Advances in Infectious Diseases
Vol.06 No.02(2016), Article ID:67504,7 pages
10.4236/aid.2016.62009
The Prevalence of Drug-Resistant Tuberculosis among People Living with HIV (PLHIV) in Abia State
Onuka Okorie1, Ahukanna John1, M. Gidado2, Gabriel Akang3, Ubochioma Emperor3, Enogu Rupert3, Ibeziako Vivian4, Emmanuel Meribole4, Pius Osakwe5
1TB Control Program, Ministry of Health, Umuahia, Nigeria
2KNCV, Abuja, Nigeria
3Institute of Human Virology, Abuja, Nigeria
4National TB Control Program, Abuja, Nigeria
5World Health Organization, Enugu, Nigeria

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 February 2016; accepted 17 June 2016; published 20 June 2016
ABSTRACT
Tuberculosis (TB) is a chronic disease caused by mycobacterium tuberculosis and transmitted from person to person, through inhalation of droplet nuclei aerosolized by coughing of an infected person. It reached epidemic proportions in Europe and North America during the 18th and 19th centuries. The incubation period is 2 - 6 weeks and the control has been complicated with emergence of HIV and drug-resistant TB. In 1993, World Health Organization (WHO) declared TB a global emergency. However, despite the concerted effort of National TB control programs, adoption and implementation of Stop TB strategy, TB has remained a major public health challenge with high mortality rate, especially in developing countries. Methodology: This is a descriptive study, evaluated using the positivist/quantitative approach. The study was conducted at Federal Medical Centre Umuahia, a tertiary specialist hospital with comprehensive TB/HIV treatment services. All the presumptive drug-resistant TB cases and symptomatic PLHIV were screened for HIV and their sputum specimens were tested for tuberculosis using the Gene xpert and the Ziehl- Neelsen technique for detecting Acid Fast bacilli. A pretested structured questionnaire was used to collect the demographic data and other essential data from the presumptive TB and laboratory registers such as total number of TB presumptive cases registered HIV status, AFB status and rifampicin status within the study period. Result: A total of 493 presumptive TB cases were screened in the study, 49.9% were HIV positive while 50.05% were HIV negative. More so, 77.85% of the screened cases were AFB negative and 22.15% were AFB positive. Moreover, 11% of the TB/HIV co-infected patients were rifampicin positive. Interestingly among the 493 cases screened with gene xpert machine, 3.6% were rifampicin positive. Furthermore, 3.6% of the HIV negative cases were rifampicin positive while 1.6% of the HIV positive cases were rifampicin positive. Discussion: The data depict lower rifampicin resistance among HIV positive cases than HIV negative cases. The implication for public health professionals is to intensify equitable and unbiased search for resistant TB cases among smear negative and positive cases.
Keywords:
Multidrug Resistant TB, among People Living with HIV

1. Introduction
Tuberculosis (TB) is a chronic disease caused by Mycobacterium tuberculosis and transmitted from person to person, through inhalation of droplet nuclei aerosolized by coughing of an infected person. “It reached epidemic proportions in Europe and North America during the 18th and 19th centuries, earning the sobriquet, Captain among These Men of Death” [1] . The incubation period is 2 - 6 weeks and the control has been complicated with emergence of HIV and drug-resistant TB. In 1993, World Health Organization (WHO) declared TB a global emergency [2] . However, despite the concerted effort of National TB control programs, adoption and implementation of Stop TB strategy [3] , TB has remained a major public health challenge with high mortality rate, especially in developing countries.
Globally, 9.6 million people were estimated to have developed TB in 2014 including HIV positive cases, among the new estimated cases, 5.4 million were men, 3.2 million women and 1.0 million children [4] . However there were estimated 1.5 million deaths for both HIV negative and positive cases, 1.1 million deaths for only HIV negative cases and 390,000 deaths among HIV positive cases [4] . South East Asia region tops the chart of new incident cases with 4 million cases followed by African region with 2.7 million cases and Western pacific region with 1.6 million cases.
The South East Asia region still ranked first with estimated mortality of 460,000 deaths followed by African region with 450,000 deaths. Nigeria is ranked the 4th among 22 high burden countries and TB remains one of the killer diseases among children, adults and people living with HIV [4] . According to WHO (2015) report, there are estimated 570,000 new TB cases in Nigeria including the HIV positive cases which signify 15% case detection rate. Furthermore, there were 250,000 deaths of tuberculosis in Nigeria (HIV negative and HIV positive cases), 170,000 deaths among HIV negative cases while 78,000 deaths were among HIV positive cases [4] .
The collaboration among tuberculosis, HIV and emergence of resistant strain has changed the global outlook of the disease. The emergence of drug resistance TB is a setback to progress made in global TB control [5] . Multidrug-resistant TB (MDR-TB) is defined as resistance to at least to isoniazid (INH) and rifampicin (RIF) [6] . Extensively drug-resistant TB (XDR-TB) is a less common form of multidrug-resistant TB in which bacteria develop resistance to INH and RIF, as well as to second line drugs used for the management of multidrug- resistant TB (any fluoroquinolone and at least one of three injectable second-line drugs) [6] .
Fundamentally, 5% of TB cases are estimated to have MDR-TB, among these cases, 3.5% are new cases while 20.7% are previously treated cases [5] . There were estimated 480,000 MDR-TB cases globally, 123,000 were detected, 111,000 placed on treatment, 190,000 died while 50% of registered cases in 2012 were successfully treated. More so, extensive drug resistant TB was reported in 105 countries in 2014 and estimated 9.7% had MDR-TB [5] . The 2012 drug-resistant prevalence survey conducted in Nigeria showed a prevalence of 2.9% among new TB cases and 14% for retreatment TB cases [7] .
Drug-resistant TB arises as a result of improper use of antibiotics in treatment of drug-susceptible TB, administration of improper treatment regimens and poor adherence to treatment [7] . Program related factors, health system problems and patients related factors have been implicated as being responsible for poor TB case management. However, contacts of MDR-TB patients can be primarily infected by the resistant TB strain from the patients [5] .
There are various method approved by WHO for the diagnosis of susceptible and drug-resistant Tuberculosis. These include: sputum smear microscopy, sputum culture and gene xpert technology. Gene xpert is a real-time PCR assay for Mycobacterium tuberculosis that simultaneously detects rifampicin resistance developed on the Gene Xpert platform, which integrates sample processing and greatly simplifies testing [8] . In December 2010, the World Health Organization (WHO) endorsed the Xpert MTB/RIF for use in TB endemic countries and declared it a major milestone for global TB diagnosis [9] .
With the introduction of gene Xpert in National TB programs, the diagnosis of HIV-associated TB and Rifampicin resistance had been made easy and accessible. It is an automated molecular technology with 76% - 93% sensitivity and specificity of 69% - 93% [10] . The advantages of gene Xpert are varied and creditable. In addition to the high sensitivity and specificity of the technology, diagnosis can be made in two hours against 48 hours sputum smear microscopy and approximately two months for culture.
With the increasing incidence of MDR-TB among HIV patients and health care workers [11] , coupled with poor infection control practices in hospital settings, it is imperative to determine the prevalence of MDR-TB among People Living with HIV (PLHIV). It is against this backdrop that the Abia State TB control program decided to ascertain the prevalence of MDR-TB among PLHIV screened with the gene Xpert machine. Abia State is one of the States in South Eastern Nigeria and has a population of 3.9 million (projected from the 2006 census). The HIV prevalence in the State is 3.9 (2014 sentinel survey).
Objectives
・ To ascertain the prevalence of MDR-TB among PLWHIV.
・ To determine the prevalence of MDR-TB among TB/HIV co-infected Clients.
2. Methodology
Inclusion criteria: all presumptive drug resistant clients and symptomatic PLHIVs in the State. All age groups and both sexes were included in the study. This is a descriptive study, evaluated using the positivist/quantitative approach. The Study was conducted at Federal Medical Centre Umuahia, a tertiary specialist hospital with comprehensive TB/HIV treatment services. The Gene Xpert machine located in this facility serves the entire State, consequently the data generated in this study is a reflection of all clients screened with gene Xpert in the State.
All the presumptive drug-resistant TB cases and symptomatic PLHIV were screened for HIV and their sputum specimens tested for Tuberculosis using the Gene Xpert and the Ziehl-Neelsen technique for detecting Acid Fast bacilli.
2.1. Sample Size Determination
To estimate the sample size, the formula for the prevalence or proportion of a factor in a descriptive study was used [12]
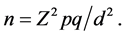
Z = the standard normal deviate, usually set at 1.96 which corresponds to the 95 per cent confidence interval.
p = the prevalence or proportion in the study population estimated to have MDR-TB. “MDR-TB was found to be significantly associated with HIV seropositive patients having a rate of 32% when compared to HIV seronegative rate of 2.2%” [13] .
The sample size in this study will be extrapolated from this value as a reference.
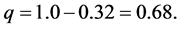
d = the precision, tolerable margin of error or the expected difference, usually set at 5% or 0.05
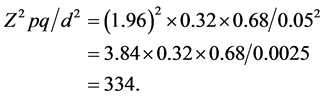
However to make room for improperly completed questionnaires and to increase the power of the study, the calculated sample size will be increased by 40% (133), to a total 467 subjects as the minimum required sample size.
2.2. Ziehl-Neelsen Staining and Microscopic Examination of Smears
Sputum collection was done according to routine procedure by the National TB Program’s guidelines for sputum-smear microscopy examination and the Standard Operating Procedure (SOPs) for sputum collection at the study centers as described below. Two sputum samples were collected from each participant. The patient was given a labelled plastic sputum collection cup to produce and submit the first specimen (spot sputum sample) on the first day of visit to the study center, a second labelled sputum collection cup will be given to the patient with the instruction to collect an early morning specimen at home and bring to the center the next day.
The smears will be flooded with strong carbon fuchsine solution and the underside of the glass slide will be flamed gently until the stain just began to steam.
The smears will be left to stain for about 10 minutes and then washed gently by flooding the slide with water. The water will be drained off and the smears decolorized with 3% acid-alcohol for 5 minutes. The smears will be rinsed with water, drained and counterstained with methylene blue for about 30 seconds. The smear will be rinsed with water, drained and left to dry in a draining rack. The dry stained sputum smears will be examined microscopically using 100x oil immersion objective. The result will be categorized as: Negative (0 AFB/100 HPF), scanty (1 - 9 AFB/100 HPF), 1 + (10 - 99 AFB/100 HPF), 2 + (1 - 10 AFB/HPF), 3+ (>10 AFB/HPF) according to WHO grading [14] .
2.3. HIV Testing
HIV counselling (pre and post) and testing were done on consented patients by following the national algorithm on HIV counselling and testing for persons. Screening test was done using determine HIV kit. Reactive sera were further tested using Unigold HIV kit. Stat-Pack was used for inconclusive result and serves as tie-breaker. Their results were recorded in the Laboratory and presumptive TB registers. The test was performed by adding 50 µl of serum or plasma or blood to the test pad at the bottom of the strip. For whole blood testing, one drop of the chase buffer was applied to the sample on the strip and the assay and the assay incubated at room temperature for 10 minutes. The result was read visually and interpreted according to the test’s instruction manual.
2.4. Gene Xpert Screening
The Xpert MTB/RIF test was performed as described by (2010) [15] and [16] . The already decontaminated or concentrated sputum sample was treated with isopropanol-containing sample reagent (SR). The SR will be added at a 2:1 ratio to the sputum samples and incubated for 15 minutes at room temperature. The closed sputum sample containers was manually agitated twice during a 15-min period at room temperature before 2 ml of the inactivated material was transferred to the test cartridge and the cartridge loaded into the gene Xpert machine.
All specimens that were culture positive and MTB/RIF assay negative and specimens that were culture negative and MTB/RIF assay positive were retested twice. The last result was used for the analysis
2.5. Ethical Authorization
Approval was gotten from the ethical committee of Federal Medical Center, Umuahia.
2.6. Data Collection
A pretested structured questionnaire was used to collect the demographic data and other essential data from the presumptive TB and laboratory registers such as total number of TB presumptive cases registered HIV status, AFB status and rifampicin status within the study period.
2.7. Test Performance Assessment and Statistical Analysis
The data was analyzed with epi info 7. The data will be analyzed using paired T test. The performance characteristics of Xpert MTB/RIF for the diagnosis of pulmonary TB will be measured by the (percentage) of patients with drug resistance tuberculosis as determined by the result of Xpert MTB/RIF.
3. Results
The results shows that 493 persons participated in the study, 287 (58.22%) were males while (206) 41.78% were females (Table 1). There were also 4.6% prevalence DR-TB among under 20 age groups and 8.8% prevalence among age groups above 60 years as well as high prevalence of (84.7%) among economic productive age group (21 - 50 years) (Table 2).
More so, a total of 493 presumptive TB cases were screened in the study 246 (49.9%) were HIV positive while 247 (50.05%) were HIV Negative (Table 3). More so, 383 (77.85%) of the screened cases were AFB negative and 109 (22.15%) were AFB positive (Table 3). Also 13 (11%) of the TB/HIV co-infected patients were rifampicin positive. Interestingly among the 493 cases screened with gene xpert machine, 13 (3.6%) were rifampicin positive (Table 4).
More so, 9 (3.6%) of the HIV negative cases were rifampicin positive while 4 (1.6%) of the HIV positive cases were rifampicin positive (Table 4). The results revealed high Prevalence of rifampicin positive cases among HIV negative cases than HIV positive cases. Also, among the 1.6% rifampicin positive HIV positive cases, 1.2% were previously treated cases and 0.4% are new cases. Paired-T test of rifampicin positivity among HIV negative and HIV positive cases were statistically significant (p-value 0.001).
4. Discussion
Multidrug-resistant tuberculosis could be due to primary infection, acquired infection, and amplified resistance
Table 1. Sex distribution of presumptive TB cases.
Table 2. Age distribution of presumptive MDR-TB cases.
Table 3. HIV, AFB & Rifampicin status of presumptive TB cases.
Table 4. Rifampicin status stratified among HIV positive and negative clients.
[17] . More so, acquired MDR-TB may result from incorrect prescription, irregular supply of drugs, noncompliance to treatment, lack of supervision and poor adherence [18] . The data shows that the prevalence of MDR- TB among the presumptive TB cases screened is 3.6%, while the prevalence is 3.6% among HIV negative cases and 1.6% among PLHIV respectively.
The data depicts low rifampicin resistance among HIV positive cases than HIV negative cases. The implication for public health professionals is to intensify equitable and unbiased search for resistant TB cases among smear negative and positive cases. The 11% resistant cases among TB/HIV co-infected patients is a serious concern to the National TB control program; consequently increase search for resistant cases among co-infected patients should be prioritized.
Among the 1.6% rifampicin-resistant HIV positive clients, 1.2% were previously treated cases and 0.4% arenew cases. The primary rifampicin positive HIV patients is an indication of poor infection control in the hospital setting and poor implementation of intensified case finding, isoniazid preventing therapy and infection control (3 Is) activities.
5. Conclusion
The National TB control program should intensify equitable and unbiased search for resistant TB cases among smear negative, smear positive and co-infected patients. More so, the WHO recommended that 3Is (intensified case finding, infection control and Isoniazid preventive therapy) should be effectively implemented in the facilities.
Cite this paper
Onuka Okorie,Ahukanna John,M. Gidado,Gabriel Akang,Ubochioma Emperor,Enogu Rupert,Ibeziako Vivian,Emmanuel Meribole,Pius Osakwe, (2016) The Prevalence of Drug-Resistant Tuberculosis among People Living with HIV (PLHIV) in Abia State. Advances in Infectious Diseases,06,63-69. doi: 10.4236/aid.2016.62009
References
- 1. Daniel, T.M. (2006) History of Tuberculosis. Respiratory Medicine, 100, 1862-1870.
http://dx.doi.org/10.1016/j.rmed.2006.08.006 - 2. WHO (2004) Tuberculosis.
http://www.who.int/mediacentre/factsheets/who104/en/print.html - 3. WHO (2008) Implementing the WHO Stop TB Strategy.
http://www.who.int/tb/publications/2008/who_htm_tb_2008_401_eng.pdf - 4. WHO (2015) Global Tuberculosis Report 2015.
http://www.who.int/tb/publications/global_report/en/ - 5. WHO (2015) Multidrug-Resistant Tuberculosis (MDR-TB) 2015 Update.
http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf - 6. CDC (2015) Drug Resistant TB.
http://www.cdc.gov/tb/topic/drtb/Accessed 26/01/2016 - 7. WHO (2012) Report of First National TB Prevalence Survey 2012.
http://www.who.int/tb/publications/NigeriaReport_WEB_NEW.pdf - 8. Blakemore, R., Story, E., Helb, D., Kop, J., Banada, P., Owens, M.R., Charkravorty, S., Jones, M. and Alland, P. (2010) Evaluation of the Analytical Performance of the Xpert MTB/RIF Assay. Journal of Clinical Microbiology, 48, 2495-2501.
http://dx.doi.org/10.1128/JCM.00128-10 - 9. World Health Organization (2010) Roadmap for Rolling Out Xpert MTB/RIF for Rapid Diagnosis of TB and MDR-TB. Geneva, Switzerland.
- 10. Scott, L., Kerrigan, M., Natasha, G., Matilda, N., Annelies, V., Lan, V., Willem, V., Adrian, D. and Wendy, S. (2011) Comparison of Xpert MTB/RIF with Other Nucleic Acid Technologies for Diagnosing Pulmonary Tuberculosis in a High HIV Prevalence Setting: A Prospective Study. PLoS Medicine, 8, e1001061.
- 11. CDC (1992) Meeting the Challenge of Multidrug-Resistant Tuberculosis: Summary of a Conference.
http://www.cdc.gov/mmwr/preview/mmwrhtml/00031277.htm - 12. Ibrahim, T. (2009) Research Methodology and Dissertation Writing for Health and Allied Health Professionals. Cress Global Link Limited, Abuja, 74-75.
- 13. Oluwaseun, E., Akinniyi, A.P. and Afolabi, O. (2013) Primary Multi-Drug Resistant Tuberculosis among HIV Seropositive and Seronegative Patients in Abeokuta, Southwestern Nigeria. American Journal of Research Communication, 1, No. 10.
- 14. WHO (1998) Laboratory Services in Tuberculosis Control. Part II. Microscopy. World Health Organisation, Geneva, Switzerland.
- 15. Helb, D., Jones, M. and Story, E. (2010) Rapid Detection of Mycobacterium tuberculosis and Rifampin Resistance by Use of On-Demand, Near-Patient Technology. Journal of Clinical Microbiology, 48, 229-237.
http://dx.doi.org/10.1128/JCM.01463-09 - 16. Arzu, N.Z., Sezai, T. and Cengiz, C. (2011) Evaluation of the Gene Xpert MTB/RIF Assay for Rapid Diagnosis of Tuberculosis and Detection of Rifampin Resistance in Pulmonary and Extra Pulmonary Specimens. Journal of Clinical Microbiology, 49, 4138-4141.
http://dx.doi.org/10.1128/JCM.05434-11 - 17. Nwokeukwu, H.I., Okafor, P.N., Okorie, O. and Ukpabi, I.K. (2013) Paediatric Multidrug-Resistant Tuberculosis with HIV Coinfection: A Case Report. Case Reports in Medicine, 201, Article ID: 756152.
- 18. Park, K. (2011) Textbook of Preventive and Social Medicine. 21st Edition.
http://www.goodreads.com/book/show/16247589-park-s-textbook-of-preventive-and-social-medicine2011
Written Consent Note for Data Collection
Title of Research: The Prevalence of Drug-Resistant Tuberculosis among People Living with HIV (PLHIV) in Abia State
Patient’s name and address ______________________________________________
・ All personal information are strictly confidential: the only people who may see my information are the research team.
・ All personal information may be stored on a computer and will not affect the confidentiality of this information. All such storage of information must comply with Data Protection Act.
・ However if there is any problem, I can contact: Dr Okorie Onuka, the principle investigator.
Patient’s/Legally authorized representative’s Signature ____________________________
Witness Name ____________________________
Witness Signature _________________________
Date ____________________________________
As the investigator for this research, I confirm that I have explained to the volunteer named above the nature and purpose of the research to be undertaken
Investigator’s Name ________________________
Investigator Signature ______________________Date ______________________
DR-TB/HIV QUESTIONNAIRE
CODE ________________________
We are studying the problem drug-resistant tuberculosis among people living with HIV in Abia State. Information collected with the questionnaire will be handled confidentially.
Thank you.
Part A: Socio-demographic Data
・ Date of birth: ______________ Age ______________ (yrs)
・ Sex: Male ______________ Female ______________ (Specify)
・ Tribe: Ibo ________ Hausa ________ Yoruba ________ Other ________ (Please specify)
・ Religion: Christianity ________ Islam ________ Traditional ________ Other ________ (Please specify)
・ Level of Education: None ________ Primary ________ Secondary ________ Tertiary ________ (Specify)
・ Employment status: Public servant, self-employed, unemployed ________ (Specify)
・ LGA ____________________________ (Specify)
Part B: data from the presumptive and laboratory register.
・ Total number of presumptive TB cases
・ The number of HIV positive cases among presumptive cases
・ The number of HIV negative cases among presumptive cases
・ The number of rifampicin positive cases among presumptive TB cases
・ The number of rifampicin negative cases among presumptive TB cases
・ The number of rifampicin positive cases among HIV positive cases
・ The number of rifampicin negative cases among HIV positive cases
・ The number of rifampicin positive cases among dually co-infected cases


