Open Journal of Anesthesiology
Vol.2 No.3(2012), Article ID:20561,5 pages DOI:10.4236/ojanes.2012.23018
Differences of Heart Rate Variability during Sevoflurane Anesthesia in Children by Age
![]()
1Department of Anesthesiology and Pain Medicine, College of Medicine, Seoul National University, Seoul, Korea; 2Department of Anesthesiology and Pain Medicine, College of Medicine, Inha University, Incheon, Korea.
Email: dami0605@snu.ac.kr
Received April 3rd, 2012; revised May 6th, 2012; accepted May 30th, 2012
Keywords: Children; Heart Rate Variability; Sevoflurane
ABSTRACT
Background: The child’s central nervous system develops with aging, and heart rate variability (HRV), which is controlled by the brain, differs from that of adults. We investigated changes in HRV during sevoflurane anesthesia in children. Methods: One 138 children aged from 2 - 12 years without major underlying problems were enrolled. During maintenance with 2 - 2.5 vol% sevoflurane anesthesia, electrocardiographic data were obtained and power spectral analysis, approximate entropy (ApEn) or Hurst exponent were analyzed and compared in three groups (age 2 - 5 years, 6 - 9 and 10 - 12 years of age). Results: The RR interval increased with aging, but low-frequency powers did not. Highfrequency power was greater in the oldest children (P < 0.05), while ApEn and Hurst exponents were lower (P < 0.05). Conclusion: Change in HRV is one of the characteristics of development in children.
1. Introduction
Heart rate variability (HRV) is a physiologic change of heart rate or electrocardiographic R-R interval regulated by the autonomic nervous system [1], and is therefore under direct or indirect control of the brain [2,3] . HRV changes with aging, [4] anesthesia [5-7] and exercise [8, 9] . Indices of various nonlinear dynamic analyses such as approximate entropy (ApEn) or Hurst exponent (H) for HRV are useful for diagnosis or predictors of prognosis in some clinical situations [10-13] . The human brain develops continuously from birth to adolescence, and thus HRV should also change with aging. HRV becomes less random with increasing age in normal subjects in normal condition [14]. Special condition such as under general anesthesia, it may reflect the brainstem component [15]. Sevoflurane is commonly used in children and, during induction of anesthesia may depress parasympathetic activity more than halothane [6]. We hypothesized that in children, HRV might vary with age during sevoflurane anesthesia.
2. Methods
This prospective observational study of children requiring orthopedic surgery was done after institutional review board approval. Informed consent was obtained from parents or guardians. We recruited 138 children aged from 2 to 12 years (group T: 2 - 5, group P: 6 - 8, group S: 9 - 12). They were classified as American Society of Anesthesiologists physical status I. Exclusion criteria were neurologic abnormalities, history of epilepsy, concurrent antiepileptic drug, developmental delay, or heart problems (Table 1).
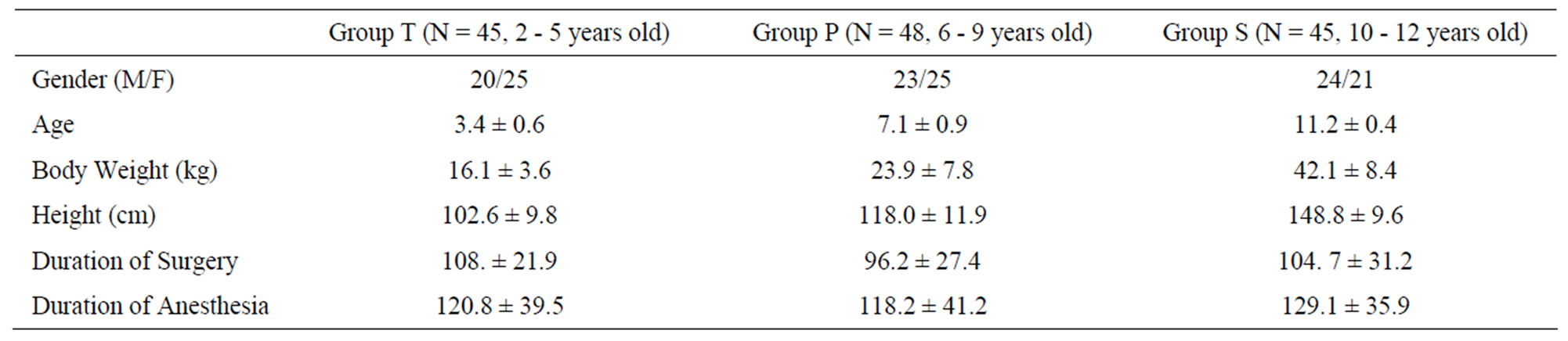
Table 1. Demographic characteristics.
Patients were fasted according to guidelines (at least 8 hours for solid food, 2 hours for clear fluid) and were appropriately hydrated. They arrived in the operating room without any premedication. Blood pressure was measured and the electrocardiogram (ECG), SpO2 (Solar 8000, GE, Milwaukee, WI) was attached. Another lead II ECG (MP100A-CE, Biopac Systems, Santa Barbara) was monitored for data collection. After preoxygenation, anesthesia was induced with pentothal 6 mg/kg and atropine 0.02 mg/kg (maximum dose: 0.5 mg) intravenously. Patients were ventilated with 8 vol% of sevoflurane in 100% of oxygen via mask after loss of consciousness. After full anesthesia and paralysis with 0.6 mg/kg of rocuronium, a laryngeal mask airway (LMA) of appropriate size was inserted or intubation was performed. Anesthesia was maintained with 2.5% sevoflurane in 35% oxygen in air with total flow of 3 l/min. Mechanical ventilation was at a tidal volume of 7 - 10 ml/kg and respiratory rate was adjusted by age (group T: 18/min, group P: 15/min, group S: 12/min) to give an end-tidal carbon dioxide tension of 35 - 40 mmHg. Lactated Ringer’s solution was administered at 8 ml/kg/h. When the inspiratory and end-tidal sevoflurane concentration had been maintained at 2.5 vol% for more than 10 minutes before surgery, we collected the ECG data for 15 minutes and stored it on a personal computer. At the same time, blood pressure and heart rate were checked and recorded at 5 minute intervals. The LMA or endotracheal tube was removed after surgery and the patients were transferred to the postanesthetic care unit (PACU) when fully awake and opening their eyes on verbal command.
2.1. Data Processing
All ECG data were reviewed by visual inspection. Segments with signal loss, significant noise or with atrial or ventricular extrasystoles were discarded. R peak was determined by the algorithm of Pan and Tompkins [16]. RR intervals (RRI) were measured and tested for the presence of outliers (HR < 30 or >200 beats/min), which were removed. All RRI data were linearly interpolated at 1000 Hz to construct a real-time series of RRIs, and then re-sampled at 2 Hz. The 1800 points (15 min of data) were used for calculating HRV indices. All data processing, including visual inspection, spectral analysis, and Hurst analysis, was conducted using Matlab 7.0.1 (Mathwork Inc., MA, USA).
2.2. Power Spectral Analysis
The RR interval data were divided into 512 data point sections (4.26 min of data). After being detrended, these sections were fast Fourier transformed [17] and averaged to form a power spectral density function. Power spectral analysis of HRV is regarded as the index of autonomic nervous systemic regulation for sinus rhythm. [18,19] We calculated low-frequency (LFP) and high-frequency power (HFP) by integrating the power spectral density curve in the 0.04 - 0.15 and 0.15 - 0.4 Hz ranges. LFP is regarded as representative of activity of sympathetic and parasympathetic activity and HFP of the parasympathetic.
2.3. Approximate Entropy (ApEn) [20,21]
The methodologic details for computing approximate entropy, ApEn (m, r, N), have been published elsewhere [20,21] and will be described briefly. ApEn (m, r, N) is approximately equal to the negative average natural logarithm of the conditional probability that 2 sequences are similar for m points remain similar, that is, within a tolerance, r, at the next point. Thus, a low value of ApEn reflects a high degree of regularity.
2.4. Hurst Analysis Using Structure Function [22,23]
The tth RRI was denoted as RRI(t) and we calculated the fluctuations in the difference as the structure function of RRI 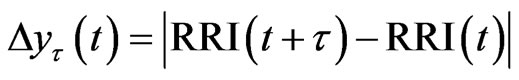 for different time increments, τ s. Δyτ(t) is the magnitude of the change in RRI after t s. In order to perform Hurst analysis, we estimated the average of these local variations, which depended on the scaled time increments as shown in the following equation:
for different time increments, τ s. Δyτ(t) is the magnitude of the change in RRI after t s. In order to perform Hurst analysis, we estimated the average of these local variations, which depended on the scaled time increments as shown in the following equation:
 (1)
(1)
where 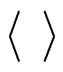 indicates the time average and ~ indicates the presence of the power law behavior of
indicates the time average and ~ indicates the presence of the power law behavior of 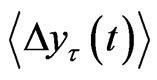 against τ
against τ
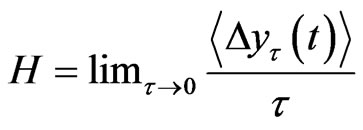
In order to quantify the scaling behavior of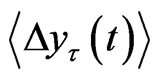 this value was plotted for
this value was plotted for . The scaling region was then obtained and the slopes of
. The scaling region was then obtained and the slopes of 
were calculated against t at the scaling region, thus yielding the Hurst exponent, H. Since H is the slope of 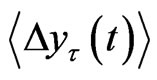 against τ in the log-log scale and H > 0, this value measures the rate of increase in the mean RRI difference over time τ. In other words, the exponent measures how the mean RRI changes as time advances.
against τ in the log-log scale and H > 0, this value measures the rate of increase in the mean RRI difference over time τ. In other words, the exponent measures how the mean RRI changes as time advances.
2.5. Statistics
Repeated-measures ANOVA was performed to compare the differences in HRV indices between groups; P-values < 0.05 were considered significant. All values shown are means (SD). All statistical analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Three patients (two in group T, one in group P) were excluded from analysis because of loss of data. RR intervals during sevoflurane anesthesia increased by 100 msec per year of age. These changes corresponded with decreases in heart rate (Figure 1) (P < 0.001). There were no significant changes in LFP. HFP in group S increased more than in the other groups (Table 2, P < 0.05).
ApEn and the Hurst exponent in group T and P were similar each other and those in group S decreased (Table 2, P < 0.05).
4. Discussion
HFP was greater in children over ten years of age than in younger ones during sevoflurane anesthesia, while ApEn and the Hurst exponent were less. The decrease in heart rate with aging has been frequently observed, and in this study RR intervals increased about 100 msec with aging (Table 2).
Signal linearity implies linear changes with time, and therefore it should be possible to predict the results of changes if we know the variation of signal changes by time.
On the other hand, it is hard to predict when the signal is nonlinear. The decrease in complexity of HRV in adults is well known [4].
In a previous study [24], power spectral analysis was performed on electrocardiographic data gathered from children and young adults aged 5 - 24 years in the supine and erect positions. LFP and HFP decreased in both positions with aging; in children of 10 - 12 years it decreased profoundly more than in those of 5 - 7 years.
In our study, HFP was maintained or increased in children of 10 - 12 years, which may imply maintenance of parasympathetic activity during sevoflurane anesthesia.
In the other study, girls (aged (9 ± 0.25) years) showed higher resting heart rate but HRV were not different between genders [25]. Our study also showed no difference between genders.
As the respiratory rate was fixed during collection of
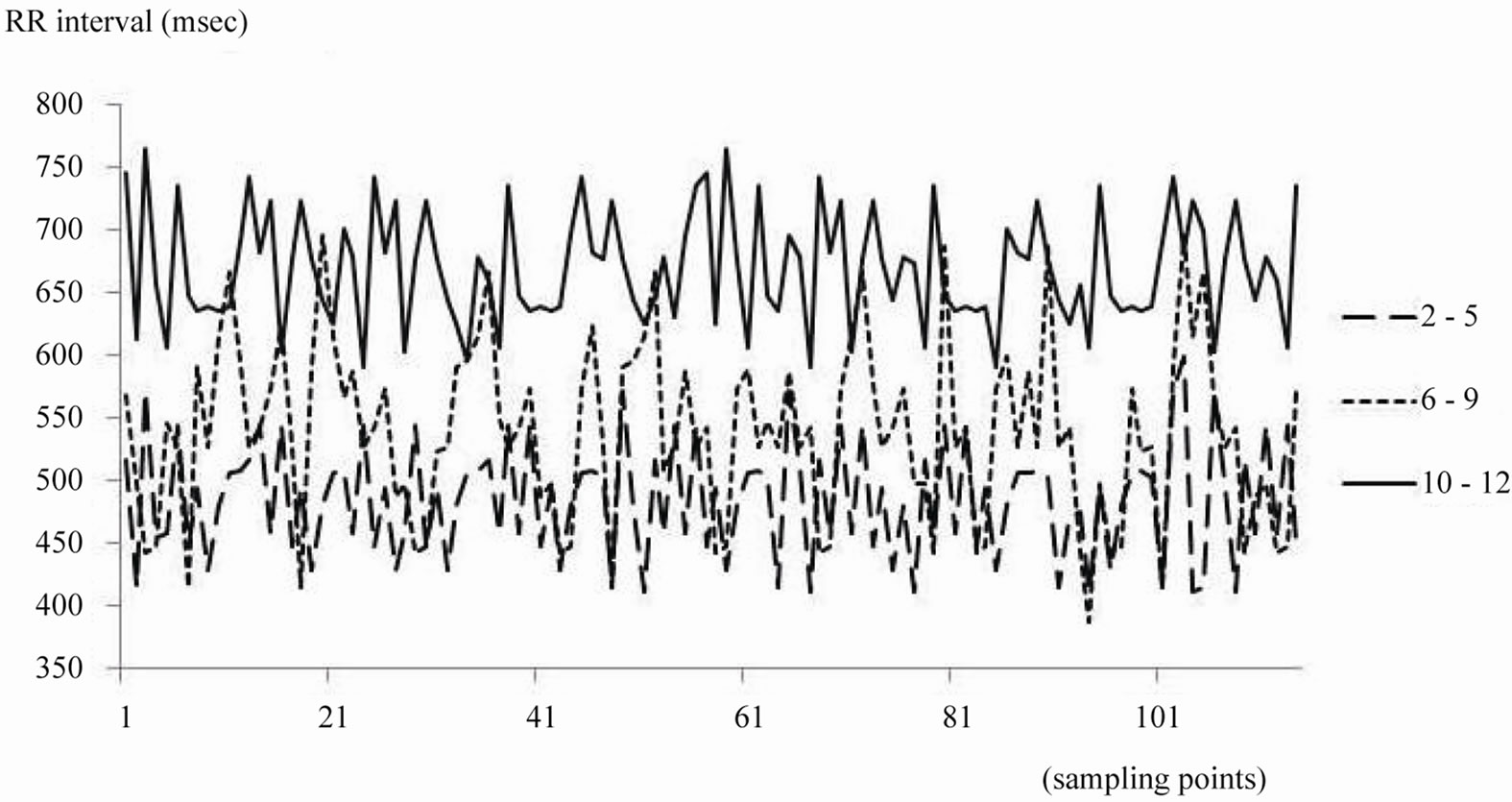
Figure 1. This shows RR intervals during sevoflurane anesthesia from 3 patients in each group.

Table 2. Parameters of cardiac autonomic nervous system.
data [26] and sevoflurane concentrations were the same in this study, it is likely that the difference in HFP is a function of age.
Nonlinearity of HRV as measured by ApEn or Hurst exponent was greatest in group S. ApEn is common used as an index of nonlinearity of signals changing over seconds [2,27] , and the Hurst exponent also expresses nonlinearity [23,28] and ApEn decreased with age [21].
Another study analyzed the ECG data from patients aged 1 - 82 years. Power spectral analysis showed that frequency domain power steadily increased until young adulthood and then decreased with age.
Generally spectral powers in children are relatively lower than those in adults but the complexity or nonlinearity of biosignals are greater than in adults. Development thus implies increasing regularity and decreasing complexity [4].
Although nonlinearity of HRV was maintained in our children under general anesthesia, activity of the autonomic nervous system, as represented by LFP or HFP, was inhibited, and thus caution should be exercised in the use of drugs which depress the autonomic nervous system.
HRV in Japanese children of 8 - 14 years suggested that the autonomic nervous system was developed fully at 10 years, and that maturation was achieved at 7 - 8 years of age [29]. Our study shows that nonlinearity of HRV changed above nine years of age during general anesthesia and this is quite similar results.
Our study had several limitations; we had ECG data only during general anesthesia and simply compared these data by age group since it is difficult to obtain data preoperatively in awaken children. Therefore we could not extend our results to awaken children. Second limitation was the arbitrary division into three groups.
In conclusion, during general anesthesia with sevoflurane, LFP was the same at all ages but HFP was greatest in children over 10 years, and the ApEn and Hurst exponent decreased above ten years of age. Thus parasympathetic activity was less well maintained in the younger children. These aging or developmentally associated changes suggest reason for caution.
5. Acknowledgements
This study was supported by Seoul National University (Fund No. 0420060620).
REFERENCES
- J. P. Saul, R. F. Rea, D. L. Eckberg, R. D. Berger and R. J. Cohen, “Heart rate and Muscle Sympathetic Nerve Variability during Reflex Changes of Autonomic Activity,” American Journal of Physiology, Vol. 258, No. 3, 1990, pp. H713-H721.
- C. Liu, P. Shao, L. Li, X. Sun, X. Wang and F. Liu, “Comparison of Different Threshold Values r for Approximate Entropy: Application to investigate the Heart Rate Variability between Heart Failure and Healthy Control Groups,” Physiological Measurement, Vol. 32, No. 2, 2011, pp. 167-180. doi:10.1088/0967-3334/32/2/002
- N. Haddad, R. B. Govindan, S. Vairavan, E. Siegel, J. Temple, H. Preissl, C. L. Lowery and H. Eswaran, “Correlation between Fetal Brain Activity Patterns and Behavioral States: An Exploratory Fetal Magnetoencephalography Study,” Experimental Neurology, Vol. 228, No. 2, 2011, pp. 200-205. doi:10.1016/j.expneurol.2011.01.003
- A. C. Takahashi, A. Porta, R. C. Melo, R. J. Quiterio, E. Da Silva, A. Borghi-Silva, E. Tobaldini, N. Montano and A. M. Catai, “Aging Reduces Complexity of Heart Rate Variability Assessed by Conditional Entropy and Symbolic Analysis,” Internal and Emergency Medicine, Vol. 7, No. 3, 2012, pp. 229-235. doi:10.1007/s11739-011-0512-z
- D. L. Toweill, W. D. Kovarik, R. Carr, D. Kaplan, S. Lai, S. Bratton and B. Goldstein, “Linear and Nonlinear Analysis of Heart Rate Variability during Propofol Anesthesia for Short-Duration Procedures in children,” Pediatric Critical Care Medicine, Vol. 4, No. 3, 2003, pp. 308-314. doi:10.1097/01.PCC.0000074260.93430.6A
- E. Wodey, L. Senhadji, P. Pladys, F. Carre and C. Ecoffey, “The relationship between Expired Concentration of sevoflurane and Sympathovagal Tone in children,” Anesthesia & Analgesia, Vol. 97, No. 2, 2003, pp. 377-382. doi:10.1213/01.ANE.0000068825.96424.F3
- Y. C. Arai, M. Nakayama, N. Kato, Y. Wakao, H. Ito and T. Komatsu, “The effects of Jaw Thrust and the Lateral Position on Heart Rate Variability in Anesthetized Children with Obstructive Sleep Apnea Syndrome,” Anesthesia & Analgesia, Vol. 104, No. 6, 2007, pp. 1352-1355. doi:10.1213/01.ane.0000262041.46833.21
- A. Cooke, M. Kavussanu, D. Mcintyre and C. Ring, “Effects of competition on Endurance Performance and the Underlying Psychological and Physiological Mechanisms,” Biological Psychology, Vol. 86, No. 3, 2011, pp. 370-378. doi:10.1016/j.biopsycho.2011.01.009
- M. Mizuno, T. Kawada, A. Kamiya, T. Miyamoto, S. Shimizu, T. Shishido, S. A. Smith and M. Sugimachi, “Exercise Training Augments the Dynamic Heart Rate Response to vagal but Not Sympathetic Stimulation in rats,” American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, Vol. 300, No. 4, 2011, pp. R969-R977. doi:10.1152/ajpregu.00768.2010
- J. Hu, J. Gao, W. W. Tung and Y. Cao, “Multiscale analysis of Heart Rate Variability: A Comparison of different Complexity Measures,” Annals of Biomedical Engineering, Vol. 38, No. 3, 2010, pp. 854-864. doi:10.1007/s10439-009-9863-2
- C. Jeleazcov, J. Schmidt, B. Schmitz, K. Becke and S. Albrecht, “EEG variables as measures of arousal during Propofol Anaesthesia for General Surgery in Children: Rational Selection and Age Dependence,” British Journal of Anaesthesia, Vol. 99, No. 6, 2007, pp. 845-854. doi:10.1093/bja/aem275
- H. Nazeran, R. Krishnam, S. Chatlapalli, Y. Pamula, E. Haltiwanger and S. Cabrera, “Nonlinear Dynamics Analysis of Heart Rate Variability Signals to Detect Sleep Disordered Breathing in children,” 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, 30 August-3 September 2006, pp. 3873-3878. doi:10.1109/IEMBS.2006.260709
- V. Papaioannou, M. Giannakou, N. Maglaveras, E. Sofianos and M. Giala, “Investigation of Heart Rate and Blood Pressure Variability, Baroreflex Sensitivity, and approximate entropy in acute Brain Injury Patients,” Journal of Critical Care, Vol. 23, No. 3, 2008, pp. 380-386. doi:10.1016/j.jcrc.2007.04.006
- U. R. Acharya, N. Kannathal, O. W. Sing, L. Y. Ping and T. Chua, “Heart Rate Analysis in Normal Subjects of Various Age Groups,” BioMedical Engineering OnLine, Vol. 3, No. 1, 2004, p. 24. doi:10.1186/1475-925X-3-24
- M. Maenpaa, T. Laitio, T. Kuusela, J. Penttila, K. Kaisti, S. Aalto, S. Hinkka-Yli-Salomaki and H. Scheinin, “Delta entropy of Heart Rate Variability along with Deepening Anesthesia,” Anesthesia & Analgesia, Vol. 112, No. 3, 2011, pp. 587-592. doi:10.1213/ANE.0b013e318208074d
- J. Pan and W. J. Tompkins, “A Real-Time QRS Detection Algorithm,” IEEE Transactions on Biomedical Engineering, Vol. 32, No. 3, 1985, pp. 230-236. doi:10.1109/TBME.1985.325532
- S. Akselrod, D. Gordon, F. A. Ubel, D. C. Shannon, A. C. Berger and R. J. Cohen, “Power Spectrum Analysis of Heart Rate Fluctuation: A Quantitative Probe of beat-toBeat Cardiovascular Control,” Science, Vol. 213, No. 4504, 1981, pp. 220-222. doi:10.1126/science.6166045
- A. Fujiki, A. Masuda and H. Inoue, “Effects of Unilateral Stellate Ganglion Block on the Spectral Characteristics of Heart Rate Variability,” Japanese Circulation Journal, Vol. 63, No. 11, 1999, pp. 854-858. doi:10.1253/jcj.63.854
- R. Furlan, S. Piazza, S. Dell’orto, F. Barbic, A. Bianchi, L. Mainardi, S. Cerutti, M. Pagani and A. Malliani, “Cardiac Autonomic Patterns Preceding Occasional Vasovagal Reactions in Healthy Humans,” Circulation, Vol. 98, No. 17, 1998, pp. 1756-1761. doi:10.1161/01.CIR.98.17.1756
- S. M. Pincus, T. R. Cummins and G. G. Haddad, “Heart Rate Control in normal and Aborted-SIDS Infants,” American Journal of Physiology, Vol. 264, No. 3, 1993, pp. R638-R346.
- S. M. Pikkujamsa, T. H. Makikallio, L. B. Sourander, I. J. Raiha, P. Puukka, J. Skytta, C. K. Peng, A. L. Goldberger and H. V. Huikuri, “Cardiac Interbeat Interval Dynamics from childhood to senescence: comparison of conventional and New Measures Based on fractals and Chaos Theory,” Circulation, Vol. 100, No. 4, 1999, pp. 393-399. doi:10.1161/01.CIR.100.4.393
- M. K. Yum and J. H. Kim, “A Very-Short-Term Intermittency of Fetal Heart Rates and Developmental Milestone,” Pediatric Research, Vol. 53, No. 6, 2003, pp. 915- 919. doi:10.1203/01.PDR.0000064945.53546.8E
- M. K. Yum, J. T. Kim and H. S. Kim, “Increased nonstationarity of Heart Rate during General Anaesthesia with sevoflurane or desflurane in children,” British Journal of Anaesthesia, Vol. 100, No. 6, 2008, pp. 772-779. doi:10.1093/bja/aen080
- J. P. Finley, S. T. Nugent and W. Hellenbrand, “HeartRate Variability in children. Spectral analysis of Developmental Changes between 5 and 24 years,” Canadian Journal of Physiology and Pharmacology, Vol. 65, No. 10, 1987, pp. 2048-2052. doi:10.1139/y87-320
- B. Krishnan, A. Jeffery, B. Metcalf, J. Hosking, L. Voss, T. Wilkin and D. E. Flanagan, “Gender differences in the relationship between Heart Rate Control and adiposity in Young Children: A Cross-Sectional Study (EarlyBird 33),” Pediatric Diabetes, Vol. 10, No. 2, 2009, pp. 127- 134. doi:10.1111/j.1399-5448.2008.00455.x
- M. Pöyhönen, S. Syväoja, J. Hartikainen, E. Ruokonen and J. Takala, “The effect of Carbon Dioxide, Respiratory Rate and Tidal Volume on Human Heart Rate Variability,” Acta Anaesthesiologica Scandinavica, Vol. 48, No. 1, 2004, pp. 93-101. doi:10.1111/j.1399-6576.2004.00272.x
- A. Jovic and N. Bogunovic, “Electrocardiogram Analysis Using a combination of Statistical, Geometric, and nonlinear Heart Rate Variability Features,” Artificial Intelligence in Medicine, Vol. 51, No. 3, 2011, pp. 175-186. doi:10.1016/j.artmed.2010.09.005
- P. Abry, H. Wendt, S. Jaffard, H. Helgason, P. Goncalves, E. Pereira, C. Gharib, P. Gaucherand and M. Doret, “Methodology for Multifractal Analysis of Heart Rate Variability: From LF/HF Ratio to Wavelet Leaders,” Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, 31 August-4 September 2010, pp. 106-109. doi:10.1109/IEMBS.2010.5626124
- Y. Fukuba, H. Sato, T. Sakiyama, M. Yamaoka Endo, M. Yamada, H. Ueoka, A. Miura and S. Koga, “Autonomic Nervous Activities Assessed by Heart Rate Variability in preand Post-Adolescent Japanese,” Journal of Physiological Anthropology, Vol. 28, No. 6, 2009, pp. 269-273. doi:10.2114/jpa2.28.269

