Advances in Microbiology
Vol.3 No.6A(2013), Article ID:37937,9 pages DOI:10.4236/aim.2013.36A005
Isolation and Characterization of Cyanobacterial Community Including a Microcystin-Producing Nostoc sp. Strain in the Nile River, Egypt
1City for Scientific Research and Technology Applications (SRTACity), New Borg El Arab, Alexandria, Egypt
2IMDEA-Agua, C/Punto Net 4, Alcalá de Henares, Madrid, Spain
3Chemistry Department, Faculty of Science, King Khalid University, Abha, KSA
Email: *ranyaamer@yahoo.com, *r.amer@mucsat.sci.eg
Copyright © 2013 Ranya Amer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 23, 2013; revised August 23, 2013; accepted September 1, 2013
Keywords: Cyanobacteria; Nostoc; Microcystins; Cyanotoxins; River
ABSTRACT
The combined use of morphological identification and phylogenetic characterization employing primers that target the 16S rDNA region led to the identification of ten isolates belonging to eight cyanobacterial genera in the Nile River. 16S-23S ITS region was amplified to confirm two isolates to be affiliated to genus Nostoc. Using MALDI-TOF/MS, we detected the production of the hepatotoxic demethylated MC-LR by one isolate that clustered together with the genus Nostoc. Protein phosphatase inhibition assay has confirmed toxicity. Our results add to the rising importance of Nostoc as a hepatotoxin-producing cyanobacterium. Furthermore, our results stress that water municipalities in the studied region need to assess the potential threat of toxic cyanobacteria that may pose to human health and economy.
1. Introduction
Cyanobacteria are diverse groups of photoautotrophs capable of obtaining electrons from water and producing oxygen as a byproduct through oxygenic photosynthesis. As primary producers, they play a significant role in the global carbon cycle, and because many can fix nitrogen, they also play a significant role in the nitrogen cycle. Certain cyanobacteria are capable of mass growth forming blooms and scums in water bodies. Blooms of cyanobacteria result from eutrophication by growth-limiting nutrients such as phosphorus and nitrogen. In addition to the nuisance they cause, they deplete water of its oxygen and alter food webs. The recent rise in global temperature and the projected level of global warming are thought to act in synergy with eutrophication to convey a competitive advantage to cyanobacteria. Under such circumstances, an increase of proliferation of cyanobacteria, a shift towards dominance of toxic phenotypes and an elevated level of cellular toxicity have been proposed [1,2]. This in turn will further complicate lake restoration and the management of water sources, and may necessitate the exertion of more efforts to predict, monitor, and manage toxic cyanobacterial blooms.
Microcystins (MCs) are heptapeptide hepatotoxins of cosmopolitan distribution. MCs were found to be produced by several genera of cyanobacteria including Microcystis, Anabaena, Nostoc and Planktothrix. MCs are commonly found in eutrophic lakes, ponds and reservoirs worldwide. They act by inhibiting protein phosphatases one and two. MCs are produced non-ribosomally by a multifunctional enzyme complex consisting of peptide synthetase-polyketide synthase (PS-PKS) modules and tailoring enzymes. The PS-PKS modules are encoded by the mcy gene cluster [3,4]. Studies that take into account both the beneficial and harmful consequences of various aspects of cyanobacterial growth in water bodies have been widely carried in various regions of the world, but Africa is clearly underrepresented in international publications. In order to bring new insights into cyanobacterial diversity, ecophysiology and toxicology, it is necessary to extend our research to under-studied habitats such as those of the African continent.
The Nile River is believed to be the longest river in the world. The Nile passes through nine countries and covers approximately ten percent of Africa. In the north of Egypt the Nile forms the Nile Delta, which includes two main branches, Rosetta (Rashid) and Damietta, in addition to several canals. The river is the main source of drinking water for Egypt (population over 85 millions) as well as nine other countries. Nevertheless, it suffers from eutrophication and pollution due to heavy anthropogenic activities resulting in endemic diseases. Some studies have documented the presence of cyanobacteria in the Nile using morphological examination, while others have isolated hepatotoxic cyanobacteria from a number of irrigation canals and fishponds [5]. In our previous study [6], we used DGGE (denaturing gradient gel electrophoresis) for the first time in the region in order to characterize, on the molecular level, the potentially hepatotoxic cyanobacteria community in the Nile Delta. We demonstrated the presence of the hepatotoxic species Microsytis aeruginosa, Microcystis wesenbergii and Microcystis flos-aquae.
In this study, we undertook detailed isolation combined with molecular characterization and toxin analysis to identify and characterize other hepatotoxic-producing species in the Nile Delta water apart from Microcystis spp. This work led to the isolation of an MC-producing Nostoc strain. We stress that governmental organizations and water utility companies in the region need to incorporate into their legislation and management plans at the presence of toxic cyanobacteria in Nile water and assess the potential threat that they may pose specifically to human health and generally to the economy and the environment.
2. Material and Methods
2.1. Sample Collection
Water quality of the two Nile branches forming the Nile Delta is deteriorating due to heavy agricultural and industrial activities. Surface offshore samples were collected from the Nile branch Rosetta at the city of Kafr El Zayt (Latitude 30˚49'31.68"N Longitude 30˚48'50.10"E). Kafr El Zayat is a city with heavy agricultural, industrial and other anthropogenic activities representing the common activities in the Delta. Samples were collected using Planktonic net size 20 μm mesh during July 2009, July 2010 and July 2011.
2.2. Morphological Identification and Isolation of Unialgal Cyanobacteria Cultures
From the planktonic concentrate, 15 ml was filtered through 10 μm polycarbonate filters (PC, Whatman). Each filtrate was then passed through 2 μm pore-size PC filter. Filters were incubated in 50 ml of BG11 or BG11- N0 containing 50 mg/L cyclohexamide on a rotor-shaker at 100 rpm and 25˚C under 2000 LUX light intensity. Flasks were incubated for at least 10 days. From each flask, 30 µl was used to inoculate plates of BG11 and BG11N0 (BG11 free from combined nitrogen) solidified with 0.8 g/L agarose (molecular grade, Sigma). Growth on each plate was carefully examined under the microscope and colonies were repeatedly transferred to fresh plates to obtain unialgal cultures.
2.3. Light Microscopy and Morphological Characterization
Light microscopy was performed on glutaraldehydefixed samples using a Zeiss Axiovert 200 microscope equipped with interference contrast; images. The morphological identification of cyanobacteria was done according to Komárek and Anagnostidis [7].
2.4. DNA Isolation
DNA was extracted from environmental samples and unialgal cultures as described by [8]. DNA was further purified using GeneJET Genomic DNA Purification Kit (Fermentase, Thermo Fisher Scientific Inc., Canada) as described by the manufacturer.
2.5. PCR, Phylogenetic Reconstructions and TA-Cloning
Primers used in this work were targeting the 16S rDNA (CYA106F-CYA781R) [9], 16S-23S ITS region (P322- P340) [10] (Table 1). PCR reactions were performed as previously described for each primer pair [9,10]. All PCR reactions were performed using 5 u/ml of Dream Taq DNA polymerase (Fermentase, Thermo Fisher Scientific Inc., Canada). PCR products were visualized on 1.5% agarose gels stained with ethidium bromide.
The 16S-23S ITS PCR products were cloned into TA-cloning vector using the TA-cloning kit (Invitrogen) as described by the manufacturer. White colonies were picked and vectors were extracted using Mini Plasmid extraction kit (Qiagen). The cloned fragments were sequenced using M13 universal primers on API model 3730 XL sequencer (Bioneer Company, Korea).
Sequences were then subjected to BLAST searches (www.ncbi.nlm.nih.gov/blast) and the closely related sequences from GenBank were selected. The selected se-
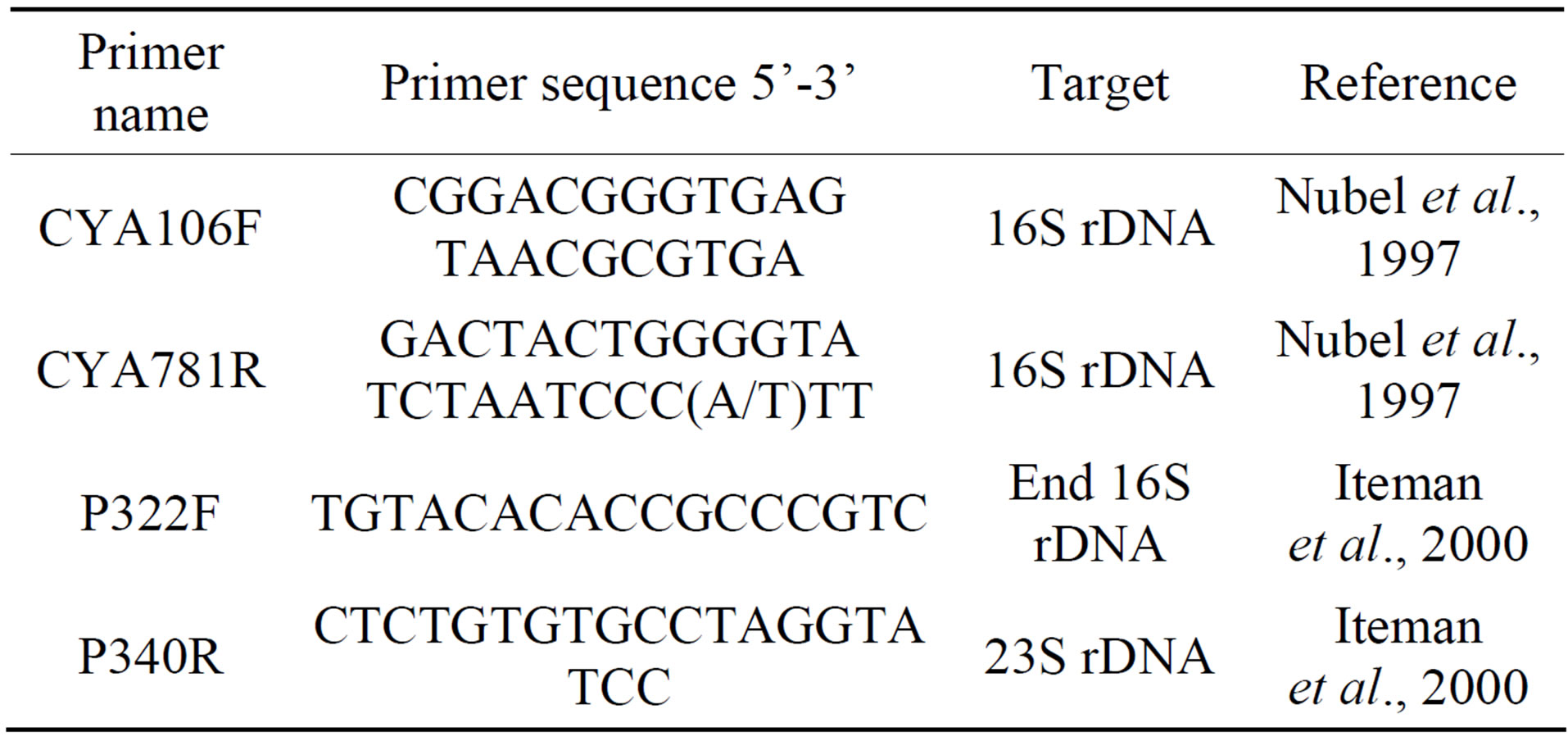
Table 1. List of primers used for PCR and sequencing in this study.
quences were aligned using CLUSTALW integrated into MEGA5.0 package [11]. Sequence comparison and phylogenetic analyses of the partial sequencing of 16S rDNA and 16S-23S ITS were performed using the software MEGA5.0 [11]. Neighbor-joining with Jukes-Cantor correction and one thousand bootstraps was used to build the corresponding phylogenetic trees.
2.6. Chemical Analysis of Water Samples
Water samples were passed through a 0.2 μm membrane filter (PC, Whatman) and stored at −20˚C till further analyses. Total nitrogen (TN) and total phosphorus (TP) were determined as described in Eaton and Franson [12].
2.7. Detections of Microcystins Using MALDI-TOF/MS
Samples for Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF/MS) analysis were prepared as described by [13]. All samples were subjected to MALDI-TOF/MS mass spectrometer “Shimadzu AXIMA Confidence” (RIPAC-Labor, GmbH, Potsdam, Germany). The samples measured in “reflectron mode” in the mass range m/z 500 - 3000 with the matrix 2,5-dihydroxy-benzoic acid. The following standard microcystins and variants were injected (MC-LR, 981.54100, MC-LR, 995.55700, MC-RR, 1038.57400, MC-RR, 1024.55800, MC-YR, 1031.5200, MC-YR, 1045.53600). Full-scan spectra were recorded from m/z 100 to 1000.
2.8. Protein Phosphatase Inhibition Assay
Mice liver extract was prepared according to [14] to be used for studying the isolate toxicity. Microcystins crude extract was prepared and the protein phosphatase inhibition assay was performed as described by [15]. The microcystin inhibition (calibration) curve was plotted as percentage activity of PP2A relative to the control, versus microcystin concentration, where
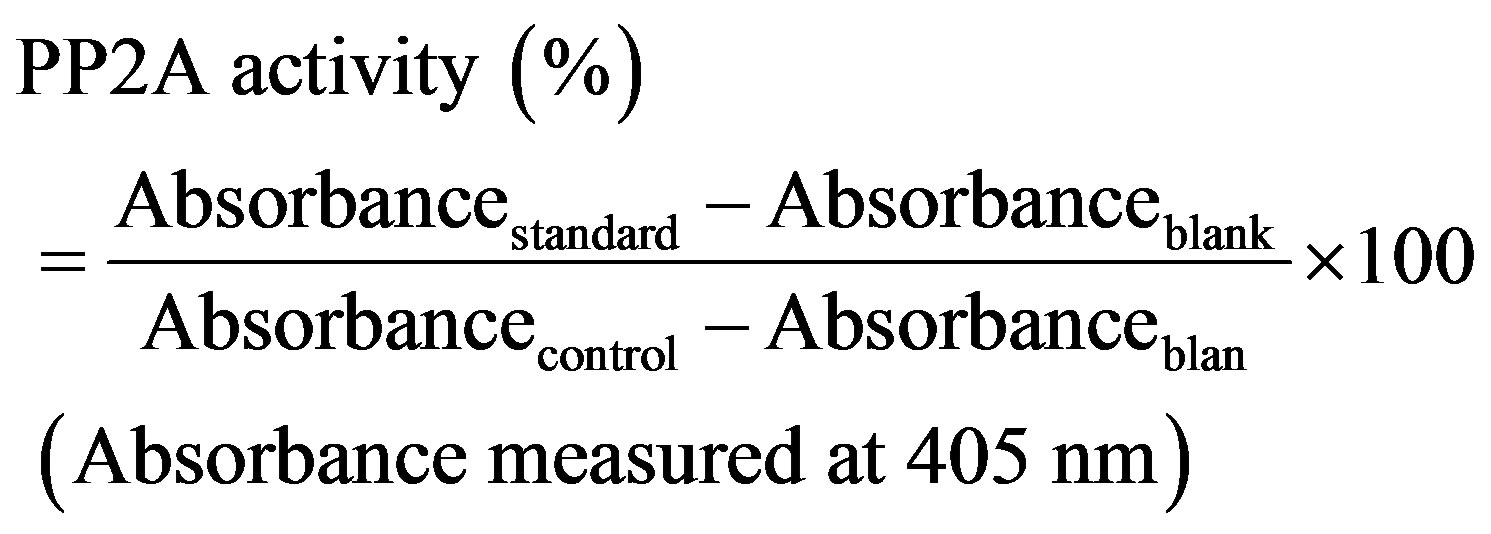
The IC50 of the extract compared to the standard MC-LR was used as a standard (Sigma-Aldrich) was determined since IC50 is the toxin concentration that resulted in 50% inhibition of PP2A activity.
Accession Numbers
The sequences generated in this study are deposited in the GenBank under the following accession numbers: JX193461, JX193462, JX193463, JX193464, JX193465, JX193466, JX193467, JX193468, JX193469, JX193470, JX193471, JX193472, JX193473, JX193459, JX193460.
3. Results and Discussion
Cyanobacteria dominate the phytoplankton communities of many freshwater and marine ecosystems. The advance made in microbial ecology through molecular and genomic approaches in recent decades has led to the discovery of various new cyanobacterial strains and continues to reveal new ecotypes and genotypes [16]. Accordingly, it is worthwhile for the international scientific community to continue to explore the diversity of cyanobacteria, especially in the less-studied regions of the world, such as the Nile River, which has ten countries in its catchment and a population of approximately 160 million inhabitants using its water as their main drinking source.
3.1. Morphological Characterization and Water Chemical Analysis
Samples from summer 2009, 2010 and 2011 contained several morphologically distinct cyanobacteria including the previously documented hepatotoxic Microcystis aeroginosa and Microcystis wesenbergii [6]. The various phenotypes were assigned to different genera on the basis of their morphological characteristics, such as cell size, presence or absence of sheath, colony shape, presence or absence of heterocysts and akinetes, according to [17].
We then attempted to isolate these cyanobacteria in cultures, separate from Microcystis, and test their hepatotoxicity. After repeatedly transferring single colonies on fresh BG11 and BG11N0 plates, unialgal colonies were obtained. The samples harbored nine different morphotypes that were tentatively identified as belonging to several cyanobacterial genera including the unicellular Synechococcus and the nitrogen-fixer Nostoc in addition to two Microcystis morphotypes. Most of the isolates are known to be non-nitrogen fixers under aerobic conditions, which were confirmed by their growth on BG11 and their failure to grow on BG11N0. This is of no surprise given that chemical analysis of water samples has shown an average TN:TP ratio of 55.45. Such a ratio does not favor growth of aerobic nitrogen-fixing cyanobacteria [18], but rather favors growth of algae [19]. Water samples also showed high concentrations of both TN and TP characteristics of eutrophied to hyper-eutrophied waters (Table 2). TN and TP are good predictors of cyanobacterial do-
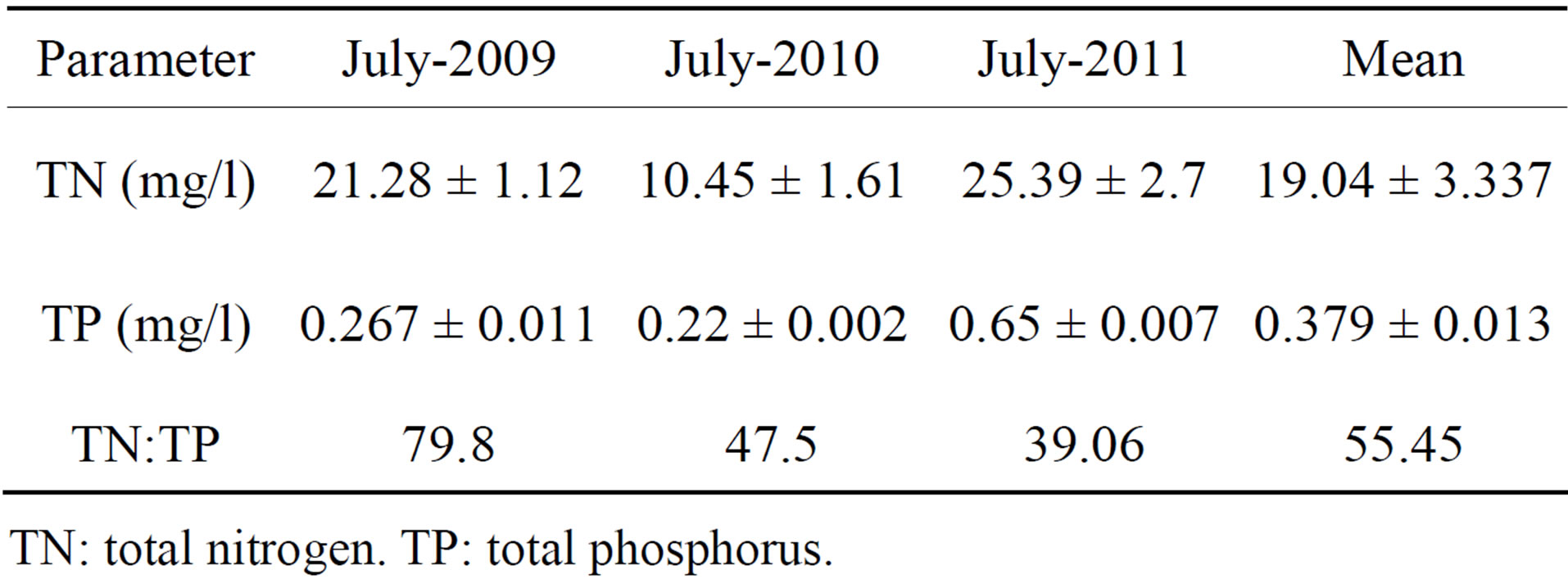
Table 2. Chemical analysis of water samples.
TN: total nitrogen. TP: total phosphorus.
minance in fresh water [20] with the exception of hypereutrophied water where availability of N and P surpass the assimilation capacity of phytoplankton [21].
3.2. Phylogenetic Reconstructions Using Partial 16S rDNA and ITS Region
To molecularly identify the isolates and to determine their phylogenetic relationships, the 16S rDNA genes were partially sequenced and analyzed. The resulting phylogenetic reconstructions are shown in Figure 1 on the basis of neighbor-joining analysis.
As shown in Figure 1, the isolates belong to distinct cyanobacterial clusters. Our analysis also revealed more cyanobacterial diversity than what was previously shown using DGGE and 16S rDNA analysis [6] and confirmed our morphological analysis.
Two of our ten isolates were clustered with Synechococcus and one isolate with Synechocystis. The presence of these genera together with the previous identification of Cyanobium phylotypes at the same sampling location [6] suggests that picocyanobacteria may be abundant in the Nile River. In fact, picocyanobacteria or the so called “non-bloom formers” are ecologically widespread and are common in freshwaters. They play an important ecological role as primary producers and as an indispensable component of the food webs [22-24].
One isolate clustered together with Phormidium and one clustered with Planktolyngbya, both belonging to the LPP group B [25]. Both genera are common in freshwaters. Phormidium usually found in mats and Plaktolyngbya commonly occurr in large mesotrophic reservoirs with several species limited to the warm areas of temperate regions [26]. Phormidium can fix nitrogen under anaerobic conditions, but the ecological relevance of this trait is not well understood [27].
Two isolates clustered with the genus Limnothrix, which is common in temperate areas [26]. Amer, Diez and El-Shehawy [6] have shown the presence of Pseudanabaena, but not Limnothrix, in the same sampling location using DGGE analysis of the 16S-ITS region. Morphologically, both genera resemble each other and both occur in eutrophic water bodies [28-30]. 16S rRNA gene data groups them in a monophyletic cluster [30,31]. Multiphasic approaches including pigmentation phenotypes, as well as analysis of the ITS and IGS, are essential to accurately distinguishing between these two genera [32].
Finally our collection of isolates comprises three filamentous heterocystous nitrogen fixers; two belong to the genus Nostoc and one to the genus Anabaena. None of these genera were previously reported in the phytoplankton community of the Nile River. This could be explained by the fact that chemical water analysis showed a high TN:TP ratio which does not favor the growth of nitrogen-fixers, hence their relative abundance is expected to be low and might not give an obvious band on the DGGE 16S rDNA analysis as studied by [6].
Our phylogenetic analysis (Figure 1) using the 16S rDNA primers revealed that isolates named as Iso-1 and Iso-2 are closely related and showed 96% and 97%, similarity to other Nostoc strains available on the GenBank, respectively. Further analysis using 16S-23S ITS region and phylogenetic reconstructions (Figure 2) has confirmed clustering of these two isolates with Nostoc. Table 3 showed designated names of the isolated cyanobacteria and their accession numbers.
3.3. Screening for the Production of Microcystins Using MALDI-TOF/MS
MALDI-TOF/MS offers the advantages of fast screening of peptide-production with small sample volumes and hence it has been shown to be useful in detecting microcystins [33,34]. The intracellular extracts of all isolates showed similar patterns for natural product compounds by MALDI-TOF/MS. A demethylated variant of MC-LR was detected in the extract of Iso-1 (identified as belonging to the genus Nostoc). The peak showed low intensity at m/z 981.4410 (M + H) (Figure 3) [35]. MC-LR have three demethylated variants; Mdha/Dha in position 7, MeAsp/Asp in position 3 and Adda/DMAdda in position 5, but MALDI-TOF/MS cannot distinguish between them [36]. Moreover, MALDI-TOF/MS cannot detect less polar microcystins such as MC-LF [36], hence the production of hydrophobic variants by this isolate cannot be ruled out.
Many toxic cyanobacteria genera can produce several variants of microcsytins and usually one or two being the dominant. Nostoc strains have been demonstrated to produce several microcystins (for example [5,37-40]). Moreover, one of these strains is producing rare and highly toxic microcystins [26]. As far as we know, no Nostoc strain was found to produce only a demethylated variant of microcystins, while that was the case for some strains of Anabaena and also Planktothrix [13,41].
3.4. Toxicity Assay
To confirm the toxicity of the isolated Nostoc sp. NR1, a crude extract of Nostoc sp. NR1 culture was prepared and its toxicity was determined by the inhibition of protein phosphatase enzyme. A standard curve was prepared using the different concentration of standard MC-LR to determine the IC50 of microcystin in the crude extract the results showed that 3.56 mg of lyophilized cells have an IC50 value of 9.507 µg/ml. This falls within the range of previously reported IC50 values for microcystins [15, 17,42]. Our finding is thus adding to the rising importance of Nostoc as microcystins-producer.
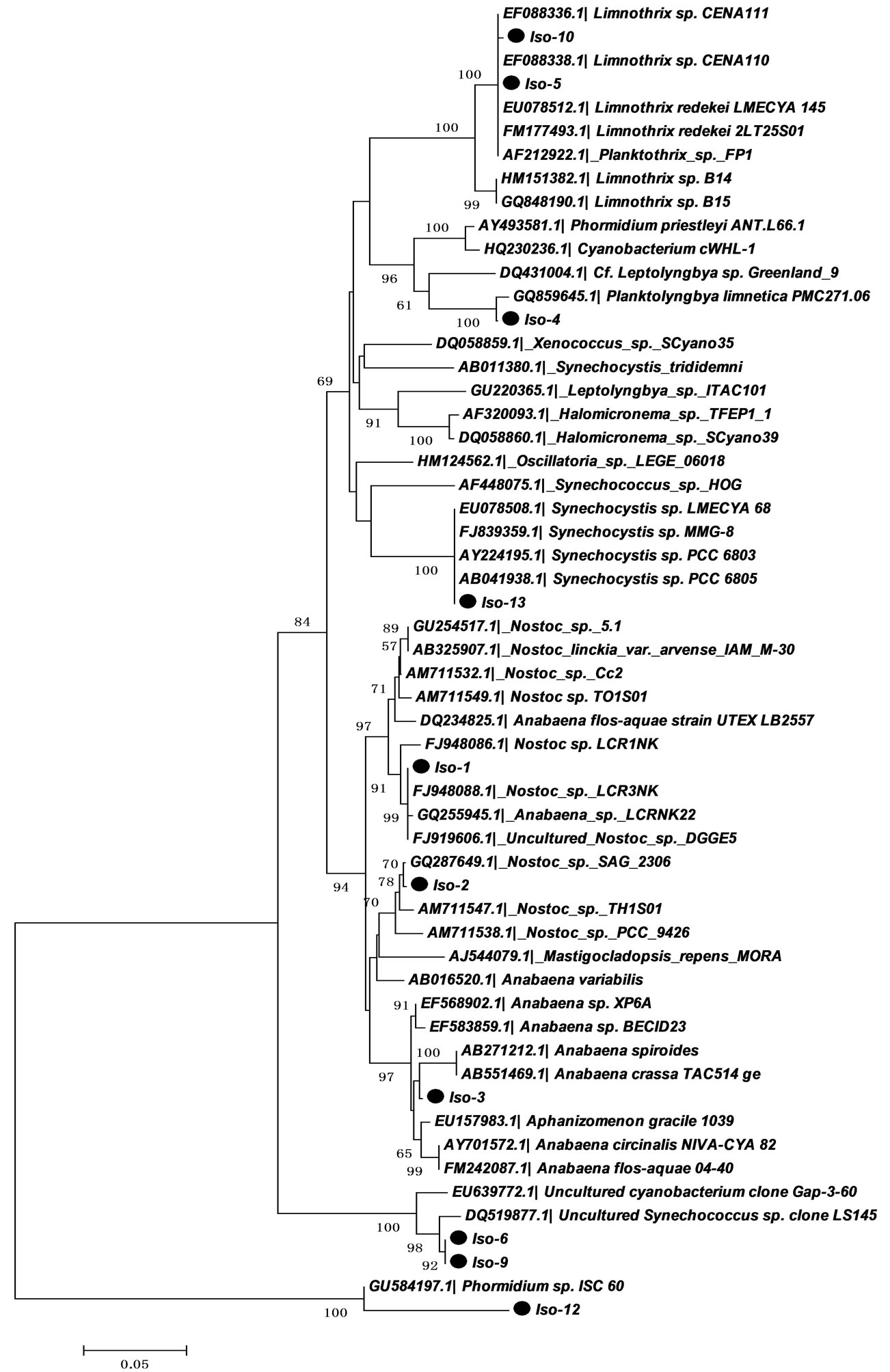
Figure 1. Neighbor-joining tree based on 16S rRNA sequences (CYA primers) of the isolated cyanobacteria from Nile River. Evolutionary distances were calculated using the Kimura 2 model using MEGA5 software. The numerals show the results of the bootstrap analysis values from 1000 replicates (only bootstrap values above 50% were shown). The sequence with  was determined in this work.
was determined in this work.
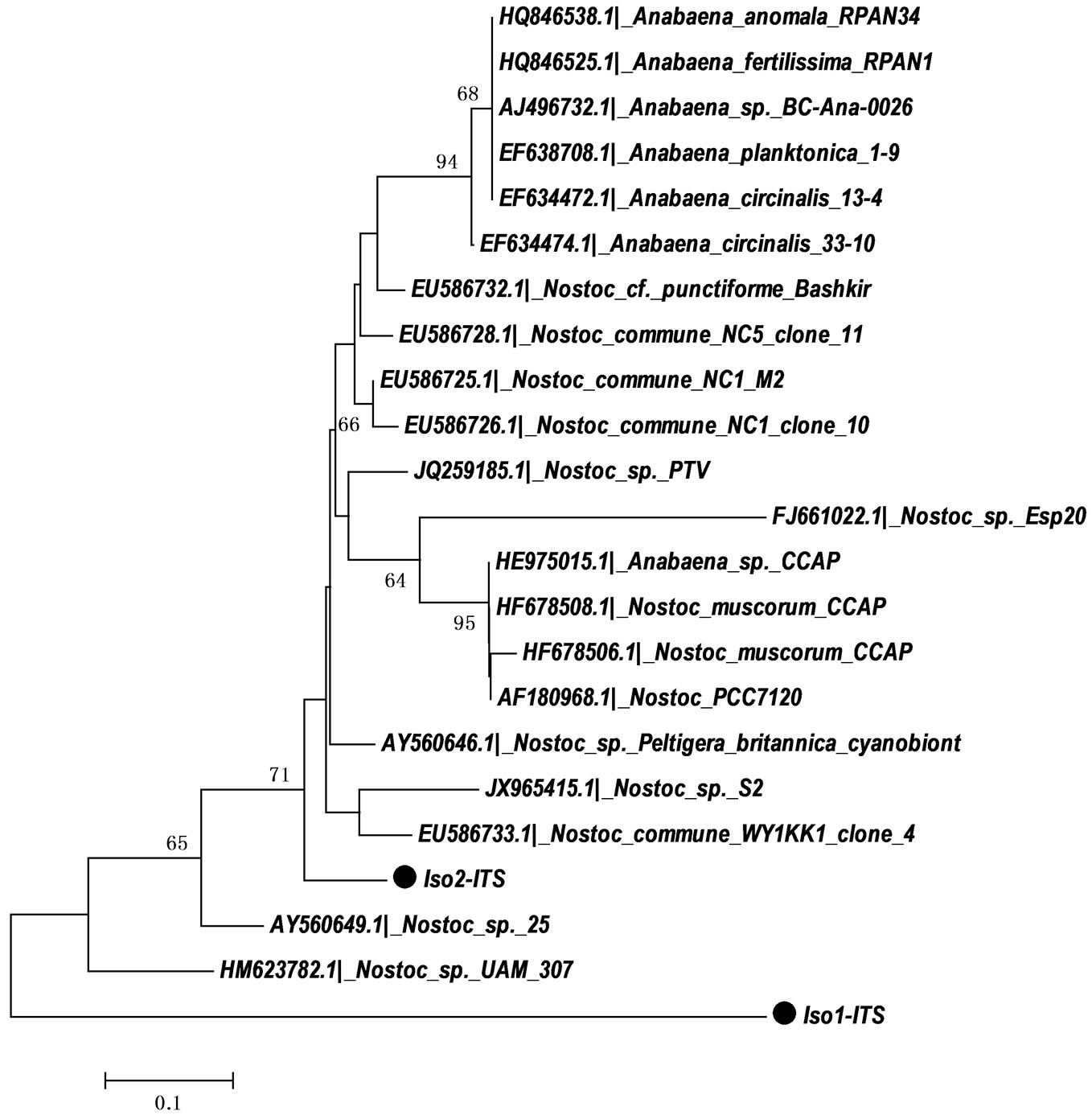
Figure 2. Neighbor-joining tree based on 16S-23S ITS region sequences (P322-P340 primers) of Iso-1 and Iso-2 isolated in this study. Evolutionary distances were calculated using the Kimura 2 model using MEGA5 software. The numerals show the results of the bootstrap analysis values from 1000 replicates (only bootstrap values above 50% were shown). The sequence with  was determined in this work.
was determined in this work.
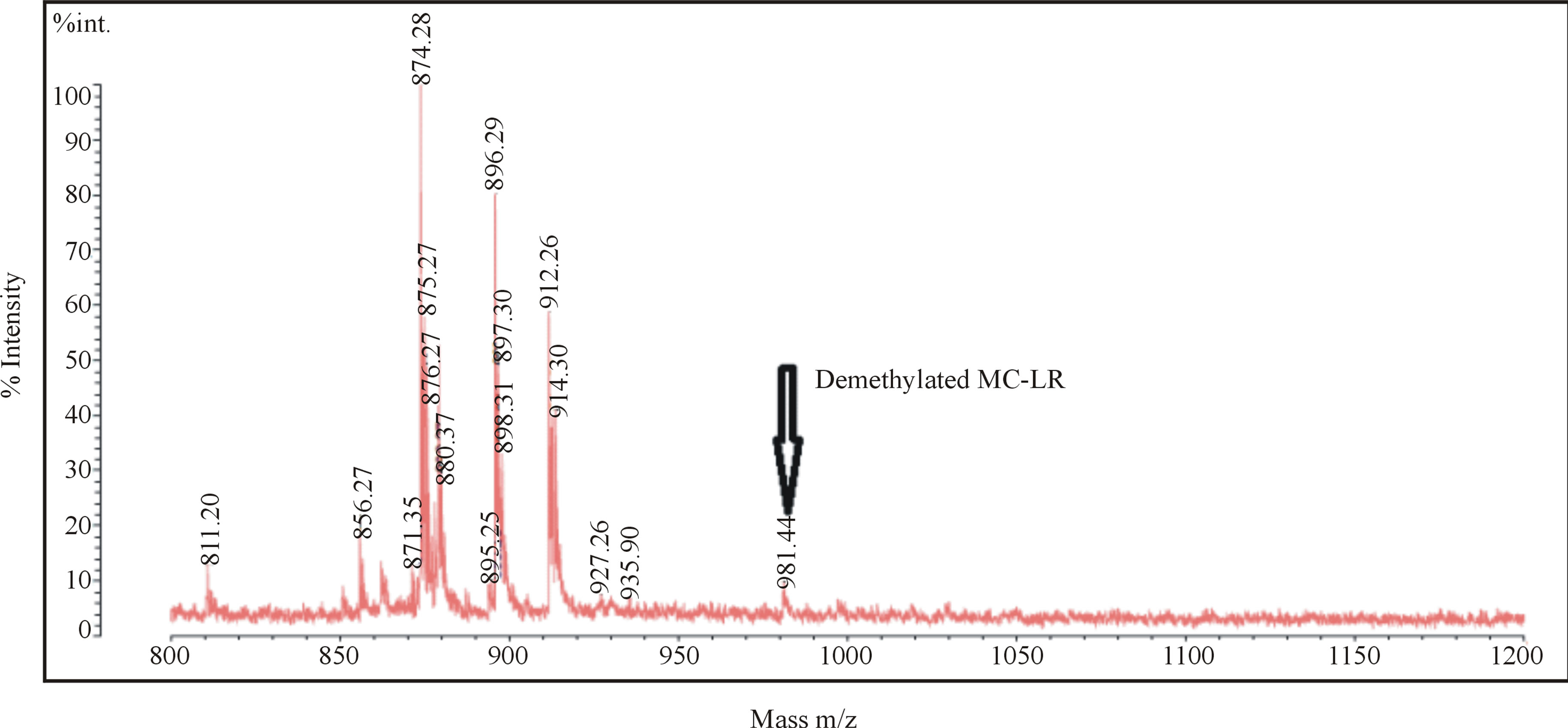
Figure 3. MALDI-TOF/MS spectra of Iso-1 extract (Nostoc sp. Iso-1) showing a peak of demethylated MC-LR at 981.44.
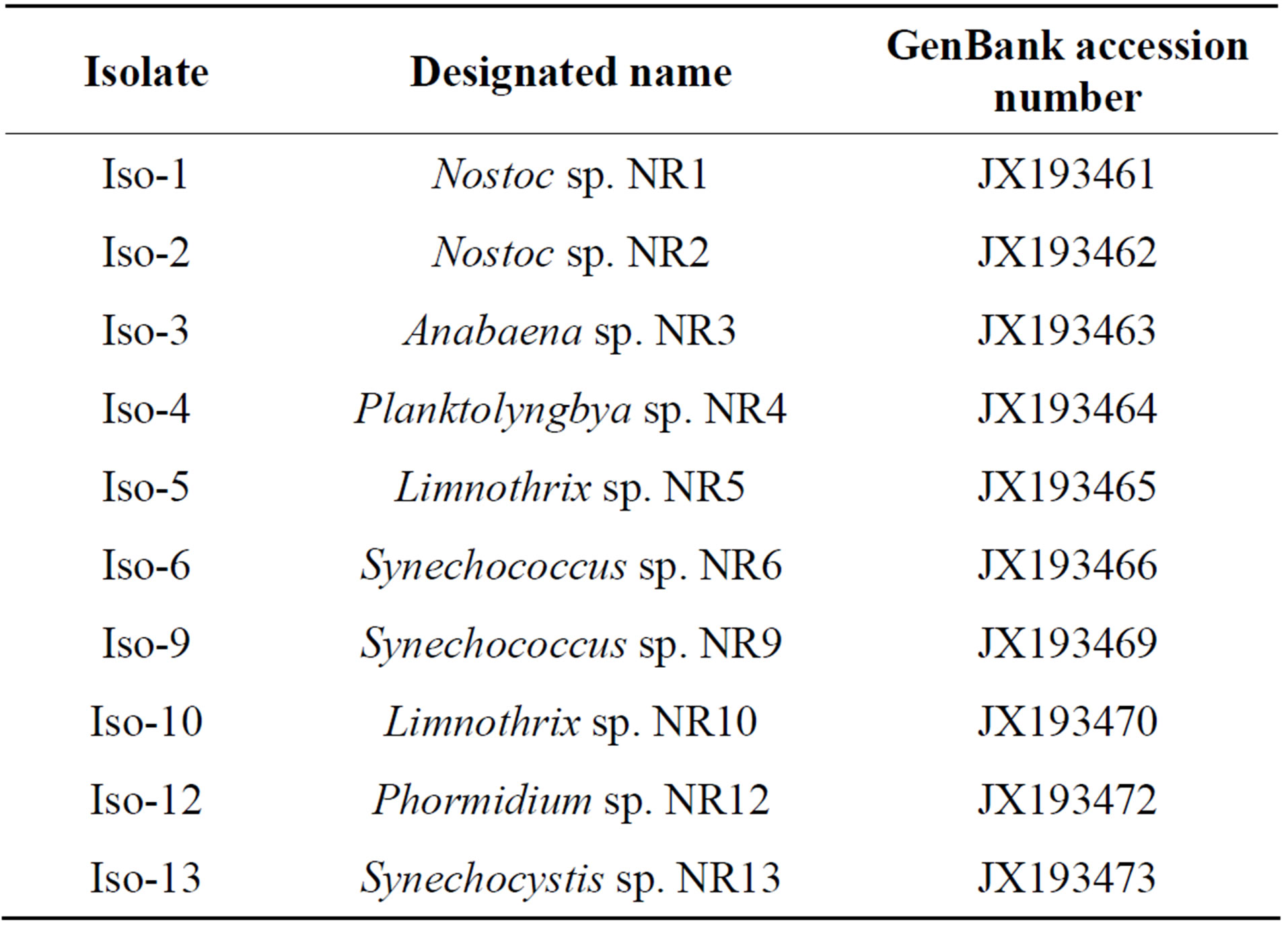
Table 3. Accession numbers and designated names of the cyanobacteria isolated in this study.
The data presented by this study make evidence on the presence of the cyanobacteria species capable of producing hepatotoxins in Nile River the main source of drinking water. This may be one of the reasons for the presence a lot of epidemic diseases in Egypt. This will be confirmed by bioassay studies to study the effect of these toxins on different organs as future work.
4. Conclusion
In this study, we undertook a detailed examination of the cyanobacterial community in the Nile River, combining morphological analysis, mass spectrometry and phylogenetic reconstructions. We were able to identify ten isolates belonging to eight different cyanobacterial genera. One of these isolates was identified as belonging to the genus Nostoc and was shown to produce a demethylated variant of MC-LR using MALDI-TOF/MS. Toxicity of the isolate was further proven by protein phosphatase inhibition assay. Our results add to the rising importance of Nostoc as hepatotoxic cyanobacterium. Moreover, our results stress the need to develop legislation that takes into consideration the presence of these potentially harmful bacteria in the River of a region that is home to approximately 160 million inhabitants.
5. Acknowledgements
This study was funded by the Science and Technology Development Fund (STDF), project No. 274.The authors wish to express their gratitude to Prof. Pilar M. Ortega, Prof. Francisca Fernández-Piñas (Universidad Autónoma de Madrid) and Ms Maria-Ángeles Lezcano Vega (IMDEA Water) for helpful opinions.
REFERENCES
- R. El-Shehawy, E. Gorokhova, F. Fernández-Piñas and F. F. Del Campo, “Global Warming and Hepatotoxin Production by Cyanobacteria: What Can We Learn from Experiments?” Water Research, Vol. 46, No. 5, 2012, pp. 1420-1429. http://dx.doi.org/10.1016/j.watres.2011.11.021
- H. W. Paerl and J. Huisman, “Blooms Like It Hot,” Science, Vol. 320, No. 5872, 2008, pp. 57-58. http://dx.doi.org/10.1126/science.1155398
- W. W. Carmichael, “Health Effects of Toxin Producing Cyanobacteria: ‘The CyanoHABs’,” Human and Ecological Risk Assessment, Vol. 7, No. 5, 2001, pp. 1393- 1407. http://dx.doi.org/10.1080/20018091095087
- J. Goldberg, H. B. Huang, Y. G. Kwon, P. Greengard, A. C. Nairn and J. Kuriyan, “Three-Dimensional Structure of the Catalytic Subunit of Protein Serine/Threonine Phosphatase-1,” Nature, Vol. 376, 1995, pp. 745-753. http://dx.doi.org/10.1038/376745a0
- Z. A. Mohamed, H. M. el-Sharouny and W. S. Ali, “Microcystin Production in Benthic Mats of Cyanobacteria in the Nile River and Irrigation Canals, Egypt,” Toxicon: Official Journal of the International Society on Toxinology, Vol. 47, No. 5, 2006, pp. 584-590.
- R. Amer, B. Diez and R. El-Shehawy, “Diversity of Hepatotoxic Cyanobacteria in the Nile Delta, Egypt,” Journal of environmental monitoring: JEM, Vol. 11, No. 1, 2009, pp. 126-133.
- J. Komárek and K. Anagnostidis, “Cyanoprokaryota,” Süßwasserflora von Mitteleuropa, Gustav Fischer, Jena Stuttgart Lübeck Ulm, 2005.
- D. Tillett and A. Neilan, “Xanthogenate Nucleic Acid Isolation from Cultured and Environmental Cyanobacteria,” Journal of Phycology, Vol. 36, No. 1, 2000, pp. 251-258. http://dx.doi.org/10.1046/j.1529-8817.2000.99079.x
- U. Nübel, F. Garcia-Pichel and G. Muyze, “PCR Primers to Amplify 16S RRNA Genes from Cyanobacteria,” Applied and Environmental Microbiology, Vol. 63, No. 8, 1997, pp. 3327-3332.
- I. Iteman, R. Rippka, N. T. de Marsac and M. Herdman, “Comparison of Conserved Structural and Regulatory Domains within Divergent 16S rRNA-23S rRNA Spacer Sequences of Cyanobacteria,” Microbiology Vol. 146, No. 6, 2000, pp. 1275-1286.
- K. Tamura, D. Peterson, N. Peterson, G. Stecher, M. Nei and S. Kumar, “MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods,” Molecular Biology and Evolution, Vol. 28, No. 10, 2011, pp. 2731- 2739. http://dx.doi.org/10.1093/molbev/msr121
- A. D. Eaton and M. A. Franson, “Standard Methods for the Examination of Water and Wastewater,” American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC, 2005, pp. 4-14-14-153.
- J. Fastner, I. Flieger and U. Neumann, “Optimised Extraction of Microcystins from Field Samples—A Comparison of Different Solvents and Procedures,” Water Research, Vol. 32, No. 10, 1998, pp. 3177-3181. http://dx.doi.org/10.1016/S0043-1354(98)00073-6
- S. J.-S. Yoo, J. M. Boylan, D. L. Brautigan and P. A. Gruppuso, “Subunit Composition and Developmental Regulation of Hepatic Protein Phosphatase 2A (PP2A),” Archives of Biochemistry and Biophysics, Vol. 461, No. 2, 2007, pp. 186-193. http://dx.doi.org/10.1016/S0043-1354(98)00073-6
- T. Heresztyn and B. C. Nicholson, “Determination of Cyanobacterial Hepatotoxins Directly in Water Using a Protein Phosphatase Inhibition Assay,” Water Research, Vol. 35, No. 13, 2001, pp. 3049-3056. http://dx.doi.org/10.1016/S0043-1354(01)00018-5
- F. Garcia-Pichel, “The Cyanobacteria: Molecular Biology, Genomics and Evolution,” In: A. Herrero and E. Flores Eds., Molecular Ecology and Environmental Genomics of Cyanobacteria, Caister Academic Press, UK, 2008, pp. 60-87.
- R. E. Honkanen, B. A. Codispoti, K. Tse and A. L. Boynton, “Characterization of Natural Toxins with Inhibitory Activity against Serine/Threonine Protein Phosphatases,” Toxicon: Official Journal of the International Society on Toxinology, Vol. 32, No. 3, 1994, pp. 339-350.
- V. H. Smith, “Nitrogen, Phosphorus and Nitrogen Fixation in Lacustrine and Estuarine Ecosystems,” Limnology and Oceanography, Vol. 35, No. 8, 1990, pp. 1852-1859.
- J. P. Jensen, E. Jeppesen, K. Olrik and P. Kristensen, “Impact of Nutrients and Physical Factors on the Shift from Cyanobacteria to Chlorophyte in Shallow Danish Lakes,” Canadian Journal of Fisheries and Aquatic Sciences, Vol. 51, No. 8, 1994, pp. 1692-1699. http://dx.doi.org/10.1139/f94-170
- J. A. Downing, S. B. Watson and E. McCauley, “Predicting Cyanobacteria Dominance in Lakes,” Canadian Journal of Fisheries and Aquatic Sciences, Vol. 58, No. 10, 2001, pp. 1905-1908. http://dx.doi.org/10.1139/f01-143
- H. W. Paerl and R. S. Fulton, “Ecology of Harmful Cyanobacteria,” In: E. Graneli and J. T. Turner, Eds., Ecological Studies, Ecology of Harmful Algae, SpringerVerlag, Berlin, Heidelberg, 2006, pp. 95-109. http://dx.doi.org/10.1007/978-3-540-32210-8
- N. D. Crosbie, M. Pöckl and T. Weisse, “Dispersal and Phylogenetic Diversity of Non-Marine Picocyanobacteria, Inferred from 16S rRNA Gene and cpcBA—Intergenic Spacer Sequence Analyses,” Applied and Environmental Microbiology, Vol. 69, No. 9, 2003, pp. 5716-5721. http://dx.doi.org/10.1128/AEM.69.9.5716-5721.2003
- A. Ernst, S. Becker, U. I. A. Wollenzien and C. Postius, “Nitrate and Phosphate Affect Cultivability of Cyanobacteria from Environments with Low Nutrient Levels,” Applied and Environmental Microbiology, Vol. 149, No. 1, 2003, pp. 217-228.
- J. Stockner, C. Callieri and G. Cronberg, “Picoplankton and Other Non-Bloom-Forming Cyanobacteria in Lakes,” In: B. A. Whitton and P. M. Eds., The Ecology of Cyanobacteria: Their Diversity in Time and Space, Kluwer Academic Publishers, Dordrecht, 2000, pp. 195-231.
- R. Rippka, J. Deruelles, J. B. Waterbury, M. Herdman and R. Y. Stanier, “Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria,” Microbiology, Vol. 111, No. 1, 1979, pp. 1-61. http://dx.doi.org/10.1099/00221287-111-1-1
- J. Komárek, “Diversita a Moderní Klasifikace Sinic (Cyanoprocaryota) [Diversity and Modern Classification of Cyanobacteria (Cyanoprokaryota),” Inaugural Dissertation, 1992, not published.
- B. Bergman, J. R. Gallon, A. N. Rai and S. L. J., “N2-Fixation by Non-Heterocystous Cyanobacteria,” FEMS Microbiology Reviews, Vol. 19, No. 3, 1997, pp. 139-185. http://dx.doi.org/10.1016/S0168-6445(96)00028-9
- J. Mayer, M. T. Dokulil, M. Salbrechter, M. Berger, T. Posch and G. E. A. Pfister, “Seasonal Successions and Trophic Relations between Phytoplankton, Zooplankton, Ciliate and Bacteria in a Hypertrophic Shallow Lake in Vienna, Austria,” Hydrobiologia, Vol. 342-343, 1997, pp. 165-174. http://dx.doi.org/10.1023/A:1017098131238
- J. Rücker, C. Wiedner and P. Zippel, “Factors Controlling the Dominance of Planktothrix Agardhii and Limnothrix Redekei in Eutrophic Shallow Lakes,” Hydrobiologia, Vol. 342-343, 1997, pp. 107-115. http://dx.doi.org/10.1023/A:1017013208039
- G. Zwart, M. P. Kamst-van Agterveld, I. van der Werff- Staverman, F. Hagen, H. L. Hoogveld and H. J. Gons, “Molecular Characterization of Cyanobacterial Diversity in a Shallow Eutrophic Lake,” Environmental Microbiology, Vol. 7, No. 3, 2005, pp. 365-377. http://dx.doi.org/10.1111/j.1462-2920.2005.00715.x
- R. Willame, C. Boutte, S. Grubisic, A. Wilmotte, J. Komarek and H. L., “Morphological and Molecular Characterization of Planktonic Cyanobacteria from Belgium and Luxembourg,” Journal of Phycology, Vol. 42, No. 6, 2006, pp. 1312-1332. http://dx.doi.org/10.1111/j.1529-8817.2006.00284.x
- S. G. Acinas, T. H. Haverkamp, J. Huisman and L. J. Stal, “Phenotypic and Genetic Diversification of Pseudanabaena spp. (Cyanobacteria),” The ISME Journal, Vol. 3, No. 1, 2009, pp. 31-46. http://dx.doi.org/10.1111/j.1529-8817.2006.00284.x
- J. Fastner, M. Erhard and H. von Döhren, “Determination of Oligopeptide Diversity within a Natural Population of Microcystis spp. (Cyanobacteria) by Typing Single Colonies by Matrix-Assisted Laser Desorption IonizationTime of Flight Mass Spectrometry,” Applied and Environmental Microbiology, Vol. 67, No. 11, 2001, pp. 5069-5076. http://dx.doi.org/10.1128/AEM.67.11.5069-5076.2001
- M. Welker and H. von Dohren, “Cyanobacterial Peptides —Nature’s Own Combinatorial Biosynthesis,” FEMS Microbiology Reviews, Vol. 30, No. 4, 2006, pp. 530-563. http://dx.doi.org/10.1111/j.1574-6976.2006.00022.x
- M. Welker, B. Maršálek, L. Šejnohová and H. von Döhren, “Detection and Identification of Oligopeptides in Microcystis (Cyanobacteria) Colonies: Toward an Understanding of Metabolic Diversity,” Peptides, Vol. 27, No. 9, 2006, pp. 2090-2103. http://dx.doi.org/10.1016/j.peptides.2006.03.014
- F. F. del Campo and Y. Ouahid, “Identification of Microcystins from Three Collection Strains of Microcystis aeruginosa,” Environmental Pollution, Vol. 158, No. 9, 2010, pp. 2906-2914. http://dx.doi.org/10.1016/j.envpol.2010.06.018
- R. Bajpai, N. K. Sharma, L. A. Lawton, C. Edwards and A. K. Rai, “Microcystin Producing Cyanobacterium Nostoc sp. BHU001 from a Pond in India,” Toxicon, Vol. 53, No. 5, 2009, pp. 587-590.
- K. A. Beattie, K. Kaya, T. Sano and G. Codd, “Three Dehydrobutyrine-Containing Microcystins from Nostoc,” Phytochemistry, Vol. 47, No. 7, 1998, pp. 1289-1292. http://dx.doi.org/10.1016/S0031-9422(97)00769-3
- I. Oksanen, J. Jokela, D. P. Fewer, M. Wahlsten, J. Rikkinen and K. Sivonen, “Discovery of Rare and Highly Toxic Microcystins from Lichen-Associated Cyanobacterium Nostoc sp. Strain IO-102-I,” Applied and Environmental Microbiology, Vol. 70, No. 10, 2004, pp. 5756- 5763. http://dx.doi.org/10.1128/AEM.70.10.5756-5763.2004
- K. Sivonen, W. W. Carmichael, M. Namikoshi, K. L. Rinehart, A. M. Dahlem and S. I. Niemelä, “Isolation and Characterization of Hepatotoxic Microcystin Homologs from the Filamentous Fresh-Water Cyanobacterium Nostoc sp. Strain-152,” Applied and Environmental Microbiology, Vol. 56, No. 9, 1990, pp. 2650-2657.
- K. Sivonen, M. Namikoshi, R. Luukkainen, M. Fardig, L. Rouhiainen, W. R. Evans, W. W. Carmichael, K. L. Rinehart and S. I. Niemela, “Variation of Cyanobacterial Hepatotoxins in Finland,” In: M. Munawar and M. Luotola, Eds., The Contaminants in the Nordic Ecosystem, Dynamics, Processes and Fate Ecovision World Monograph Series, SPB Academic Publishing, Amsterdam, 1995, pp. 163-169.
- B. S. F. Wong, P. K. S. Lam, L. H. Xu, Y. Y. Zhang and B. J. Richardson, “A Colorimetric Assay for Screening Microcystin Class Compounds in Aquatic Systems,” Chemosphere, Vol. 38, No. 5, 1999, pp. 1113-1122. http://dx.doi.org/10.1016/S0045-6535(98)00354-3
NOTES
*Corresponding author.

