Modern Research in Catalysis
Vol.3 No.3(2014), Article
ID:48238,8
pages
DOI:10.4236/mrc.2014.33013
Fischer-Tropsch Synthesis of the Promoted Co/ZSM-5 Hybrid Catalysts for the Production of Gasoline Range Hydrocarbons
Suk-Hwan Kang1*, Jae-Hong Ryu1, Jin-Ho Kim1, Hyo-Sik Kim1, Chan-Gi Lee1, Yun-Jo Lee2, Ki-Won Jun2
1Plant Engineering Center, Institute for Advances Engineering (IAE), Yongin-si, Korea
2Petroleum Displacement Technology Research Center, Korea Research Institute of Chemical Technology (KRICT), Yuseong, Daejeon, Korea
Email: *shkang@iae.re.kr
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 25 May 2014; revised 25 June 2014; accepted 2 July 2014
ABSTRACT
Fischer-Tropsch synthesis (FTS) reaction for the direct production of gasoline range hydrocarbons (C5-C9) from syngas was investigated on Ru, Pt, and La promoted Co/ZSM-5 (Si/Al = 25) catalysts. The hybrid catalysts were characterized by BET surface area, XRD, H2-TPR, NH3-TPD and XPS analyses. These physico-chemical properties were correlated with activity and selectivity of the catalysts. The promoted Co/ZSM-5 hybrid catalysts were found to be superior to the unpromoted Co/ZSM-5 catalyst in terms of better C5-C9 selectivity. Pt-Co/ZSM-5 exhibited the highest catalytic activity because of the small cobalt particle size.
Keywords:Fischer-Tropsch Synthesis, Cobalt Hybrid Catalyst, Promoter, Gasoline, ZSM-5, Syngas

1. Introduction
The production of olefins by Fischer-Tropsch synthesis (FTS), using syngas derived from coal, natural gas or other carbon-containing materials, has recently received considerable attention for obtaining clean fuels and chemicals as a consequence of fast depletion of crude oil. Thus, FTS has emerged as one of the most promising routes to solve the energy crisis [1] . However, the polymerization mechanism (known as Anderson-Schulz-Flory (ASF) distribution) of FTS reaction is inherently found to yield a wide-range of hydrocarbon distribution from methane to heavy waxy products as the distribution is governed by the chain growth probability. In general, the selectivity towards gasoline range products is known to be limited to a maximum of 48 mol% [2] . To obtain branched hydrocarbon selectively through FTS reaction directly without any upgradation, especially for highoctane gasoline production, many intensive efforts have been made by modifying cobalt-based catalysts with the addition of acidic components. Some researchers have investigated the hybrid or composite systems consisting of cobalt-based FTS catalysts and acidic zeolites as cracking catalysts by using the following preparation methods: 1) cobalt-based FTS catalyst physically mixed with zeolites (It is prepared easily; but molding is difficult); 2) zeolite supported cobalt-based hybrid catalyst prepared by wet-impregnation method (the large quantities production is possible, but the concentration of cobalt is limited and zeolite can be changed to amorphous phase by metal precursor solution of a strong acid); and 3) zeolite membrane-coated Co/SiO2 catalyst (It has excellent performance of the catalyst, but it is difficult to get large quantities production) [3] -[8] . However, marginal success has been achieved leaving scope for further improvement.
In the present investigation, we studied the promoted Co/ZSM-5 catalytic systems for the direct production of gasoline range hydrocarbons from syngas by using the promoted Co/ZSM-5 hybrid catalysts. The presence of weak acid sites on Co/ZSM-5 catalyst with large pore size and small cobalt particle size are mainly responsible for showing a high catalytic performance due to the high reducibility of cobalt particles with the presence of large amount of acid sites on Co/ZSM-5 catalyst possessing a low Si/Al ratio of 15 [3] . The objective of the present investigation is to find the effect of the addition of promoter on the performance of the hybrid catalyst during FTS reaction. The catalysts were characterized by surface area, pore size distribution, temperature-programmed reduction (TPR), XRD, acidity (TPD of ammonia) and XPS measurements. An attempt is made to correlate the properties with activity and selectivity of the catalysts.
2. Experiments
2.1. Catalyst Preparation
The Co/ZSM-5 catalyst was prepared by the conventional wet-impregnation method using the proton-type ZSM- 5 (supplied by Zeolyst having Si/Al ratios of 25) with a cobalt nitrate precursor of required composition in deionized water solvent. The impregnation on ZSM-5 was carried out by stirring the slurry containing the zeolite continuously for 12 h at room temperature. The sample was subsequently dried in a rotary evaporator and then subjected to calcination at 500˚C in air for 5 h. The promoted catalysts were prepared by co-impregnation of the chloride or nitrate (Ru-nitrate, Pt-nitrate, Pt-chloride and La-nitrate) precursors of the promoter and cobalt nitrate of required concentrations. Drying and activation procedures were the same as described above. The ratio of cobalt to zeolite and the promoter metal component to that of ZSM-5 in the finished catalyst was fixed at 20/100 and 0.3/100 by weight.
2.2. Catalyst Characterization
The BET surface area was estimated from nitrogen adsorption isotherm data obtained at −196˚C on a Micromeritics, ASAP-2400 equipment. The calcined sample was degassed at 300˚C in a He flow for 4 h before the measurement. The pore volume and pore size distribution of the sample was determined by the BJH (Barett-Joyner-Halenda) model from the data of desorption branch of nitrogen isotherm. The powder X-ray diffraction (XRD) patterns of fresh and used catalysts were obtained with a Rigaku diffractometer using Cu-Kα radiation to identify the various phases of supported cobalt catalysts.
The temperature-programmed desorption of ammonia (NH3-TPD) experiments were performed to determine the surface acidity of various fresh FTS catalysts. Typically about 0.1 g of catalyst was flushed in a He flow at 250˚C for 2 h, cooled to 100˚C and saturated with NH3. After NH3 adsorption, the sample was purged with a He flow until equilibrium and the TPD run was carried out from 100˚C to 800˚C at a heating rate of 10˚C/min.
Temperature programmed reduction with hydrogen (H2-TPR) was performed to determine the reducibility of the supported metal oxides. Prior to the H2-TPR experiment, the sample was pretreated in helium flow up to 400 ˚C and kept for 2 h to remove the adsorbed water and other contaminants followed by cooling to 50˚C. The reducing gas containing 5% H2/Ar mixture was passed over the sample at a flow rate of 30 ml/min, with the heating rate of 10˚C/min, up to 850˚C.
The surface metal species and the carbon species with their electronic states after FTS reaction were characterized by using X-ray photoelectron spectroscopy (XPS; ESCALAB MK-II) analysis. During the experiment, the Al Ka monochromatized line (1486.6 eV) was adopted and the vacuum level was kept around 10−7 Pa. The XPS analysis was carried out with a step size of 0.02 eV and scanned twice. The powder samples after FTS reaction were previously passivated with 1% O2/He mixed gas and pressed in air atmosphere to thin pellet before characterization. The binding energy (BE) was corrected with the reference BE of C1s (284.4 eV).
2.3. Catalytic Activity Measurement
Catalytic activity test was carried out in a tubular fixed bed reactor (O.D. = 12.7 mm) with a catalyst of 0.3 g. Prior to the reaction, the catalyst was reduced at 450˚C for 12 h in a flow of 5% H2 balanced with nitrogen. After reduction, the synthesis gas (H2/CO = 2) was fed into the reactor. The FTS reaction was carried out subsequently under the following reaction conditions; T = 240˚C, P = 2.0 MPa and SV = 3000 ml/gcat/h. The effluent gas from the reactor was analyzed by an online gas chromatograph (Young Lin Acme 6000 GC) employing GS-GASPRO capillary column connected with flame ionized detector (FID) for the analysis of hydrocarbons and a Porapak Q/molecular sieve (5A) packed column connected with TCD for the analysis of carbon oxides, hydrogen, methane and internal standard gas of Ar.
3. Results and Discussion
In previous studies, we highlighted the influence of Si/Al ratio on the activity of impregnated Co/ZSM-5 hybrid catalysts. At higher Si/Al ratio (greater than 25), the reducibility of cobalt species and the density of weak acid sites were observed to be lower, thus reducing the CO conversion and C5-C9 selectivity [3] . In this study, therefore, we used ZSM-5 with Si/Al ratio of 25 as a support.
3.1. Textural Properties of Co/ZSM-5 Hybrid Catalysts
The BET surface area and BJH pore size distribution results are summarized in Figure 1 and Table1 As shown in Figure 1, the pores in all catalysts are the mesopores falling in the region of 2 - 5 nm. The intensity of dV/dlog(D) is almost similar for all catalysts. However, surface area of the promoted catalysts slightly increased compared to that of Co/ZSM-5 catalyst. Large specific surface area and pore volume of the catalyst affects its reducibility and the catalytic performance during FTS reaction.
3.2. Surface Acidity Measurement by NH3-TPD
The promoted Co/ZSM-5 hybrid catalysts were examined by NH3-TPD to elucidate the surface acidity. The effects of addition of promoters on surface acidity are possibly responsible for different catalyst performance, especially the product distribution. The desorption patterns of NH3 are shown in Figure 2 and the quantitative values of acidic sites (mmol NH3/g) are presented in Table2 The desorption peaks could be separated into three peaks, even though the resolution of peaks is not significant. The low temperature desorption peak (below 250˚C) is assigned to weak acidic sites and the high temperature desorption peak to strong acidic sites. The high temperature desorption peak is not responsible for FTS reaction and product distribution; it mainly contributes to water evolution by dehydration of hydroxyl groups on surfaces [9] . Therefore, by considering the first peak only (weak acid sites), it can be observed that the number of weak acid sites is higher on CoPt(N)/ZSM-5 hybrid catalyst. Further, the total number of acid sites also varied in order of Pt(N) > La(N) > Ru(N) > Pt(Cl), and the variation of acidity can be further related to the hydrocarbon selectivity of promoted Co/ZSM-5 hybrid catalysts.
3.3. Reducibility of Active Metals Measured by TPR
TPR experiments were carried out to understand the reduction behavior of cobalt oxides as the metallic cobalt surface sites are responsible for FTS activity. Figure 3 shows the H2-TPR profiles of the Co/ZSM-5 hybrid catalysts with different promoters. The TPR profiles of promoted Co/ZSM-5 hybrid catalysts exhibit two or three distinct peaks. In most of the promoted hybrid catalysts, the reduction process in H2 occurs in two distinct stages. The first stage is attributed to the reduction of Co3O4 to CoO and the second stage is assigned to the reduction of CoO to metallic cobalt.
In the case of Co/ZSM-5 hybrid catalysts with promoted Ru(N) and Pt(N), the TPR profiles show the first low
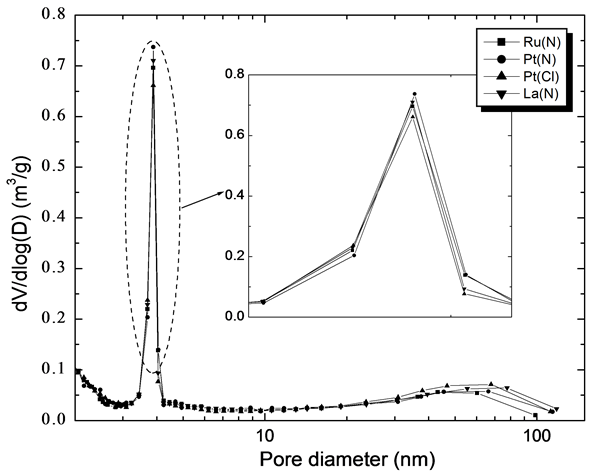
Figure 1. Pore size distribution of fresh Co/ZSM-5 hybrid catalysts.
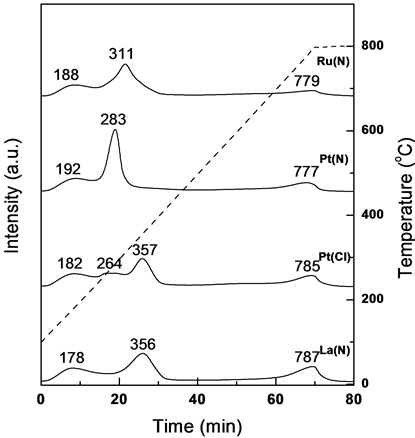
Figure 2. Results of NH3-TPD of fresh Co/ZSM-5 hybrid catalysts.
temperature peak around 120˚C. However, TPR profile of the Co-La(N)/ZSM-5 hybrid catalyst shows a distinctive shoulder peak around 270˚C and broad second peak with Tmax in the range of 316˚C - 359˚C. The small peak intensity at high temperature region (above 600˚C) suggests the facile reduction of cobalt oxide with
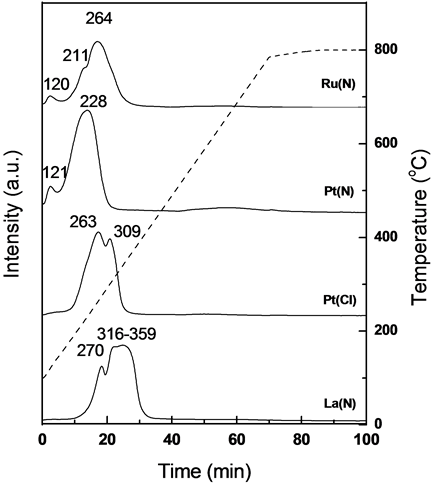
Figure 3. H2-TPR profiles of fresh Co/ZSM-5 hybrid catalysts.
The surface acidity of fresh Co-based hybrid catalyst was displayed in two separate regions according to the desorption temperature of NH3.
Table 3. Hydrogen consumption of Co-based hybrid catalysts measured by H2-TPR.
a little formation of inactive cobalt aluminates or silicates. The two reduction degrees were separately calculated based on total hydrogen consumption and the theoretical value (Co3O4 + 4H2 = 3Co + 4H2O; 3.68 mmol H2/gcat) by considering the actual Co content. Peak I (%) was expressed as the ratio of the amount of hydrogen consumption below 300˚C to the theoretical hydrogen consumption on the fresh promoted Co/ZSM-5 hybrid catalysts. The difference between two reduction degrees suggests the possible formation of inactive cobalt species such as cobalt aluminate or silicate on Co/ZSM-5 catalysts [10] . As can be seen in Table 3, the total H2 uptakes from TPR experiments are estimated as 2.039, 0.760, 2.711 and 2.955 mmol H2/gcat on Co/ZSM-5 catalyst having promoters of Ru(N), Pt(N), Pt(Cl) and La(N), respectively. Although, the variation in the reduction degree of the Co-Pt(N)/ZSM-5 catalysts measured by TPR runs below 300˚C is not considerable, Co/ZSM-5 catalyst having Pt(N) as a promoter shows higher value for the reduction degree up to 300˚C.
3.4. Measurement of Cobalt Particle Size by XRD
In order to understand the dependence of activity for the promoted Co/ZSM-5 hybrid catalysts on their physicochemical properties, XRD study was also carried out and Figure 4 displays the diffraction patterns of fresh promoted hybrid Co/ZSM-5 catalysts. All hybrid catalysts before the reaction show the characteristic reflection peak at 2θ = 36.8˚ due to the presence of Co3O4 phase. The particle size of Co3O4 is calculated by using the X-ray line broadening method with the help of Scherrer’s equation. The crystallite size of Co3O4 for the hybrid catalysts promoted by -Ru(N), -Pt(N), -Pt(Cl) and -La(N) is 32.4, 25.2, 29.6 and 27.2 nm, respectively. In general, the cobalt particle size of promoted Co/ZSM-5 catalysts is higher than 16.8 mm of Co/ZSM-5 catalyst. The smaller particle size of the easily reducible cobalt species uniformly distributed inside the relatively larger pores are reported to be responsible for the higher activity of promoted Co/ZSM-5 hybrid catalysts [3] .
3.5. The Activity and Selectivity of Co/ZSM-5 Catalysts
The catalytic performance of promoted Co/ZSM-5 hybrid catalysts was measured at 240˚C and 260˚C, 2.0 MPa, 3000 ml/gcat∙h and H2/CO = 2. The activity of the catalysts was tested for over 40 h. CO conversion and the product distribution data obtained on hybrid catalysts are presented in Table 4 as steady state average values obtained after 30 h. CO conversion proportionately decreases with the crystallite size of cobalt oxides and its reducibility as shown in Table1 As is well known, the catalyst having a large surface area with a large pore diameter is beneficial for obtaining a small cobalt crystallite size and for the facile transport of heavy hydrocarbons formed during FTS reaction. Also, the large pores on FTS catalysts have been suggested to be linked to less coke or wax deposition [11] . In Table 4, the difference in CO conversion can be possibly attributed to the difference in metallic surface area of cobalt and its reducibility (low reduction temperature from H2-TPR) because of the presence of larger cobalt particles of size above 20 nm on all hybrid catalysts. At a reaction temperature of 260˚C, Co-Pt(N)/ZSM-5 hybrid catalyst possessing a smaller cobalt particle size (high metallic surface area) and facile reducibility shows higher catalytic activity, as confirmed by the XRD data [12] .
As reported in our previous work different product distribution was obtained on promoted Co/ZSM-5 hybrid catalysts. Co-Pt(N)/ZSM-5 and Co-La(N)/ZSM-5 hybrid catalysts showed higher values of CO conversion than that of Co/ZSM-5 catalyst taken as a reference in the FTS reaction at temperature of 240˚C. This can be explained by the presence of acidic sites of different strength on the catalyst leading to hydrocracking of olefins. The high content of weak acidic sites is also responsible for high yields of C5-C9 hydrocarbons, due to the possible catalytic cracking of higher molecular-weight olefins on acidic sites of zeolites [3] [10] .
The selectivity data of the catalysts are also presented in Table4 Increase of FTS temperature increased CO conversion and C5-C9 selectivity, however, with lower C10+ selectivity. Co-Pt(N)/SiO2 catalyst showed higher values for conversion and yield of C5-C9 at 260˚C than that of Co/ZSM-5 catalysts and the other hybrid catalysts. However, CO conversion to CO2 by the water gas shift (WGS) reaction on the promoted hybrid catalysts appeared to be much lower (below 1.9) than that of Co/ZSM-5 catalyst. In the present work, CO conversion to hydrocarbons showed more than 98.1% for all promoted Co/ZSM-5 catalysts.
The Co-Pt(N)/ZSM-5 hybrid catalyst shows the highest selectivity towards C2-C4 (15.74%) and C5-C9 (26.28%) in the hydrocarbon products and also the yield of C5-C9 (14.1%) at temperature of 260˚C. The olefin selectivity is also found to be lower (7.8%) on that catalyst at 260˚C. Whereas the Co-Pt(Cl)/ZSM-5 hybrid catalyst has exhibited the lowest selectivity of C2-C4 (10.82%) and C5-C9 (15.63%). This reduced selectivity towards lower hydrocarbons could be correlated with the suppressed olefin cracking properties of heavy olefinic products due to the presence of less number of acidic sites [13] . Also, the presence more number of weak acidic sites (assigned to first peak in NH3-TPD experiments) on Co-Pt(N)/ZSM-5 hybrid catalyst seems to be responsible for obtaining high selectivity to C2-C9 hydrocarbons.
Figure 5 shows the variation of hydrocarbon selectivity with increasing density of weak acid sites in the promoted and un-promoted Co/ZSM-5 catalyst. The high content of weak acidic sites is also responsible for high yields to C5-C9 hydrocarbons, due to the possible catalytic cracking of higher molecular-weight olefins on acidic sites of zeolites [11] . As can be seen from the figure, the increase of the weak acidic site density for the promoted four catalysts is responsible for increasing C2-C4 and C5-C9 selectivity, and a simultaneous decrease in C10+ selectivity. Thus, the promotion of Co/ZSM-5 catalyst with Pt, Ru and La improved the selectivity and yield of the gasoline range hydrocarbons due to higher concentration of weak acid sites.
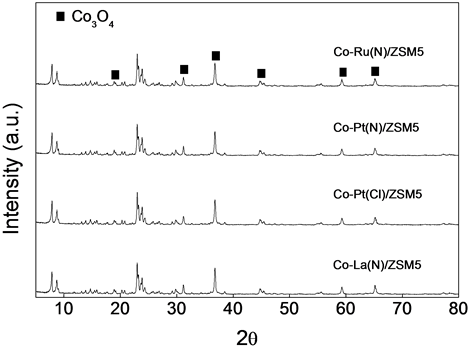
Figure 4. XRD patterns of fresh Co/ZSM-5 hybrid catalysts.
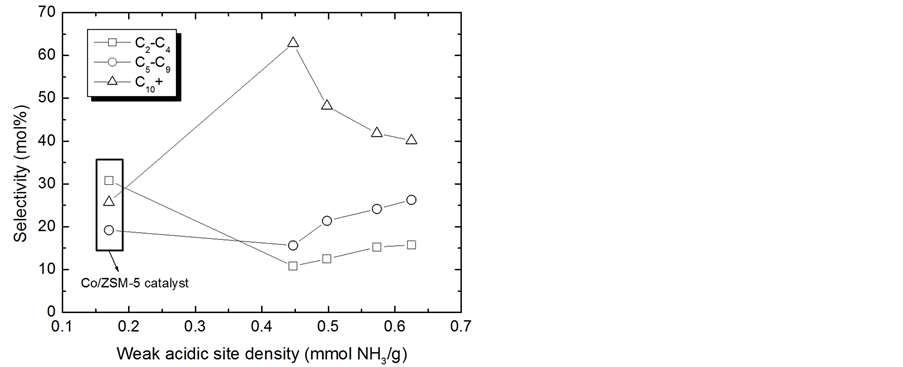
Figure 5. Correlation between the surface activity and the selectivity of hydrocarbons.
Table 4. Catalytic activity and hydrocarbon distribution over the Co/ZSM-5 hybrid catalysts a.
aCatalytic performance was measured at the following fixed reaction conditions; H2/CO = 2, SV = 3000 ml/g∙h and P = 2.0 MPa. bHC stands for the total hydrocarbon selectivity. cO/(O + P) stands for the olefin-to-paraffin ratio in the range of C2-C4 hydrocarbons. dYield of C5-C9 stands for the converted CO moles to the C5-C9 hydrocarbon products.
4. Conclusion
The activity and C5-C9 selectivity during FTS reaction of the hybrid catalysts were obtained to the different results according to the promoters. The Co-Pt(N)/ZSM-5 hybrid catalyst offers higher CO conversion and C5-C9 selectivity. The reducibility of cobalt particles and the concentration of weak acid sites are higher, thus consequently increasing CO conversion and C5-C9 selectivity. The presence of weak acid sites on Co-Pt(N)/ZSM-5 hybrid catalyst with large pore size and pore volume, and small cobalt particle size are mainly responsible for showing a high catalytic performance due to the high reducibility of cobalt particles.
Acknowledgements
This work was supported by Korea Institute of Energy Technology Evaluation and Planning (KETEP) under “Energy Efficiency & Resources Programs” (Project No. 2010201010008A) of the Ministry of Knowledge Economy, Republic of Korea.
References
- Keshav, T.R. and Basu, S. (2007) ZSM-5 Supported Cobalt Catalyst for the Direct Production of Gasoline Range Hydrocarbons by Fischer-Tropsch Synthesis. Fuel Processing Technology, 88, 493-500. http://dx.doi.org/10.1016/j.fuproc.2006.12.006
- Dry, M.E. (2002) The Fischer-Tropsch Process: 1950-2000. Catalysis Today, 71, 227-241. http://dx.doi.org/10.1016/S0920-5861(01)00453-9
- Kang, S.H., Ryu, J.H., Kin, J.H., Sai Prasad, P.S., Bea, J.W., Cheon, J.Y. and Jun, K.W. (2011) ZSM-5 Supported Cobalt Catalyst for the Direct Production of Gasoline Range Hydrocarbons by Fischer-Tropsch Synthesis. Catalysis Letters, 141, 1464-1471. http://dx.doi.org/10.1007/s10562-011-0626-y
- Liu, Z.W., Li, X., Asami, K. and Fujimoto, K. (2007) Syngas to Iso-Paraffins over Co/SiO2 Combined with Metal/Zeolite Catalysts. Fuel Processing Technology, 88, 165-170. http://dx.doi.org/10.1016/j.fuproc.2006.02.009
- Yang, G.H., He, J.J., Yoneyama, Y., Tan, Y.S. and Tsubaki, N. (2007) Syngas to Iso-Paraffins over Co/SiO2 Combined with Metal/Zeolite Catalysts. Applied Catalysis A: General, 329, 99-105. http://dx.doi.org/10.1016/j.apcata.2007.06.028
- Bao, J., He, J.J., Zhang, Y., Yoneyama, Y. and Tsubaki, N. (2008) A Core/Shell Catalyst Produces a Spatially Confined Effect and Shape Selectivity in a Consecutive Reaction. Angewandte Chemie International, 47, 353-356.http://dx.doi.org/10.1002/anie.200703335
- Li, X.G., He, J.J., Meng, M., Yoneyama, Y. and Tsubaki, N. (2009) One-Step Synthesis of H-β Zeolite-Enwrapped Co/Al2O3 Fischer-Tropsch Catalyst with High Spatial Selectivity. Journal of Catalysis, 265, 26-34.http://dx.doi.org/10.1016/j.jcat.2009.04.009
- He, J.J., Yoneyama, Y., Xu, B.L., Nishiyama, N. and Tsubaki, N. (2005) Designing a Capsule Catalyst and Its Application for Direct Synthesis of Middle Isoparaffins. Langmuir, 21, 1699-1702.http://dx.doi.org/10.1021/la047217h
- Cheon, J.Y., Kang, S.H., Bae, J.W., Park, S.J., Jun, K.W., Dhar, G.M. and Lee, K.Y. (2010) Effect of Active Component Contents to Catalytic Performance on Fe-Cu-K/ZSM5 Fischer-Tropsch Catalyst. Catalysis Letters, 134, 233-241.http://dx.doi.org/10.1007/s10562-009-0241-3
- Kang, S.H., Ryu, J.H., Kim, J.H., Jang, I.H., Kim, A.R., Han, G.Y., Bae, J.W. and Ha, K.S. (2012) Catalysis Letters, 26, 6061.
- Kang, S.H., Bea, J.W., Sai Prasad, P.S. and Jun, K.W. (2008) Fischer-Tropsch Synthesis Using Zeolite-Supported Iron Catalysts for the Production of Light Hydrocarbons. Catalysis Letters, 125, 264-270.http://dx.doi.org/10.1007/s10562-008-9586-2
- Chu, W., Chernavskii, P.A., Gengembre, L., Pankina, G.A., Fongarland, P. and Khodakov, A.Y. (2007) Journal of Catalysis, 252, 215-230. http://dx.doi.org/10.1016/j.jcat.2007.09.018
- Lee, Y.J., Park, J.Y., Jun, K.W., Bae, J.W. and Viswanadham, N. (2008) Enhanced Production of C2-C4 Olefins Directly from Synthesis Gas. Catalysis Letters, 126, 149-154. http://dx.doi.org/10.1007/s10562-008-9597-z
NOTES

*Corresponding author.


