Open Journal of Medical Microbiology
Vol.2 No.3(2012), Article ID:22820,7 pages DOI:10.4236/ojmm.2012.23011
Sterilization Efficacy of Demolizer Technology for Onsite Treatment of Sharps and Other Regulated Medical Waste against Staphylococcus aureus, Escherichia coli, Candida albicans, Mycobacterium phlei and Bacillus subtilis Spores
Department of Animal Science and Industry, Kansas State University, Manhattan, USA
Email: jmarsden@ksu.edu
Received June 15, 2012; revised July 20, 2012; accepted July 28, 2012
Keywords: Regulated Medical Waste; Onsite Treatment; Sharps; Demolizer Technology
ABSTRACT
This study was performed to evaluate the efficacy of the DemolizerÒ technology for the on-site sterilization of low volumes of regulated medical waste. The objective was to demonstrate a minimum of 6 log10 reduction of the dry heat sterilization process applied by the DemolizerÒ II system for the representative organisms, Staphylococcus aureus, Escherichia coli, Candida albicans, Mycobacterium phlei, and Bacillus atrophaeus spores (formerly Bacillus subtilis) on simulated medical waste consistent with numerous regulatory standards for medical waste treatment. The system cycle was heat treatment at a minimum temperature of 350˚F and held at or above this temperature for a minimum of 90 minutes. Upon completion of treatment, there was no evidence of growth in the bacterial species after treatment. Given the minimum detection level of 4 CFU/ml, the DemolizerÒ II system demonstrated a minimum sterilization efficacy of 6.6 log10 for both S. aureus and E. coli as representative gram-positive and gram-negative bacteria species. Candida albicans (6.7 log10 CFU/ml), Mycobacterium phlei (9.0 log10 CFU/ml) and Bacillus subtilis (6.3 log10 CFU/ml) were completely eliminated after sterilizing representative medical waste in the DemolizerÒ II system for 90 minutes at a minimum temperature of 350˚F. Also, the DemolizerÒ II exceeded typical recognized standards for medical waste treatment of a 6 log10 reduction of Mycobacteria and a 4 log10 reduction of the appropriate Bacillus endospore.
1. Introduction
Regulated medical waste as defined in the OSHA Blood borne Standard includes: liquid or semi-liquid blood or other potentially infectious materials; contaminated items that would release blood or other potentially infectious materials in a liquid or semi-liquid state if compressed; items that are caked with dried blood or other potentially infectious materials and are capable of releasing these materials during handling contaminated sharps, and pathological and microbiological wastes containing blood or other potentially infectious materials. Certain factors are considered to be necessary for the induction of disease, such as; presence of a pathogen of sufficient virulence, dose, portal of entry, and resistance of the host [1].
Approximately 1000 tons per day (365,000 tons/year) of infectious medical waste were generated in US hospitals [2,3]. In 1997, the AMA reported that the generation of medical waste had increased 27% to 465,000 per year [4]. Various industry reports set the current generation rate between 600,000 to 1,000,000 tons per year.
Numerous studies have shown presence of bacterial species such as gram negative rod shaped bacteria, Streptoccocci group D, and facultative anaerobes up to 5 - 6 logs in hospital wastes from outpatient surgery, laboratories, internal medicine and surgical wards [5]. Hospital waste is a heterogenous mixture of materials like plastics (14% by weight), dry cellulosic solids (45% by weight), wet cellulosic solids (18% by weight), noncombustibles (20% by weight), and others [6]. Contaminated sharps addressed in OSHA’s Bloodborne Pathogen standard, are the main medical waste associated with infectious disease transmission. This is due to the intrinsic capability of sharps to disrupt skin’s integrity and introduce infectious agents into the wound [7,8].
A number of sterilization and disinfection techniques are employed in the medical industry to treat the medical waste before it can be disposed safely in the environment with the most prevalent being steam sterilization, incineration, and chemical treatment [9]. Recommended conditions for hospital sterilization are processing for 12 min contact at 121˚C saturated steam that ensures 99.9999% reduction in number of Bacillus stearothermophilus viable spores. Several investigators have recommended a processing time of 45 min or longer in autoclave for waste placed in an autoclave bag and steel container with water to facilitate steam penetration [10]. In the early 1990s, 64% - 93% of regulated medical waste in US hospitals was commonly treated by the incineration method [2] based on EPA’s research data and industry operating experience [11]. Similarly, in the 1980s about one-third of US hospitals used steam sterilization to treat their microbiological waste, and about one-fourth poured liquid blood down the drain connected to a sanitary sewer. Non sanitary waste was discarded through sanitary landfill [12]. With the advent of state level regulations, the trend across the US has shifted away from onsite treatment to commercial transport and offsite treatment/disposal and more recently to a renewed interest in alternative onsite treatment technologies.
Several concerns have been raised regarding steam sterilization efficacy of solidified suction containers after commercial treatment [13]. While there is not a uniform standard for sterilization, most states define minimum treatment standards of a 4 log10 reduction of the appropriate Bacillus endospore indicator for the technology and a 6 log10 reduction of other important indicators, including a representative Mycobacteria such as Mycobacterium phlei, Mycobacterium fortuitum or Mycobacterium bovis. These bacterial strains typically represent the most resistant organisms to heat or chemical sterilization, the techniques most commonly used for medical waste treatment. Some states require efficacy demonstration for additional less resistant indicators including Staphylococcus aureus, Candida albicans, Psuedomonas aerugenis, etc. A 4 log10 reduction of a resistant Bacillus endospore and a 6 log10 reduction of other bacterial organisms are consistent with the EPA Guide for Infectious Waste Management (1986) and the State and Territorial Association on Alternative Treatment Technologies’ (STAATT) Technical Assistance Manual: State Regulatory Oversight of Medical Waste Treatment Technologies (1998).
This study was performed with to evaluate the efficacy of the DemolizerÒ technology for the on-site sterilization of low volumes of regulated medical waste. The objective was to demonstrate a minimum of 6 log10 reduction of the dry heat sterilization process applied by the DemolizerÒ II system for the representative organisms, Staphylococcus aureus, Escherichia coli, Candida albicans, Mycobacterium phlei, and Bacillus atrophaeus spores (formerly Bacillus subtilis) on simulated medical waste consistent with numerous regulatory standards for medical waste treatment. Bacillus atrophaeus was selected because it is the recognized USP and ISO indicator organism for dry heat sterilization processes.
The DemolizerÒ technology treats small quantities of regulated medical waste using a dry heat treatment (or thermal inactivation). Regulated medical waste collector once full is sealed with a heat resistant and tamper proof lid and placed into the DemolizerÒ heat chamber; the door is locked; and a treatment cycle begins. The system is heated to a minimum temperature of 350˚F and held at or above this temperature for a minimum of 90 minutes. Upon completion of treatment, the system automatically enters a cool down phase until the system temperature falls below a safe handling temperature. Two certification labels are then automatically printed. The first label is placed on the treated collector and contains critical process data and compliance information. The second label contains additional process data and is placed in a Process Log Book to meet state regulatory record-keeping requirements. The properly labeled, treated collector is then disposed as solid waste.
The DemolizerÒ II system has been developed to address the seven principles of Hazard Analysis Critical Control Point (HACCP) through a robust integrated parametric monitoring and control system [14]. The hazard posed by infectious waste, including sharp waste is critically controlled at two points using dry heat, and thermal process with temperature and time. A minimum temperature of 350˚F for 90 minutes has been established as the minimum process conditions for effective treatment, a minimum 6 log10 reduction of vegetative bacteria, viruses, yeast/fungi, parasitic organisms, and mycobacterium and a minimum 6 log10 reduction of resistant endospores. The critical process data generated can be used to support the appropriate record keeping standards under HACCP.
2. Materials and Methods
For this study, three full scale commercial DemolizerÒ medical waste sterilization units were used.
Challenge Organisms—The bacterial cultures used for the study were obtained from the Kansas State University bacterial culture collection. The organisms used included, Methicillin Resistant Staphylococcus aureus, Escherichia coli, Candida albicans, and Mycobacterium phlei. A cell suspension was prepared for each microbial species by transferring independently each frozen culture to 5 ml growth medium. After incubation at appropriate growth conditions (Table 1), 1 ml cell suspensions were transferred to 45 ml of growth medium and incubated in triplicate in an orbital incubator at 100 RPM at the specified temperatures. To prepare inocula, microbial cultures were centrifuged (10,000 RPM, 4˚C, 10 min) and suspended in 6.5 ml 0.1% peptone water (Difco). Initial
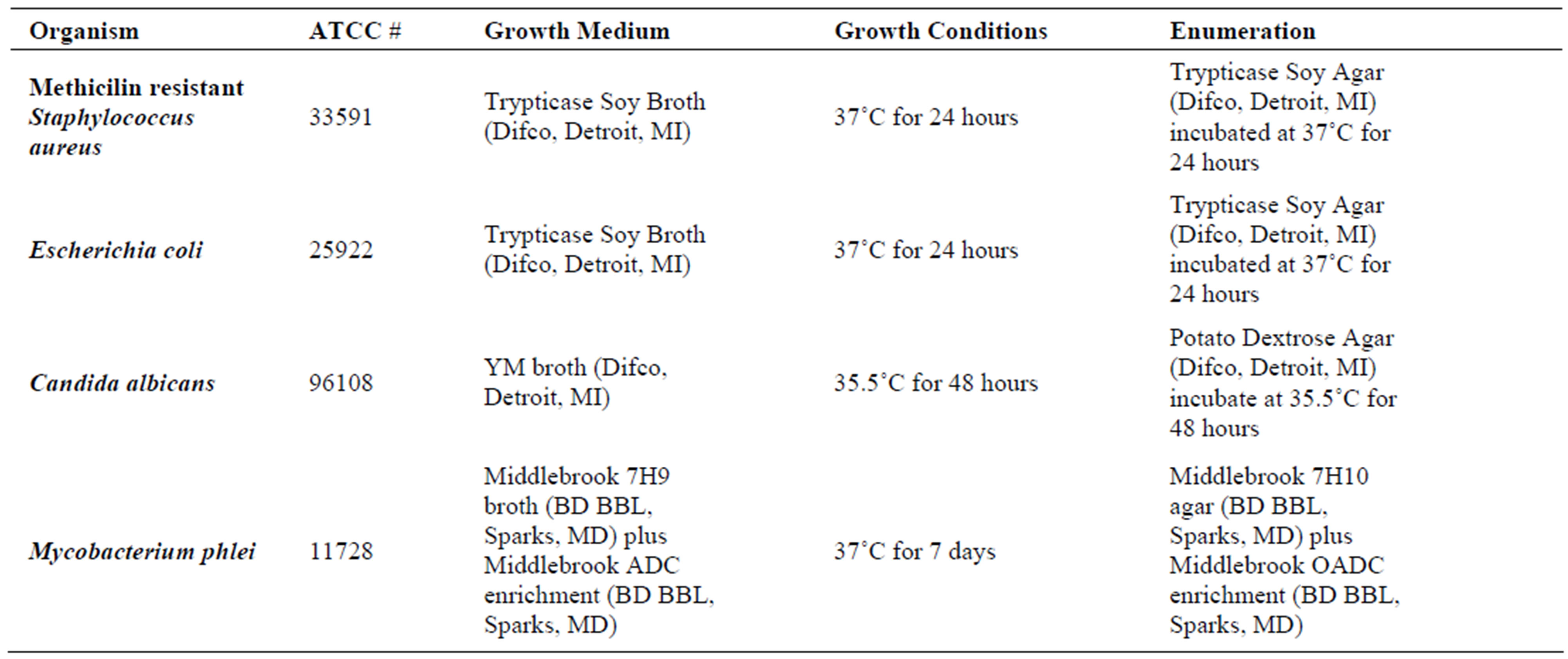
Table 1. Growth conditions and specification of challenge microorganisms.
inocula were determined in 1.5 ml microbial suspension and same volumes (1.0 ml) were transferred to five glass vials that were partially sealed and stored at 40˚C for no more than 20 minutes prior to thermal treatment.
Mycobacterium phlei was grown in 5 ml of growth medium and incubated at the required growth conditions. Thereafter, it was streaked for isolation on the same media used for enumeration. Isolated colonies were suspended in 6.5 ml peptone water to a MacFarland Standard 2. Then the bacterial suspension was transferred to glass vials and initial inoculum enumerated as discussed.
Bacterial spores were purchased from Steris Corporation. Specifically, Bacillus atrophaeus formerly Bacillus subtilis var. niger spores were obtained from Steris (Spordi, Steris) as spore strips contained in glassine envelopes. The spore strip indicators were subsequently grown in commercially available culture media (Spordex, Steris) at 35˚C for 7 days. Inoculum level for the certified spore strips was 1.9 × 106 CFU.
A carrier experimental approach was utilized to maximize the recovery of microorganisms and to simulate challenging test conditions. Carriers were placed along the geometric center of the collector, away from the hot, radiating sides and surrounded by insulating medical waste material. Upon recovery, these carriers were then removed and enumerated.
Treatment of microbial carriers—Microbial inocula contained in five microbial carriers (glass vials with partially closed silicone stoppers, representative of tubing or blood vials) or Bacillus subtilis spore strips contained in glassine envelopes were suspended with thin-wire, metallic clips from each one of three DemolizerÒ II collector rims. The carriers were located towards the collector’s geometric center. Specifically, the carriers were placed along the geometric center axis of the collector with the top of the small glass vials approximately 1 - 2” below the maximum fill-line capacity marked on the collector biohazard label. This location was selected since it was well insulated in the center of the waste load at a distance the farthest away from the hot metal sides of the collector.
Two different waste loads were simulated: 1) a sharps waste load comprised of syringes with needles attached and a small quantity of residual liquid; and 2) a red bag waste load comprised of various adsorbent and non-adsorbent items and organic material. The simulated sharps load contained 370 g of syringes and 50 ml of sterile water. This was a sufficient quantity of syringes to fill the collector to capacity as marked on the collector biohazard label.
For the red bag waste simulated load, the following items were randomly mixed and added to the collector: 1) 170 g of adsorbent material comprised of 120 g of 3-ply gauze pads and 20 g of cotton balls pre-moistened with approximately 50 g of H2O; 2) 125 g of non-adsorbent material comprised of approximately 82.9 g of syringes plus 42.1 g of gloves in a count of 6 gloves to simulate typical plastic and non-adsorbent material that is discarded in a red bag waste load such as tubing, IV bags, gloves, etc.; and 3) 125 g of organic material comprised of 62.5 g of equine serum and 62.5 g of TSB. This resulted in a ratio by weight of 40% adsorbent, 30% non-adsorbent and 30% organic materials. By volume, the ratio was actually skewed substantially with the load comprised of approximately 80% adsorbent material, 8% non-adsorbent material and 12% organic material. These conditions are considered very challenging. The test load compositions were selected to be consistent with test waste load conditions specified in the various state requirements as representative of those conditions encountered during commercial use. The loading procedure was similar for both the sharps and red bag waste loads. The simulated waste was weighed to the composition described above using a calibrated scale. Each collector was filled to approximately 2/3 capacity as marked on the biohazard collector label. The carriers (one set of 5 per repetition/machine process), either contained in glass vials or the glassine envelopes, were numbered and suspended across the central, long axis of each of three rectangular collectors from a stiff, thermally stable wire that was stretched tightly across the collector rims. The carriers were suspended from this central wire by a simple thin wire clip. The carriers were placed along the axis in such a manner as to be as far as possible from the hot, radiating metal sides of the collector. Finally, the remaining 1/3 of the material was placed around and on top of the carriers to further insulate them from the metal sides of the collector and to fill the collector to capacity as marked by the maximum fill line on the collector biohazard label.
Manufacturer procedures for the operation of the DemolizerÒ II System were then followed. At the completion of the cooling and label printing cycle, the collectors were carefully removed. The collector lids were removed and the simulated medical waste was removed. The carriers were recovered with care using aseptic techniques for handling. The carriers were then immediately sampled.
Sampling of microbial carriers—Initial inoculum levels for all microbial species were determined by spiral plating the remaining 1.5 ml of 6.5 ml suspension in which the bacterial pellets were suspended. 5 ml of the bacterial suspension was transferred to five glass vials containing 1.0 ml each. Four spiral plates per bacterial suspension were performed at the time of transfer using the designated agar. The plates were then incubated at the appropriate conditions and populations enumerated as presented in Table 2.
For the B. subtilis trials, the initial mean population was determined from the certification report provided by the manufacturer. For the non-spore bacterial species, the glass vials were collected from the DemolizerÒ II units at the end of each cycle. One-milliliter bacterial suspensions were recovered per glass vial or the volume was completed to one milliliter with sterile peptone water when evaporation occurred. Each one of five glass vials was spread plated onto four agar plates (250 ul per plate). Negative media growth controls of agar and peptone water were plated and incubated as outlined previously. Strict aseptic techniques were employed during all microbial sampling procedures. For the endospore trials, spore bioindicators were collected from each one of the three DemolizerÒ II systems. Aseptically, the glassine envelope was opened on one side with the legend “peel open” to 2/3 of its length. The strip was held by the envelope (the unopened 1/3) and inserted into a sterile glass vial containing the growth media (Sporodex, Steris). The spore strips were then incubated at 35˚C for 7 days.
3. Results and Discussion
The results of the study are shown in Tables 2-8. For vegetative bacteria species, S. aureus and E. coli, bacterial suspensions averaged 7.15 log10 CFU/ml for S. aureus and 7.2 log10 CFU/ml for E. coli. In all replications, no growth was observed following incubation at 37˚C for 24 hours. Given the minimum detection level of 4 CFU/ml, the DemolizerÒ II system demonstrated a minimum sterilization efficacy of 6.6 log10 for both S. aureus and E. coli as representative gram-positive and gram-negative bacteria species.
For representative yeast and mold species, the DemolizerÒ II system resulted in a 6.1 log10 reduction of Candida albicans based on an initial population of 4.84 × 106 CFU/ml/carrier. Following treatment at the standard manufacturer conditions, no growth in the fifteen carriers was observed after incubation at 35˚C for a minimum of 48 hours. All plates for S. aureus, E. coli, and C. albicans were held for a total of 7 days under incubation conditions and no growth was seen in any of the plates at the end of this time period.
For mycobacterium representative species, Mycobacterium phlei, initial populations were enumerated at 9.0 log10 CFU/ml. After incubation for 7 days at 37˚C, all fifteen carriers subjected to the DemolizerÒ II treatment process showed no growth when spread plated on appropriate agar. Given the minimum detection limit of 4 CFU/ml, the DemolizerÒ II inactivated Mycobacterium phlei at a minimum 8.4 log10 level. Finally, inactivation of Bacillus Subtilis resistant endospores, the USP recognized indicator organism for dry heat processes, was demonstrated at a 6.2 log10 reduction level. Initial populations in the spores acquired from Steris Corporation were certified at 1.9 × 106 CFU/ml. Upon treatment in the DemolizerÒ II System, all 15 carriers for both representative sharps and red bag waste loads showed no evidence of growth after incubation at 35˚C for 7 days.
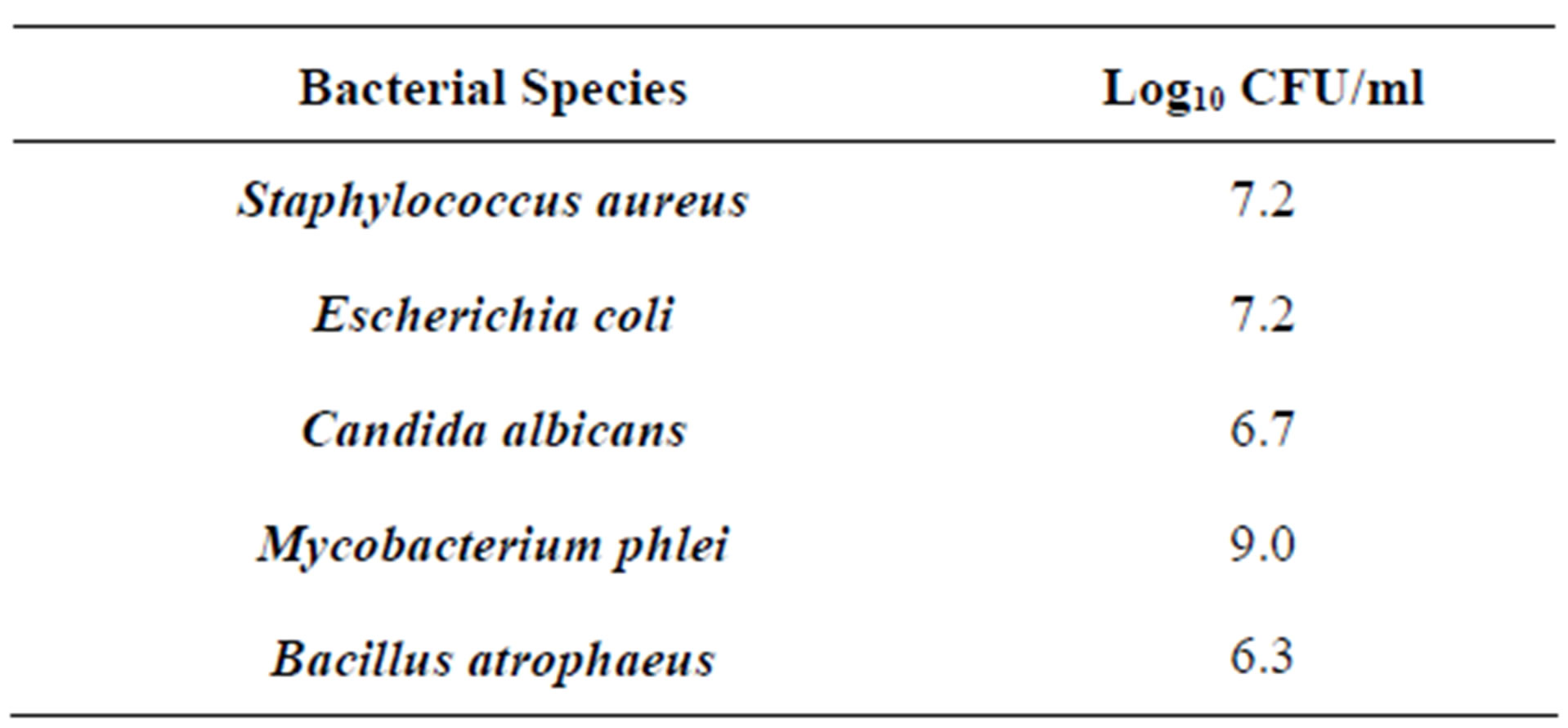
Table 2. Initial inoculum levels.

Table 3. Inactivation of Staphylococcus aureus in the DemolizerÒ II.
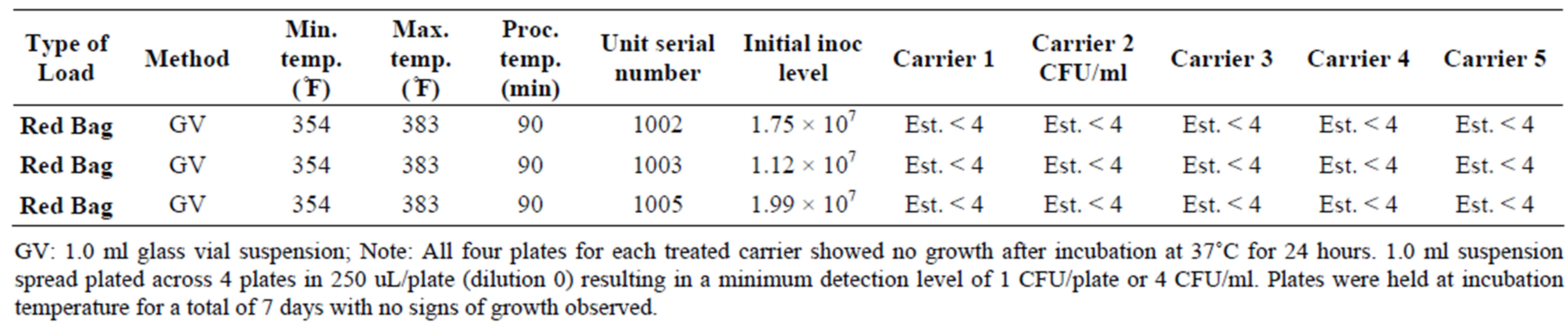
Table 4. Inactivation of Escherichia coli in the DemolizerÒ II.
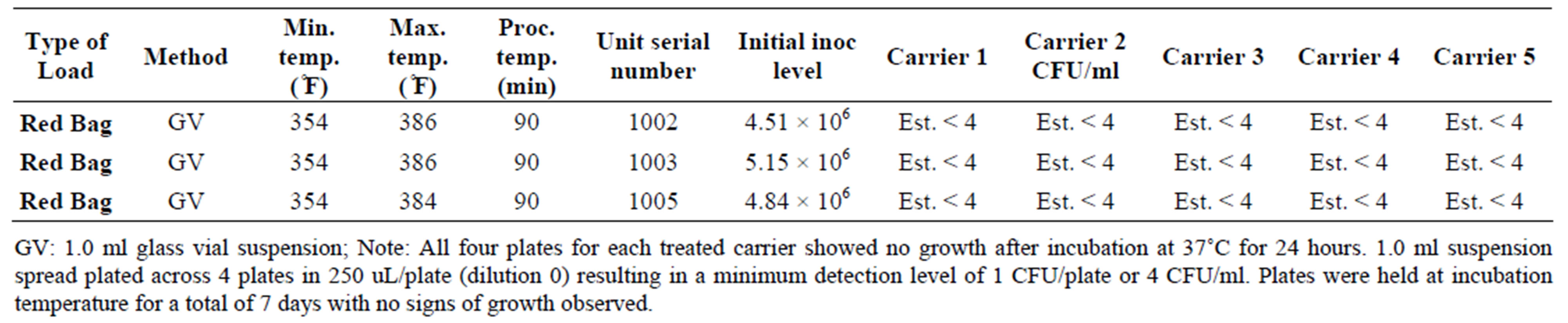
Table 5. Inactivation of Candida albicans in the DemolizerÒ II.
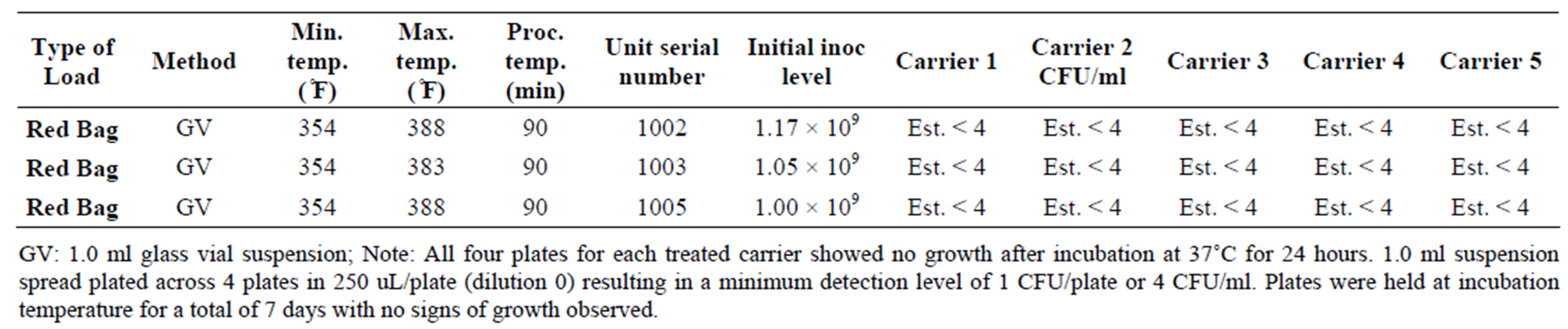
Table 6. Inactivation of Mycobacterium phlei in the DemolizerÒ II.
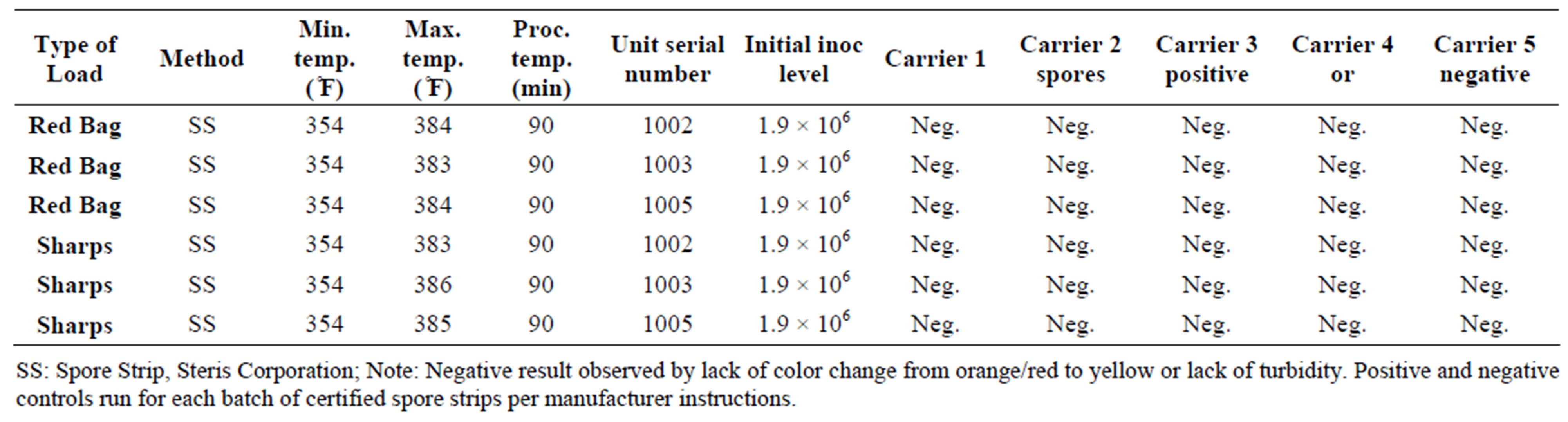
Table 7. Inactivation of Bacillus atrophaeus in sharps and red bag waste loads in the DemolizerÒ II.
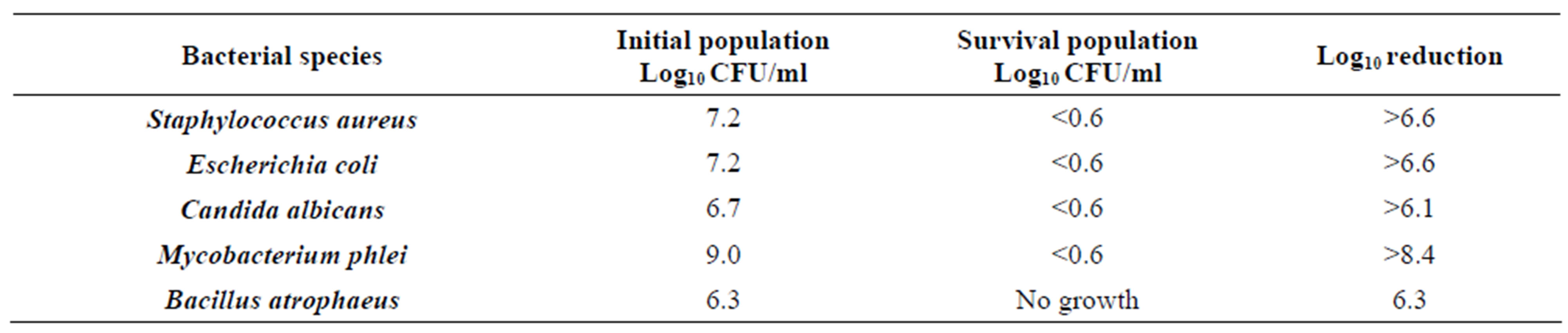
Table 8. Calculated Log10 reduction in microbial species after treatment in the DemolizerÒ II.
Previous tests at various independent laboratories have demonstrated that the DemolizerÒ dry heat technology inactivates B. subtilis, S. aureus, E. coli, P. aeruginosa, C. albicans, M. fortuitum and M. bovis at a minimum 6 log10 level1-4 when operated under the at a minimum treatment temperature of 350˚F and minimum treatment duration of 90 minutes. Further, inactivation of Duck Hepatitis was demonstrated at Stanford University in support of early regulatory approval in New York State [15-17].
The inactivation of Staphylococcus aureus, Escherichia coli, Candida albicans, Mycobacterium phlei, and Bacillus subtilis was demonstrated at a minimum 6 log10 level for the DemolizerÒ II system operating under manufacturer specifications. In conclusion, this study demonstrates that the DemolizerÒ technology inactivates a broad range of microbiological species including resistant endospores. Staphylococcus aureus and Escherichia coli (7.2 log10 CFU/ml), Candida albicans (6.7 log10 CFU/ml), Mycobacterium phlei (9.0 log10 CFU/ml) and Bacillus subtilis (6.3 log10 CFU/ml) were completely eliminated after sterilizing representative medical waste in the DemolizerÒ II system for 90 minutes at a minimum temperature of 350˚F. Further, the change in the shape of the one-gallon capacity, DemolizerÒ collector from cylindrical to rectangular does not adversely impact the sterilization efficacy of the technology. Importantly, the DemolizerÒ II exceeded typical recognized standards for medical waste treatment of a 6 log10 reduction of Mycobacteria and a 4 log10 reduction of the appropriate Bacillus endospore. Sharps were also destroyed so they were no longer usable as a result of the treatment process through a slow melting of the plastic components of the treated sharp item.
REFERENCES
- United States Environmental Protection Agency, “EPA Guide for Infectious Waste Management,” National Technical Information Service, Springfield, 1986.
- W. A. Rutala and C. G. Mayhall, “Medical Waste: The Society for Hospital Epidemiology of America Position Paper,” Infection Control and Hospital Epidemiology, Vol. 13, No. 1, 1992, pp. 38-48.
- W. A. Rutala, R. L. Odette and G. P. Samsa, “Management of Infectious Waste by US Hospitals,” The Journal of American Medical Association, Vol. 262, No. 12, 1989, pp. 1635-1640. doi:10.1001/jama.1989.03430120089027
- K. K. Hanley and M. D. Chair, “Report of the Council on Medical Service: Expense of Medical and Biohazardous Waste Removal,” CMS Report 2-I-98, American Medical Association, 1998.
- G. Kalnowski, H. Weigand and H. Rüden, “Microbial Contamination of Hospital Waste,” Zentralblatt Bakteriologie, Mikrobiologie und Hygiene 1. Abt. Originale B, Hygiene, Vol. 178, No. 4, 1983, pp. 364-379.
- US Congress Office of Technology Assessment, “Finding the Rx for Managing Medical Wastes,” US Government Printing Office, Washington DC, 1990.
- Occupational Safety and Health Standards, 29 CFR (Code of Federal Regulations), Bloodborne Pathogens, Subpart Z, “Toxic and Hazardous Substances,” 2009. http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=10051
- Agency for Toxic Substances and Disease Registry (ATSDR), “The Public Health Implications of Medical Waste: A Report to Congress,” Public Health Service, US Department of Health and Human Services, Washington DC, 1990.
- C. C. Lee, G. L. Huffman and R. P. Nalesnik, “Medical Waste Management,” Environmental Science and Technology, Vol. 25, No. 3, 1991, pp. 360-363. doi:10.1021/es00015a607
- P. Layne, “Review and Evaluation of Existing Literature on Generation, Management and Potential Health Effects of Medical Waste,” Draft Report, Research Triangle Park, Durham, 1988.
- E. T. Oppelt, “Incineration of Hazardous Waste: A Critical Review,” Journal of Air control Pollution Association, Vol. 37, No. 5, 1987, pp. 558-586. doi:10.1080/08940630.1987.10466245
- W. A. Rutala and F. A. Sarubbi, “Management of Infectious Waste from hospitals,” Infection Control, Vol. 4, No. 4, 1983, pp. 198-204.
- California Department of Health Services (CDHS), “Steam Sterilization of Suction Canisters,” All Medical Waste Generators and Offsite Treatment Facilities, 2005.
- V. N. Scott and K. E. Stevenson, “HACCP—A Systematic Approach to Food Safety,” 4th Edition, GMA, Washington DC, 2006.
- Leberco, “Efficacy Testing of the Demolizer System Medical Waste Treatment System,” Leberco Testing, Inc., Roselle Park, 1992.
- Leberco, “Efficacy Testing of the Demolizer System Medical Waste Treatment System against Pseudomona aeruginosa,” Leberco Testing, Inc., Roselle Park, 1992.
- Leberco, “Efficacy Testing of the Demolizer System against Bacillus subtilis Spores Contained on Sporodex Strips,” Leberco Testing, Inc., Roselle Park, 1993.

