Open Journal of Acoustics
Vol.04 No.03(2014), Article ID:49738,6 pages
10.4236/oja.2014.43014
Measurement of Elastic Parameters of Lithium Hydroxylammonium Sulphate Single crystal, by ultrasonic Pulse Echo Overlap technique
George Varughese1*, Santhosh Kumar2
1Department of Physics, Catholicate College, Pathanamthitta, India
2SPAP, Mahatma Gandh University, Kottayam, India
Email: *gvushakoppara@yahoo.co.in
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 25 July 2014; revised 16 August 2014; accepted 12 September 2014
ABSTRACT
Ultrasonics is the most established and precise technique to determine the elastic parameters of materials. Elastic constants are important parameters of a crystal which provide valuable information about the bonding characteristic between adjacent atomic planes and the anisotropic character of the bonding and structural ability. Second order elastic constants can be measured by measuring the velocity of the ultrasonic pulses of different polarization along different symmetry directions. Elastic Constants of Lithium Hydroxylammonium Sulphate [LHAS] single crystal by ultrasonic Pulse Echo Overlap [PEO] technique are reported for the first time. Large single crystals of LHAS of size 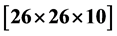 mm3 have been grown from supersaturated aqueous solution of the salt by slow evaporation technique over a period of 40 - 45 days at 305 K. Absolute velocities at room temperature (303 K) have been measured for the selected direction and modes with McSkimin
mm3 have been grown from supersaturated aqueous solution of the salt by slow evaporation technique over a period of 40 - 45 days at 305 K. Absolute velocities at room temperature (303 K) have been measured for the selected direction and modes with McSkimin  criterion. The anisotropy in the elastic properties of LHAS is well studied by measuring ultrasonic velocity in the crystal in certain specified crystallographic directions. The elastic stiffness constants,
criterion. The anisotropy in the elastic properties of LHAS is well studied by measuring ultrasonic velocity in the crystal in certain specified crystallographic directions. The elastic stiffness constants, 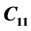 ,
, 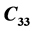 ,
, 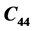 ,
,  and
and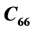 , and Acoustic impedance constants and Rao’s constants in specified directions are evaluated.
, and Acoustic impedance constants and Rao’s constants in specified directions are evaluated.
Keywords:
Inorganic compounds, Crystal growth, Ultrasonic velocity, Elastic constants

1. Introduction
The second order elastic stiffness constants play an important role in determining the strength of materials, provide valuable information about the bonding characteristic between adjacent atomic planes and the anisotropic character of the bonding and structural ability. The elastic behavior of an asymmetric crystal is determined by 21 independent elastic constants. The number of independent elastic constants will vary with symmetry of the crystal. It is 9 for orthorhombic crystal and they are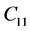 ,
,  ,
, 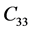 ,
, 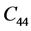 ,
, 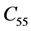 ,
, 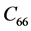 ,
, 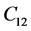 ,
, 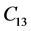 , and
, and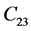 . Ultrasonics is the most established technique to determine the elastic moduli of both static and dynamic properties. Measurements of the elastic constants of a crystal as a function of temperature enable one to locate phase transition points. Second order elastic constants can be measured by measuring the velocity of the ultrasonic pulses of different polarization along different symmetry directions. Different techniques have been developed for precise measurement of velocity. Of these Pulse Echo Overlap (PEO) technique is the most accurate and precise one. In Lithium Hydroxylammonium Sulphate [LiNH3OHSO4] crystal, no work has been reported on elastic properties.. In the present study Elastic constants are measured, by ultrasonic Pulse Echo Overlap [PEO] technique for the first time. It crystallizes in the orthorhombic symmetry with space group Pbca with lattice parameters are reported to be
. Ultrasonics is the most established technique to determine the elastic moduli of both static and dynamic properties. Measurements of the elastic constants of a crystal as a function of temperature enable one to locate phase transition points. Second order elastic constants can be measured by measuring the velocity of the ultrasonic pulses of different polarization along different symmetry directions. Different techniques have been developed for precise measurement of velocity. Of these Pulse Echo Overlap (PEO) technique is the most accurate and precise one. In Lithium Hydroxylammonium Sulphate [LiNH3OHSO4] crystal, no work has been reported on elastic properties.. In the present study Elastic constants are measured, by ultrasonic Pulse Echo Overlap [PEO] technique for the first time. It crystallizes in the orthorhombic symmetry with space group Pbca with lattice parameters are reported to be



2. Material and Methods
2.1. Crystal Growth
Single crystal of LHAS have been grown by slow evaporation method from an aqueous solution of Hydroxylamine Sulphate (NH3OH)2 SO4 and Lithium Sulphate (Li2SO4) in stoichiometric quantities at 45˚C. figure 2 and figure 3 depict morphology and photograph of solution grown crystal. There are difficulties in growing the crystal. Spurious bubbles are forming in the growing medium. These bubbles may incorporate in the crystal and may cause defect in the growing crystal. By adjusting the growth temperature this can be minimized and the suitable temperature was found to be 45˚C. Increasing the speed of the solution stirrer also minimize the incorporation of the spurious air bubbles into the crystal. Another difficulty is the tendency of these crystals to grow in thin plates. But the thickness of the crystal can be increased by using a cut samples as a seed crystal. Also recrystallization by several times improves the thickness to a suitable size. Taking these factors, the growth can be controlled and single crystal of size 
The crystallographic axes are identified using the software J crystal (stereographic net) and Shape. The interfacial angles are measured using a contact goniometer. The measured angles were well in agreement with the computed values. By knowing the lattice parameters and crystal system one can construct a stereographic plot by using the computer program “Jcrystal”. The natural faces of the sample have been identified by comparing the computed value and the measured value. The grown crystal is cut into required thickness along different crystallographic directions using a slow speed Diamond Wheel Saw. An interesting feature was noted while cutting the crystal was, that the crystal could be easily peeled along the c-direction even with a sharp blade. This is because of the crystal have a cleavage plane perpendicular to c-axis. Thus cutting the crystal along b-direction is difficult; and hence measurement along that direction was forced to avoid. Also care should be taken while cutting the crystal in a-direction. Bulk samples have been cut using a slow speed diamond wheel saw (model-650, South Bay technology, USA). The diamond saw consists of a thin metal disc with micro sized diamond powder embedded on the outer edge. The blade fixed to a rotation mechanism is driven by a speed controlled motor. The
Figure 1. Distribution of atoms in the unit cell of of LHAS crystal in the a-c plane.
Figure 2. Morphology of the LHAS crystal.
Figure 3. Photograph of the grown LHAS crystal.
crystal to be cut is glued to a precision movable arm with a goniometer and counter weight. Once the directions are carefully adjusted with respect to the blade, the arm can be lowered so that the crystal rests on the rotating blade edge. The blade is continuously cooled and cleaned by a coolant, which is kept below the tray. One advantage of this diamond wheel saw is that samples can be cut easily to have parallel faces. This is very important for ultrasonic work because the non-parallelism will bring in a non-exponentially decaying echo pattern and thereby additional errors in velocity measurements. Samples with pairs of parallel planes perpendicular to [100] and [001] directions have been prepared for ultrasonic wave velocity measurements. All cuttings are made very accurately. The error due to disorientation is below ±0.5˚. The edges of the samples have been polished carefully using cerium oxide powder to optical reflection level so as to ensure proper bonding of the transducer to the sample surface. Polishing of the sample and cleaning of the transducer are very important for successful bonding. To make the bond thin, the transducer has to be held pressed to the sample under spring action.
2.2. Measurements of Elastic constants
The elements of determinantal equation are defined by elastic constants and the direction cosines of the direction of propagation [6] [7] . Non zero elastic constants are shown in the determinant.
LHAS being an Orthorhombic crystal, has the following nine second order elastic stiffness constants
















direction,

Then the three pure modes will be, 













These are also pure modes with 






The elastic constant 

The elastic constant 


where 


Acoustic Impedance constants [11] of the crystal in specified direction are given by

where 


where 


2.3. Ultrasonic Velocity measurements
The ultrasonic velocities are measured using the PEO technique [9] . The details of measurement technique are by Papadakis [10] . A MATEC model 7700 pulse modulator and receiver system with its associated subunits has been used for the velocity measurements. 


3. Results and Discussions
Of the 18 propagation modes, velocity measurements of 12 modes are sufficient to evaluate all the six independent second-order elastic constants and three dependent constants with cross checks possible using the remaining modes. Whereas the present studies have been reported 5 elastic constants. Considering all experimental uncertainties, the absolute accuracy of elastic constant value is estimated to be better than 0.2%. In all velocity measurements, the correct overlap identification is made and McSkimin 
The diagonal elastic constant 
Table 1. Measured velocities and Elastic constants of LHAS crystal at 303 K.
The abbreviation used have the following meaning: L-Longitudinal; T-Transverse. Then v is the velocity of propagation of respective mode and ρ is the density of the sample = 2002 kg/m3. and molar mass 0.137 kg for LHAS crystal.
Table 2. Measured velocities and Acoustic impedance constants of LHAS crystal at 303 K.
Table 3. Measured velocities and Rao’s constants (Molar velocity) of LHAS crystal at 303 K.
stants are the components obtained from the inverse matrix of elastic constants.
4. Conclusion
LHAS crystal of large size has been grown using slow evaporation solution growth technique. PEO technique has been successfully implemented for evaluating 5 elastic constants










References
- Vilminot, S., Anderson, M.R. and Brown, D. (1973) The Crystal Structure of Lithium Hydroxylammonium Sulphate. Acta Crystallographica, B29, 2628. http://dx.doi.org/10.1107/S056774087300720X
- Brown, I.D. (1964) The Crystal Structure of Lithium Hydrazinium Sulfate. Acta Crystallographica, 17, 654-660. http://dx.doi.org/10.1107/S0365110X64001591
- Anderson, M.R., Brown, I.D. and Vilminot, S. (1973) The Crystal Structure of Lithium Hydrazinium Fluoroberyllate. Acta Crystallographica, B29, 2625-2627. http://dx.doi.org/10.1107/S0567740873007193
- Chung, S.J. and Hahn, T. (1972) Tetrahedral-Framework Structures of NH4LiBeF4 and CsLiBeF4. Materials Research Bulletin, 7, 1209-1217. http://dx.doi.org/10.1016/0025-5408(72)90100-6
- Mahadevan Pillai, V.P. and Pradeep, T. (1992) Vibrational Spectra of LiNH3OHSO4 and LiND3ODSO4. Journal of Raman Spectroscopy, 23, 235-237. http://dx.doi.org/10.1002/jrs.1250230408
- JCPDS Card No: 29-792 (1976).
- Truell, R., Elbaum, C. and Chick, B.B. (1969) Ultrasonic Methods in Solid State Physics, Academic Press, New York, 370. http://dx.doi.org/10.1016/B978-1-4832-3318-5.50003-1
- Nye, N.F. (1957) Physical Properties of Crystals. Oxford Univ. Press, London, 145. http://www.mediafire.com/?3wx2faaheaepkyx
- Papadakis, E.P. (1967) Ultrasonic Pulse Velocity by the Pulse-Echo Overlap Method Incorporating Diffraction Phase Correlation. The Journal of the Acoustical Society of America, 42, 1045.
- Papadakis, E.P. (1976) Ultrasonic Velocity and Attenuation: Measurement Methods with Scientific and Industrial Applications. In: Mason, W.P. and Thurston, R.N., Eds., Physical Acoustics: Principles and Methods, Academic Press, New York, 227-374.
- Ali, A. and Nain, A.K. (1996) Ultrasonic Study of Molecular Interactions in N, N-Dimethylacetamide+ Ethanol Binary Mixtures at Various Temperatures. Acoustics Letters, 19, 181-187.
- Godfrey, L., Philip, J. and Sebastian, M.T. (1994) Elastic Constants and High-Temperature Elastic Anomalies near 425 K in Lithium Hydrazinium Sulfate. Journal of Applied Physics, 75, 2393.
- May Jr., J.E. (1958) Circuit Theory; Ultrasonic engineering. IRE National Convention Record, 6, 134-142.
- McSkimin, H.J. (1961) Pulse Superposition Method for Measuring Ultrasonic Wave Velocities in Solids. The Journal of the Acoustical Society of America, 33, 12. http://dx.doi.org/10.1121/1.1908386
- McSkimin, H.J. and Andreatch, P. (1962) Analysis of the Pulse Superposition Method for Measuring Ultrasonic Wave Velocities as a Function of Temperature and Pressure. The Journal of the Acoustical Society of America, 34, 609. http://dx.doi.org/10.1121/1.1918175
- McSkimin, H.J. (1964) Ultrasonic Methods for Measuring the Mechanical Properties of Liquids and Solids. In: Mason, W.P., Ed., Physical Acoustics, Academic Press, New York, 271.
- Godfrey, L. and Philip, J. (1995) Numerical Technique for Bond Correction in Ultrasonic Measurements. Acoustics Letters, 19, 111-114.
NOTES
*Corresponding author.







