˚21'28.83'' W, datum 1984), Bío Bío region. The insects corresponded to those described by Maskell [27]. Insects were treated and placed in cylinders made of polyester film PROFIM® (4 cm in diameter × 10 cm long), they are close on one side with a tulle (0.34 µm). The capture of insects was carried out with the help of an entomological aspirator and then transferred five adult insects per polyester cylinder.
2.3. Treatments
Once the plants reached 50 cm tall they were randomized obtaining 9 plants from each of the following treatments:
T0: Control treatment.
T1: Direct entomological induction. Plants of this treatment were exposed to the direct attack by the insect. A polyester cylinder was put and ties with five adults of C. eucalypti in the apical section of each of the 9 plants.
T2: Indirect entomological induction. Application of C. eucalypti macerate. The macerate was obtained by grinding 30 insects in 2 ml. of deionized water; the solution was applied with a paintbrush to the first 6 leaves of the apical section of each of the 9 plants.
T3: Induction by exposure to volatile. Plants from this treatment were subjected to stay with a group of 27 plants, also E. globulus, massively attacked by C. eucalypti and separated by a tulle (0.34 µm) in a chamber built with PVC tubes (20 mm ø) and closed with greenhouse plastic (0.2 mm thick), (1 m length × 1 m wide × 0.9 m in height).
Each induction was applied for periods of 24 hours, followed by 48 hours without induction. The cycle was repeated nine times for each treatment.
2.4. Chemical Analysis
Once the third, sixth, and ninth inductions of each treatment were completed, two leaves were extracted from the apical section of each plant in each treatment. The leaves were sectioned manually and placed in vials with 50 mL n-hexane and stored at 4˚C for 48 hours, for the extraction of compounds. Later, chromatographic profiles were obtained in a gas chromatograph Agilent 6890 A with an FID detector and a chromatograph Hewlett Packard Mod. 5890 Series II (California, USA) with a mass detector HP model 5972. The gas carrier was helium, with a flux of 1 mL·seg–1; injector temperature was 275˚C; detector temperature was 300˚C; and the program for oven temperature was 60˚C for the first 5 min, increasing 10˚C per minute until reaching 275˚C, with a final time of 15 min. A 30-m chromatographic fused silica column HP-5MS (J&W Scientific) with an internal diameter of 0.25 mm and a phase thickness of 0.25 μm was used to compare the retention times with commercial standards (SIGMA) and the database EPA-NIST 98 was used to compare the mass spectra.
3. Results and Discussion
Thirty-two compounds were found and identified (Figure 1). Mainly oxygenated monoterpenes, an aromatic carboxylic acid and a quinone, were also found but in less amount (Table 1). In the T1 treatment, (direct entomological induction), the plants expressed four different compounds, in comparison with the control. They were; borneol, exo-2-hydroxy cineole, thymol and the quinone 6-acethyl-flaviolin. For the treatment T2, (indirect ento-
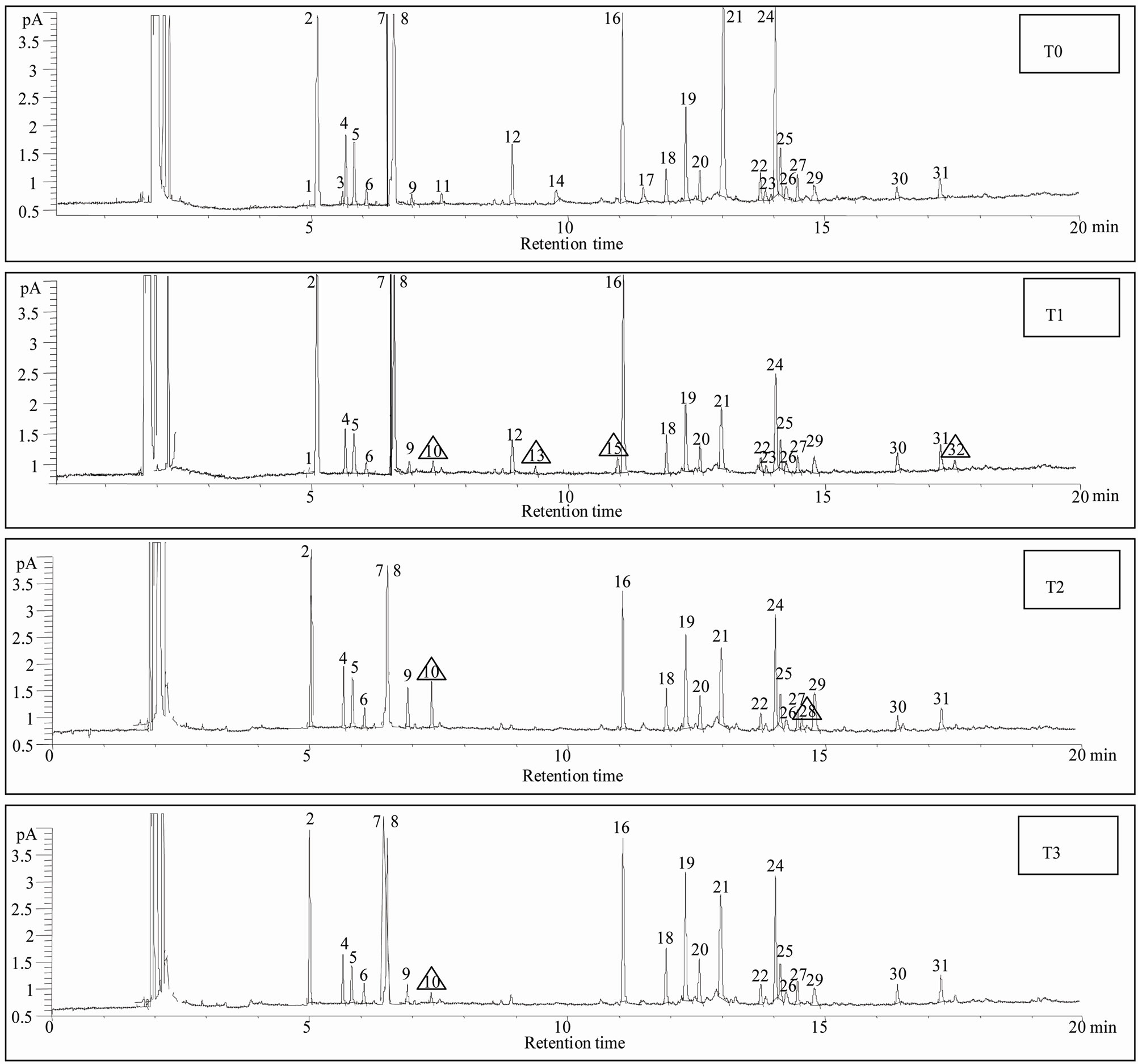
Figure 1. Chromatograms with peaks of detected compounds: (T0) control, (T1) direct entomological induction (T2) indirect entomological induction, (T3) induction by exposure to volatile compound. The triangles indicate those compounds in which the treatment differed from the control (see Table 1).

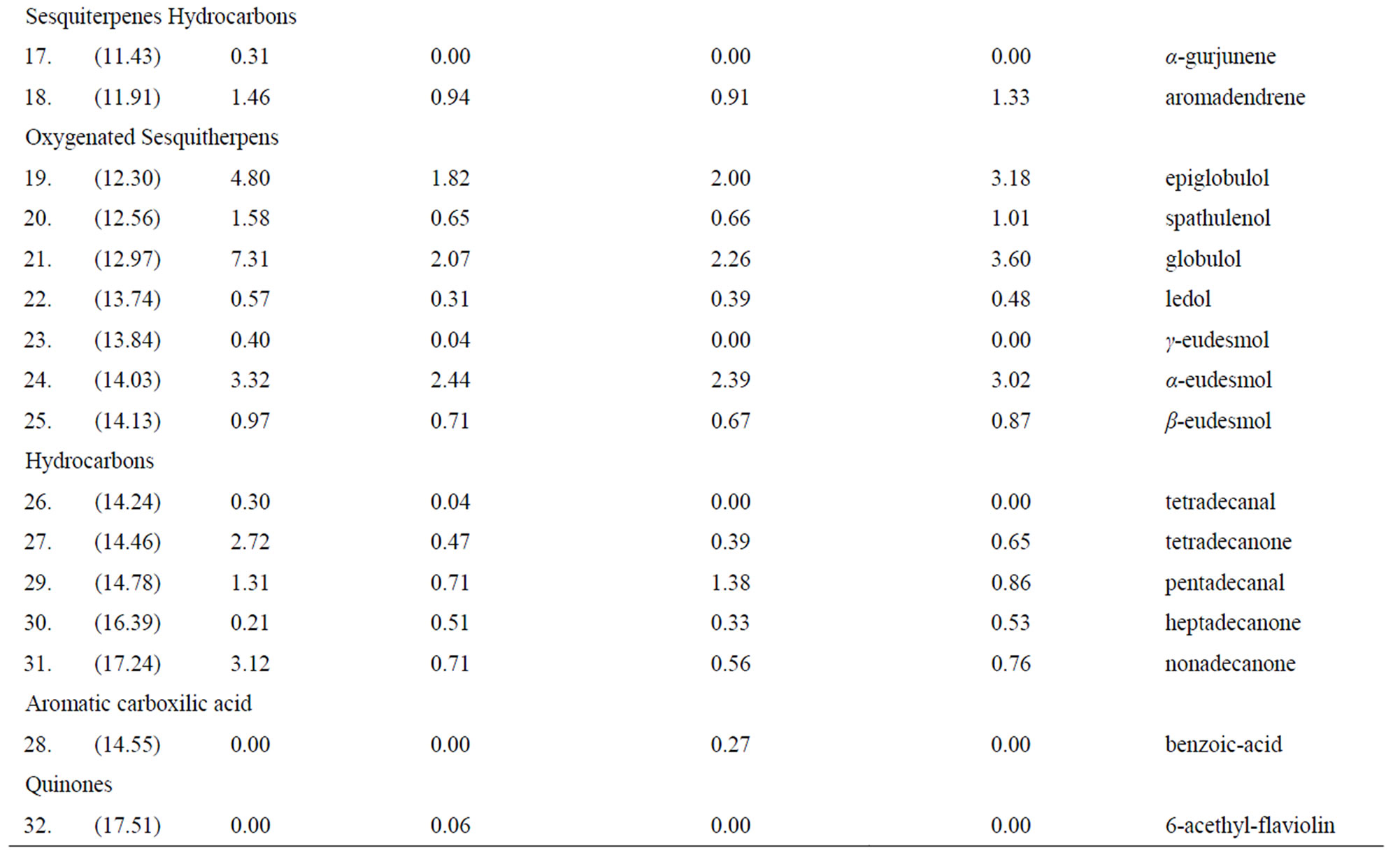
Table 1. GC-MS, identification of compounds.
mological induction) plants synthetized two compounds that differed from the control; borneol and benzoic acid. In T3 treatment (induction by exposure to volatile) plants synthesized borneol, the only one compound which was not detected in the control.
The biosynthesis of specific compounds (Figure 1), as is the case of oxygenated monoterpenes exo-2-hydroxy cineole, thymol and the quinone 6-acetyl-flaviolin, presented only in treatment with direct entomological induction (T1), consistent with those found by Reymond et al. [28] in Arabidopsis thaliana plants treated with larvae of Pieris rapae and by Korth and Dixon [29], in Solanum tuberosum, induced with larvae of Manduca sexta. This selectivity in the expression of compounds is attributable only to changes in gene expression and in most defensive secondary metabolites [1].
Effects of oral secretions from herbivorous insects, have considered the role of insect saliva in plant-insect interactions, which enables the plant to differentiate insect damage from mechanical damages. For example, by damaging Tobacco plants (Nicotiana tabacum) with larvae of Helicoverpa zea, in that the salivary gland was removed, they have shown evidences that salivary secretions qualitatively affect the defense responses of plant against this insect [20]. Recently, a new class of sulfated fatty acid called caeliferin was identified in oral secretions of Shistocerca americana, caeliferins induce the release of volatile terpenes in corn seedlings [30]. The background stated above are closely related to the response found in T2 (indirect entomological induction) and benzoic acid biosynthesis.
This volatile compound has been documented in chemical defenses of the plants, in studies of beetles that attack willow leaves [31-33]. It is very likely that the expression of this compound is due, to the insect macerate used for this induction has had compounds belonging to the saliva of C. eucalypti insect, and these have been perceived by the treated plants, promoting the synthesis of benzoic acid.
The oxygenated monoterpene borneol, detected in the treatments of direct entomological induction (T1), indirect entomological induction (T2) and also in the induction by exposure to volatile (T3) has been described as an effective repellent insects [34]. The presence of this compound in these three treatments, places it as widespread action to the different biotic stimuli.
Some monoterpene hydrocarbons, which although are present in the control treatment, they were presented at a higher concentration (overexpression), at least in one of the other treatments (Table 1). This is the case of α- pinene and p-cymene, which showed a higher relative concentration in T1, T2 and T3, these compounds have a A proven insecticide effect. In the case of α-pinene during studies on adult females of Musca domestica [35] and p-cymene as adulticide females and larvae of Thrips Frankliniella occidentalis [36], while the carene compound that showed a higher relative concentration in T2 and T3 is a volatile compound that acts within the indirect defenses of the plant, causing the attraction of insect predators of insects that cause damage to the plant [37].
The same happened with the oxygenated monoterpenes cineole, linalool and α-terpenil-acetate. The compound cineole which was overexpressed in T1, T2 and T3, as linalool that was overexpressed in T2 possess insecticidal effect [6], while α-terpenil-acetate, with repellent effectiveness [38], was overexpressed in T1, T2 and T3.
Although the results of this study demonstrate the capability of plants to express defensive responses to different stimuli, there remain at least three stages of research to develop, those that allow the application of knowledge found. A major research phase to develop, is to isolate from the saliva of the insect C. eucalypti, which are the compounds that act as chemical signal, recognizable by plants and that trigger defensive responses from the secondary metabolism, as the compound “volicitina” isolated from oral secretions of Spodoptera exigua larvae, a substance that has the capability to induce the release of volatile compounds in corn plants, found by Halitschke et al., (2001) [39], or as the studios made by Schmelz et al., (2007) [40] which sought to induce ethylene production in Vigna unguiculata plants and identified proteolytic fragments of the subunit ATP synthase-g into oral secretions from larvae of Spodoptera frugiperda.
An important aspect in a second stage of research is to determine whether these compounds from oral secretions of the insect C. eucalypti can be replicated synthetically, in order to apply them in young plants to induce defense responses artificially.
Be seen from the above a third stage of research, unavoidable, which is the determination of the persistence of defensive responses in time (as an immunological memory). While, the defensive success of a plant against an attack provoked by a pathogen or an insect is based on the speed with which the plant is able to recognize aggression and trigger the defensives chemical responses, there an unresolved question yet, is whether the plants remain predisposed to react more rapidly to future attacks, acquiring a certain level of resistance (systemic) higher, at least during the first years of life that are most important to protect. These are questions to be resolved in future studies.
4. Conclusions
The E. globulus plants, induced by different types of entomological stimuli, expressed defense compounds coming from the secondary metabolism. The biosynthesis of defense compounds in plants with inductions in which there was not a wound in the tissues, show their capability to perceive stimuli and express defensive compounds, without any previous mechanical damage.
Although most of the detected compounds are constitutive secondary metabolites of E. globulus species, was observed an over expression in their relative concentrations in front of the different performed inductions, when were compared with the control, while a smaller number of compounds were synthesized in plants only in front to stimuli caused.
Considering that in our country, a large number of plants of E. globulus are produced by vegetative propagation, the importance of the results found, challenges us to continue studies to elucidate whether these defensive responses persists in the vegetative propagation, in order to be able to produce seedlings with a higher level resistance to C. eucalypti, from mother plants previously induced.
5. Acknowledgements
We thank CONICYT and the Graduate Council of the University of Concepcion for funding granted during the development of Mr. Troncoso’s doctoral studies, CONICYT INSERCION 79100025 and Proyecto de Financiamiento Basal PFB-27.
REFERENCES
- G. Camarena, “Signals in Plant Insect Interaction,” Magazine Chapingo Series of Forestry and Environment Sciences Series, Vol. 15, No. 1, 2009, pp. 81-85.
- L. Mattiacci, B. Rocca, N. Scascighini, M. D’alessandro, A. Hern and S. Dorn, “Systemically Induced Plant Volatiles Emitted at the Time of Danger,” Journal of Chemical Ecology, Vol. 27, No. 11, 2001, pp. 2233-2252. doi:10.1023/A:1012278804105
- P. Pare and J. Tumlinson, “Plant Volatiles as a Defense against Insect Herbivores,” Plant Physiology, Vol. 121, No. 2, 1999, pp. 325-331. doi:10.1104/pp.121.2.325
- F. Gozzo, “Systemic Acquired Resistance in Crop Protection: From Nature to a Chemical Approach,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 16, 2003, pp. 4487-4503. doi:10.1021/jf030025s
- E. Shaaya, M. Kostjukovski, J. Eilberg and C. Sukprakarn, “Plant Oils as Fumigants and Contact Insecticides for the Control of Stored-Product Insects,” Journal Stored Products Research, Vol. 33, No. 1, 1997, pp. 7-15. doi:10.1016/S0022-474X(96)00032-X
- B. Lee, W. Choi, S. Lee and B. Park, “Fumigant Toxicity of Essential Oils and Their Constituent Compounds towards the Rice Weevil Sitophilus oryzae (L.),” Crop Protection, Vol. 20, No. 4, 2001, pp. 317-320. doi:10.1016/S0261-2194(00)00158-7
- A. Tapondjou, C. Adler, D. Fontem, H. Bouda and C. Reichmuth, “Bioactivities of Cymol and Essential Oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val,” Journal Stored Products Research, Vol. 41, No. 1, 2005, pp. 91-102. doi:10.1016/j.jspr.2004.01.004
- L. Taiz and E. Zeiger, “Secondary Metabolites and Plant Defense,” In: L. Taiz and E. Zeiger, Eds., Plant Physiology, Sinauer Associates, Inc., Sunderland, 2006, pp. 283- 308.
- I. Baldwin, R. Halitschke, A. Kessler and U. Schittko, “Merging Molecular and Ecological Approaches in PlantInsect Interactions,” Current Opinion in Plant Biology, Vol. 4, No. 4, 2001, pp. 351-358. doi:10.1016/S1369-5266(00)00184-9
- M. De Vos, V. Van Oosten, R. Van Poecke, J. Van Pelt and M. Pozo, “Signal Signature and Transcriptome Changes of Arabidopsis during Pathogen and Insect Attack,” Molecular Plant-Microbe Interaction, Vol. 18, No. 9, 2005, pp. 923-937. doi:10.1094/MPMI-18-0923
- C. Voelckel, W. Weisser and I. Baldwin, “An Analysis of Plant-Aphid Interactions by Different Microarray Hybridization Strategies,” Molecular Ecology, Vol. 13, No. 10, 2004, pp. 3187-3195. doi:10.1111/j.1365-294X.2004.02297.x
- I. Major and C. Constabel, “Molecular Analysis of Poplar Defense against Herbivory: Comparison of Woundand Insect Elicitor-Induced Gene Expression,” New Phytologist, Vol. 172, No. 4, 2006, pp. 617-635. doi:10.1111/j.1469-8137.2006.01877.x
- J. Knudsen, L. Tollsten and L. Bergstrom, “Floral Scent— A Checklist of Volatile Compounds Isolated by HeadSpace Techniques,” Phytochemistry, Vol. 33, No. 2, 1993, pp. 253-280. doi:10.1016/0031-9422(93)85502-I
- N. Dudareva, E. Pichersky and J. Gershenzon, “Biochemistry of Plant Volatiles,” Plant Physiology, Vol. 135, No. 4, 2004, pp.1893-1902. doi:10.1104/pp.104.049981
- T. Bukovinszky, M. Posthumus, L. Vet and J. Lenteren, “Variation in Plant Volatiles and Attraction of the Parasitoid Diadegma semiclausum (Hellen),” Journal of Chemical Ecology, Vol. 31, No. 3, 2005, pp. 461-480. doi:10.1007/s10886-005-2019-4
- Y.-G. Lou, B. Ma and J.-A. Cheng, “Attraction of the Parasitoid Anagrus nilaparvatae to Rice Volatiles Induced by the Rice Brown Planthopper Nilapavata lugens,” Journal of Chemical Ecology, Vol. 31, No. 10, 2005, pp. 2357-2372. doi:10.1007/s10886-005-7106-z
- A. Kessler and I. Baldwin, “Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature,” Science, Vol. 291, No. 5511, 2001, pp. 2141-2144. doi:10.1126/science.291.5511.2141
- C. De Moraes, M. Mescher and J. Tumlinson, “Caterpillar-Induced Nocturnal Plant Volatiles Repel Nonspecific Females,” Nature, Vol. 410, No. 6828, 2001, pp. 577-580. doi:10.1038/35069058
- G. Arimura, C. Kost and W. Boland, “Herbivore-Induced, Indirect Plant Defences,” Biochimica et Biophysica Acta, Vol. 1734, No. 2, 2005, pp. 91-111.
- R. Musser, E. Farmer, M. Peiffer, S. Williams and G. Felton, “Ablation of Caterpillar Labial Salivary Glands: Technique for Determining the Role of Saliva in InsectPlant Interactions,” Journal of Chemical Ecology, Vol. 32, No. 5, 2006, pp. 981-992. doi:10.1007/s10886-006-9049-4
- A. Mithöfer, G. Wanner and W. Boland, “Effects of Feeding Spodoptera littoralis on Lima Bean Leaves. II. Continuous Mechanical Wounding Resembling Insect Feeding Is Sufficient to Elicit Herbivory-Related Volatile Emission,” Plant Physiology, Vol. 137, No. 3, 2005, pp. 1160-1168. doi:10.1104/pp.104.054460
- T. Olivares, “Ctenarytaina eucalypti (Maskell 1890): The Blue Gum Psyllid in Chile (Hemiptera: Sternorryncha: Psylloidea: Spondyliaspininae),” Gayana, Vol. 64, No. 2, 2000, pp. 239-241.
- F. Rodriguez y F. Saiz, “Parasitoidism of Psyllaephagus pilosus Noyes (Hym.: Encyrtidae) on the Blue Gum Psyllid, Ctenarytaina eucalypti (Maskell) (Hem.: Psyllidae) in V Region Eucalypts Plantations,” Chilean Journal of Agricultural Research, Vol. 66, No. 4, 2006, pp. 342-351.
- E. Brennan, S. Weinbaum, J. Rosenheim and R. Karban, “Heteroblasty in Eucalyptus globulus (Myricales: Myricaceae) Affects Ovipositonal and Settling Preferences of Ctenarytaina eucalypti and C. spatulata (Homoptera: Psyllidae),” Environmental Entomology, Vol. 30, No. 6, 2001, pp. 1144-1149. doi:10.1603/0046-225X-30.6.1144
- H. Ramezani, H. Singh, D. Batish and R. Kohli, “Antifungal Activity of the Volatile Oil of Eucalyptus citridora,” Fitoterapia, Vol. 73, No. 3, 2002, pp. 261-262. doi:10.1016/S0367-326X(02)00065-5
- G. Sacchetti, S. Maietti, M. Muzzoli, M. Scaglianti, S. Manfredini, M. Radice and R. Bruni, “Comparative Evaluation of 11 Essential Oils of Different Origin as Functional Antioxidants, Antiradicals and Antimicrobials in Foods,” Food Chemistry, Vol. 91, No. 4, 2005, pp. 621- 632. doi:10.1016/j.foodchem.2004.06.031
- D. Burckhardt, “Generic Key to Chilean Jumping PlantLice (Homoptera: Psylloidea) with Inclusion of Potential Exotic Pests,” Revista Chilena de Entomologia, Vol. 21, No. 5, 1994, pp. 57-67.
- P. Reymond, H. Weber, M. Damond and E. Farmer, “Differential Gene Expression in Response to Mechanical Wounding and Insect Feeding in Arabidopsis,” Plant Cell, Vol. 12, No. 5, 2000, pp. 707-720.
- K. Korth and R. Dixon, “Evidence for Chewing InsectSpecific Molecular Events Distinct from a General Wound Response in Leaves,” Plant Physiology, Vol. 115, No. 4, 1997, pp. 1299-1305.
- H. Alborn, T. Turlings, T. Jones, G. Stenhagen, J. Loughrin and J. Tumlinson, “An Elicitor of Plant Volatiles from Beet Armyworm Oral Secretion,” Science, Vol. 276, No. 5314, 1997, pp. 945-949. doi:10.1126/science.276.5314.945
- J. Smiley, J. Horn and N. Rank, “Ecological Effects of Salicin at Three Trophic Levels: New Problems from Old Adaptations,” Science, Vol. 229, No. 4714, 1985, pp. 649- 651. doi:10.1126/science.229.4714.649
- J. Pasteels, M. Rowell-Rahier and M. Raupp, “PlantDerived Defense in Chrysomelid Beetles,” In: P. Barbosa and D. K. Letourneau, Eds., Novel Aspects of Insect— Plant Interactions, John Wiley & Sons, Inc., New York, 1988, pp. 235-272.
- R. Denno, S. Larsson and K. Olmstead, “Role of Enemy Free Space and Plant Quality in Host-Plant Selection by Willow Beetles,” Ecology, Vol. 71, No. 1, 1990, pp. 124- 137. doi:10.2307/1940253
- M. Jacobson, “Glossary of Plant-Derived Insect Deterrents,” 1990. http://www.scielo.br/scielo.php?script=sci_nlinks&ref=15740184&pid=S0100-204X200500100000200007&lng=en
- M. Leyva, J. Tacoronte, M. Marquetti and D. Montada, “Insecticidal Activity of 3 Essential Oils of Plants in Domestic Musca (Diptera: Muscidae),” Revista Cubana de Medicina Tropical, Vol. 60, No. 3, 2008. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0375-07602008000300005&lng=es&nrm=iso
- A. Janmaat, W. Jan de Kogel and E. Woltering, “Enhanced Fumigant Toxicity of p-Cymene against Frankliniella occidentalis by Simultaneous Application of Elevated Levels of Carbon Dioxide,” Pest Management Science, Vol. 58, No. 2, 2002, pp. 167-173. doi:10.1002/ps.432
- G. Corrado, R. Sasso, M. Pasquariello, L. Iodice, A. Carretta, P. Cascone, L. Ariati, M. Digilio, E. Guerrieri and R. Rao, “Systemin Regulates Both Systemic and Volatile Signaling in Tomato Plants,” Journal of Chemical Ecology, Vol. 33, No. 4, 2007, pp. 669-681. doi:10.1007/s10886-007-9254-9
- B. Joseph, and R. Priya, “Phytochemical and Biopharmaceutical Aspects of Psidium guajava (L.) Essential Oil: A Review,” Research Journal of Medicinal Plant, Vol. 5, No. 4, 2011, pp. 432-442. doi:10.3923/rjmp.2011.432.442
- R. Halitschke, U. Schittko, G. Pohnert, W. Boland and I. Baldwin, “Molecular Interactions between the Specialist Herbivore Manduca Sexta (Lepidoptera, Sphingidae) and Its Natural Host Nicotiana attenuata. III. Fatty AcidAmino Acid Conjugates in Herbivore Oral Secretions Are Necessary and Sufcient for Herbivore-Specific Plant Responses,” Plant Physiology, Vol. 125, No. 2, 2011, pp. 711-717. doi:10.1104/pp.125.2.711
- E. Schmelz, M. Carroll, S. Leclere, S. Phipps and J. Meredith, “Fragments of ATP Synthase Mediate Plant Perception of Insect Attack,” Proceedings of the National Academy of Sciences USA, Vol. 103, No. 23, 2006, pp. 8894-8899. doi:10.1073/pnas.0602328103
NOTES
*Corresponding author.

