Open Journal of Obstetrics and Gynecology
Vol. 2 No. 1 (2012) , Article ID: 18348 , 7 pages DOI:10.4236/ojog.2012.21005
Elective oocyte freezing for the preservation of fertility
![]()
1Reproductive Medicine Associates of New York, New York, USA
2Reproductive Endocrinology, Mount Sinai School of Medicine, New York, USA
Email: *acopperman@rmany.com
Received 11 January 2012; revised 10 February 2012; accepted 29 February 2012
Keywords: Human Oocyte Cryopreservation; Fertility Preservation; Oocyte Aging; Ovarian Reserve; Follicle Stimulating Hormone; Basal Antral Follicle Count
ABSTRACT
Oocyte cryopreservation has recently emerged as an option for women to preserve their fertility for medical (e.g. treatable malignancy) or elective indications (e.g. advancing age). This report describes an IRBapproved study of over 200 oocyte cryopreservation cycles at one center. Patients presenting for oocyte cryopreservation (January 2005 to 2010) were analyzed for day 3 follicle stimulating hormone (FSH), basal antral follicle count (BAFC), gonadotropin usage and the number of oocytes retrieved and cryopreserved. New patient consultations were performed on 516 women, of whom 175 (34%) proceeded to initiate a total of 233 cryopreservation cycles. Twenty-four cycles were cancelled (10%) after starting follicular stimulation due to poor ovarian response or self-withdrawal of the patients. Patients whose cycles were cancelled demonstrated a higher Day 3 FSH and a lower BAFC than patients who completed cycles (p < 0.01). In the 209 completed cycles, the most important predictors of a successful cycle included BAFC (r = 0.36), FSH (r = –0.25) and age (r = –0.18) with the mean number of oocytes cryopreserved at 13.6 ± 8.8. Information about long-term fertility preservation must reach both patients and health care providers so that more women can be educated about the benefits of proactive early physiological reproductive assessment and possible interventions available.
1. INTRODUCTION
The first successful birth following oocyte cryopreservation was reported in 1986 [1]. Advances in the technique were slow to occur over the next decade with only a limited number of individuals attempting to freeze and thaw the largest cell in the human body [2-4]. Due to the human oocyte’s size, precisely organized cytoplasmic architecture and its subsequent large water content; the process of slow freezing and rapid thawing initially failed to produce acceptable survival rates and desired outcomes [2]. Attempts to revitalize research into freezing human oocytes was undertaken after newly enacted reproductive governing laws instituted by the Italian government in 2004 [5], in which only a very limited number of retrieved oocytes could undergo attempted fertilization and no embryo cryopreservation was permitted. The remaining retrieved oocytes were discarded without attempting fertilization, thereby reducing the potential for successful pregnancies and live births. Investigations into modifications of cryopreservation techniques to improve water removal from the oocyte and the use of increased levels of cryoprotectants in the freezing mediums were undertaken to preserve the potential of the oocytes. For example, the increase in sucrose levels in both the freezing and thawing media allowed better water removal and reduced the amount of crystallization damage observed during the cryopreservation processes [6-10]. Moreover, increased concentrations of cryoprotectants, such as 1,2-propanediol, strengthened the cytoplasmic structural stability [2,4].
While the combinations of increased sucrose and cryoprotectants have raised the survival of the oocytes, a significant difference in the fertilization rate was observed between fresh oocytes and those that had undergone oocyte cryopreservation [4,6]. It was believed that the zona pellucida was altered during the freezing process, thus inhibiting the entry of sperm during attempted normal fertilization. Additionally, confocal microscopy demonstrated the reassembly of the oocyte cytoplasmic architecture was found to take hours following the thaw process [7,9]. These issues were overcome by first allowing the thawed oocyte to rest and recover for a few hours post thawing before attempting fertilization and second, using Intracytoplasmic Sperm Injection (ICSI) to achieve fertilization [4,6]. A review article by Gook and Edgar [11] reported that multiple centers now report successful freezing and thawing of oocytes using the slow freeze and rapid thaw techniques.
A continued interest in the cytoplasmic architecture following oocyte freeze/thaw has led to research into the reduction or even elimination of sodium from the media [12]. Based on animal model findings, some authors have attempted investigating a reduced sodium media in human oocytes with some success [13]. Although this change in media makeup has led to the birth of infants in some authors’ centers, the technique is still too new to evaluate its full potential or for comparison with standard cryopreservation mediums [11].
Vitrification is a newer cryopreservation technique that has recently gained popularity within the reproductive community and the international press [11]. The vitrification technique has been reported by some to demonstrate a higher survival rate for oocytes following the rewarming procedure, possibly due to prevention of ice crystal formation [14]. Others have expressed concern regarding the high concentrations of cryoprotectants necessary to achieve vitrification [11]. Further evaluation of the new vitrification technique in comparison to the well-established slow freeze/rapid thaw technique would require multi-centered well-controlled comparison studies to determine the optimal technique [11]. Towards this end 2 recent publications have demonstrated no significant differences between the 2 cryopreservation techniques for cycle outcomes or the normality of resulting offspring [15,16], while one recent publication showed higher implantation rates in the vitrification group [17].
The application of oocyte cryopreservation by reproductive practitioners has been slow and cautious [18]. Most centers have first debated and analyzed the medical, ethical and psychological application of this new experimental procedure [19]. Only after an organized and rigorously analyzed IRB approval process was our previous clinical research study of oocyte cryopreservation and thaw initiated [20]. Significant discussion within the reproductive community continues, with special attention paid to the ethical, social and medical aspects of the research itself and ultimately the likelihood of the resultants oocytes altering the long-term fertility opportunities for patients. Strong nondirective medical and psychological counseling is recommended by the oversight committee within the reproductive community before studies are undertaken or this new technique is openly offered to patients [21].
Experimental advances in human oocyte cryopreservation and the completion of intense medical and psychological evaluations have enabled this new therapeutic modality to enter the clinical arena for elective long-term female fertility preservation. After a successful IRB approved study of oocyte cryopreservation and thaw resulting in three pregnancies and five healthy babies in four attempts, our center initiated another IRB approved protocol open to women who desired elective preservation of their fertility [20]. We report here our initial experience with this treatment program at our large academic reproductive center. Collection and analysis of data and peer-reviewed publication of findings will improve awareness of progress using this new technology. The relationship between chronological aging, physiological aging and successful cycle outcomes were evaluated, with discussion of the need for patient and physician education of the application of this new fertility preservation technique.
2. MATERIALS AND METHODS
This was a retrospective analysis of an IRB approved clinical trial. Data from women presenting for elective oocyte cryopreservation were reviewed between January 2005 and January 2010. Oocyte cryopreservation was initiated only after intensive clinical and psychological counseling of the patients. Controlled ovarian hyperstimulation was performed under the direct care of reproductive endocrinologists with continual hormonal and ultrasound monitoring of cycle progress prior to the patients taking human chorionic gonadotropin (hCG).
Ovarian stimulation protocols were individualized by the treating physician. Three stimulation protocols were included in the study: down regulation, antagonist and microflare. All three protocols used recombinant FSH (Gonal F®-follitropin alfa-Serono Inc, Rockland, MA or Follistim®-follitropin beta-Organon Inc, West Orange, NJ), human menopausal gonadotropins (HMG) (Repronex® or Menopur®, Ferring Pharmaceuticals, Suffern, NY), or a combination of medications. In the down regulation protocol, patients were suppressed by the administration of a GnRH agonist (Lupron®-leuprolide acetate-TAP Pharmaceuticals, North Chicago, IL) in the mid luteal phase followed by stimulation with gonadotropins, after the onset of withdrawal bleeding. In the antagonist protocol, oral contraceptives were given for approximately 21 days, and initiation of gonadotropins occurred on cycle day 2 (after withdrawal bleeding). When the leading follicle reached 14 mm in diameter, a GnRH antagonist (Cetrotide®-cetrorelix acetate-Serono Inc, Rockland MA or Antagon®-ganirelix acetate-Organon Inc, West Orange, NJ) was administered daily. Ovarian suppression with oral contraceptives was also utilized prior to initiating the microflare protocol. A GnRH agonist (Lupron®-leuprolide acetate-TAP Pharmaceuticals, North Chicago, IL) was administered on cycle day 2 (after withdrawal bleeding) during morning and evening hours (flare effect) (50 mcg BID). On cycle day 3, gonadotropins were added to the stimulation. All ovarian stimulations were monitored by the measurement of serum estradiol concentration and by ultrasonographic assessment of the follicle diameter on a daily or every other day basis. Final oocyte maturation was achieved by the administration of human chorionic gonadotropin (hCG) (Novarel®, Ferring Pharmaceuticals, Suffern, NY or Ovidrel®-choriogonadotropin alfa-Serono Inc, Rockland, ME) when at least two of the leading follicles reached 18 mm in diameter. Oocyte retrieval was performed 36 hours after hCG using standard surgical and embryological techniques, with the evaluation of the collected oocyte maturities occurring approximately 60 minutes after retrieval [20]. Oocyte cryopreservation was performed as previously described, approximately 90 minutes after retrieval using a slow freezing technique according to the media manufacturers research protocol (Medicult, Denmark), with all cryopreserved oocytes being stored in liquid nitrogen in patient specific labeled cryovessels [20]. Cycles were cancelled when low ovarian response to stimulation of fewer than four mature follicles was visualized ultrasonographically or when the patient wished to withdraw from the cycle.
Cycle-specific data were collected and variables analyzed for cycle cancellation rate, patient age, day 3 FSH, basal antral follicle count (BAFC), stimulation protocols, number of retrieved oocytes, number of cryopreserved oocytes, and oocyte maturity at cryopreservation. Characteristics of the variables across different ages and age groups (A < 35, B = 35 - 37, C = 38 - 40, D > 40) were analyzed. Chi Square, ANOVA and Pearson’s correlation coefficient were used for statistical analysis.
3. RESULTS
Following the completion of an informed IRB consent, pre-cycle screening tests, and both medical and psychological counseling, 233 cycles of elective oocyte cryopreservation were initiated in 175 patients. 130 patients underwent 1 cycle, 32 attempted 2 cycles, and 13 initiated 3 cycles. Of these controlled ovarian hyperstimulation cycles initiated, 24 (10.3%) were cancelled due to poor ovarian response (fewer than four mature follicles) or patient directed withdrawal from the study. The mean patient age of the cancelled cycles was 38.7 ± 1.8 same, with four patients being cancelled more than once. Of the 159 patients who completed 209 cases of oocyte retrieval, the ages ranged from 19.8 to 44.0 years, with a mean of 38.0 ± 3.2. The mean number of oocytes retrieved was 13.8 ± 8.9, and ranged from 2 to 55. The mean number of oocytes cryopreserved was 13.6 ± 8.8. Oocyte cryopreservation included both mature (Metaphase II) and immature oocytes (Metaphase I or Germinal Vesicle). Figure 1 summarizes the overall mean maturity of cryopreserved oocytes across the four patient age groups.
Table 1 describes several characteristics of the oocyte cryopreservation patient population by their age groups, including day 3 FSH, BAFC and average quantity of gonadotropin units used for stimulation.
Comparison of stimulation protocols demonstrated that the majority of oocyte cryopreservation patients received the antagonist protocol (Table 2).
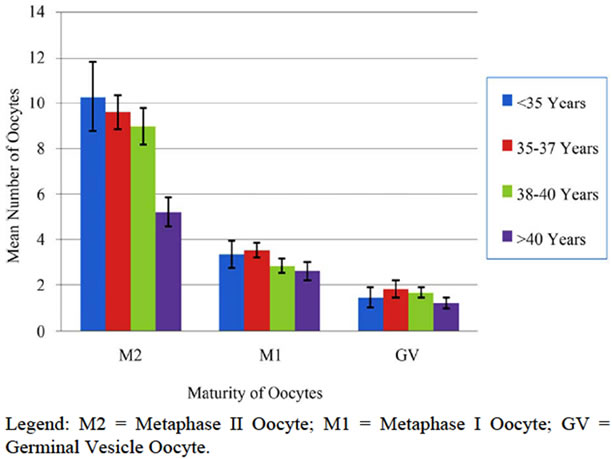
Figure 1. Maturity of oocytes cryopreserved by age group.
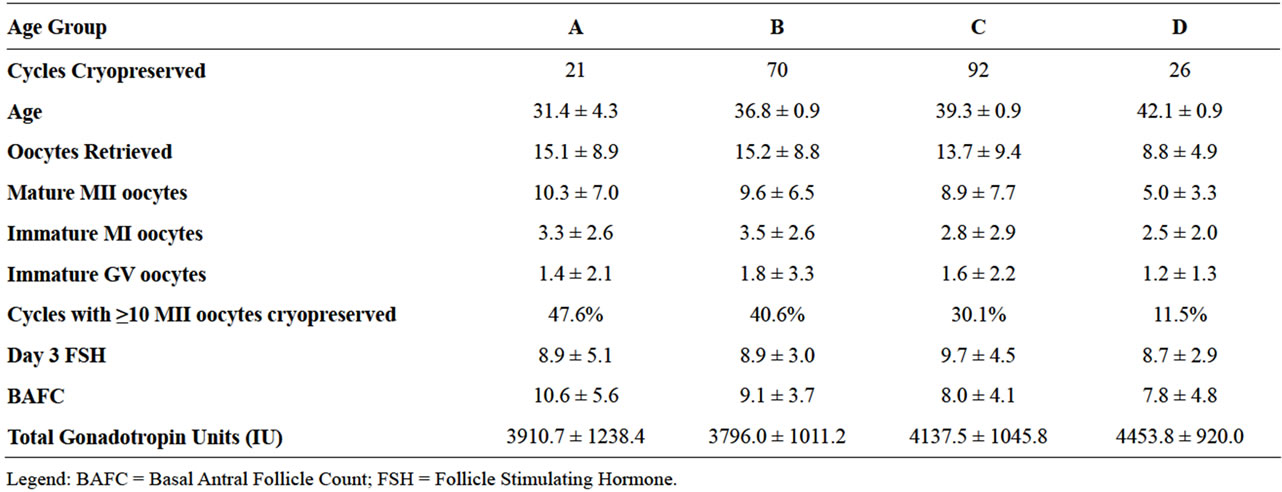
Table 1. Oocyte cycle outcomes by patient age groups.
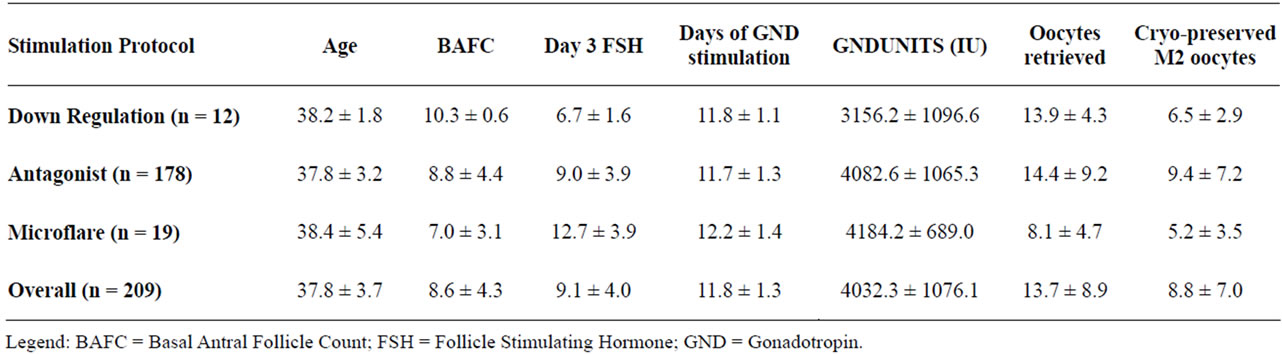
Table 2. Patient data by ovarian stimulation protocol.
Those who received the antagonist protocol had the greatest number of oocytes retrieved and produced the greatest number of mature oocytes for cryopreservation. Characteristics analyzed differed significantly among the three stimulation protocols for FSH (p < 0.001), Gonadotropin usage (p < 0.02), Oocytes retrieved (p < 0.02) and for the number of mature MII oocytes (p < 0.02).
The number of oocytes retrieved, cryopreserved, and the total number of mature oocytes were used in this study to measure the success of an oocyte cryopreservation cycle. We analyzed patient age, day 3 FSH, BAFC, peak E2, and the amount of gonadotropin used in each cycle using Pearson’s correlation coefficient. Patient age was found to be negatively associated with the numbers of retrieved, cryopreserved and of mature oocytes (p < 0.03). Day 3 FSH was associated with the number of oocytes retrieved, cryopreserved and the number of mature oocytes (p < 0.001). In addition, BAFC was shown to be indicative of the numbers of retrieved, cryopreserved and the number of mature oocytes (p < 0.001). Total gonadotropin dosage was a negative predictor of a successful oocyte cryopreservation cycle (p < 0.001), whereas peak E2 was positively associated with a successful oocyte cryopreservation cycle (p < 0.001).
Sorting the data according to the Society Associated Reproductive Technologies (SART) age groups (A < 35, B = 35 - 37, C = 38 - 40, D > 40), demonstrated that increasing maternal age was inversely correlated with number of oocytes retrieved, number of oocytes cryopreserved and number of mature oocytes (p < 0.05) (Table 1). The number of oocytes retrieved, oocytes cryopreserved, or mature oocytes identified did not vary according to controlled ovarian hyperstimulation protocol (Table 2).
Analysis of the 24 oocyte cryopreservation cycles cancelled due to low response or patient withdrawal demonstrated that all but one had undergone the antagonist stimulation protocol. The average age of these cancelled patients was 38.7 ± 1.8, their day 3 FSH was 13.3 ± 7.3 and they had 6.2 ± 2.3 antral follicles. When compared with those patients that completed cycles, those with cancelled cycles had higher day 3 FSH (p < 0.01), lower BAFC (p < 0.01) and had a trend towards increased age (p = 0.0518).
4. DISCUSSION
While the technology of oocyte preservation has recently made tremendous strides, it is imperative that there be a continued vigilance towards proving both the safety and the efficacy. Such goals are met with experimental models, test populations, and appropriate patient selection with IRB approval and informed consent. The American Society for Reproductive Medicine (ASRM) mandates that patients be counseled appropriately, and emphasizes the importance of further scientific and clinical investigation with IRB oversight. Recently, EMD-Serono launched the HOPE Registry to follow patients longitudinally from oocyte thaw to babies at one year of age [22] making it necessary to evaluate patient characteristics and establish additional parameters of patients who undergo elective egg freezing. We report here the first and largest series on such patients and describe our experience with 175 women who initiated 233 cycles of elective oocyte cryopreservation.
One striking finding includes the fact that almost twothirds of women presenting for consultation did not go forward with an attempted cycle. Of those who did, the 10% cycle cancellation rate was unexpectedly high for a supposedly fertile population. It is possible that women seeking fertility preservation are either a self-selected population with a family history of diminished ovarian reserve, or may have already noted alterations in their menstrual cycle. In our previous publication on the psychological characteristics of women presenting for elective cryopreservation of oocytes, we have previously reported that most notable was their high level of education and their overwhelming self-description as being “intelligent” and “extroverted” [23]. A number of women said they were interested in egg freezing to take the pressure off the search for relationships. For them, cryopreservation meant the freedom to wait, and to not settle for a mate because they were in a rush to conceive. The pivotal event leading to oocyte cryopresesrvation was becoming aware of advances in this technology. Had they heard that such a technology existed when they were younger, most stated that they would have made use of it [23]. Of the 159 patients who completed the 209 cycles in our study, the mean number of oocytes retrieved was 13.8, yet only 69 cycles (33%) resulted in our goal of ≥10 mature oocytes for cryopreservation.
Analysis of this cohort of patients who underwent elective oocyte cryopreservation revealed that the chronological age of the patient was a significant indicator of a successful oocyte cryopreservation cycle. Specifically, age was negatively associated with the total number of mature oocytes obtained (p < 0.05). As with infertile patients who undergo IVF, chronological age was inversely correlated with the chance of achieving a successful oocyte cryopreservation cycle. Qualitative changes in oocytes are principally responsible for the low reproductive potential in older women, as demonstrated by enormous differences in fecundity with advancing age in natural cycles in fertile couples [24]. Several theories have been proposed to explain the decline in oocyte quality that occurs with advancing maternal age. In the so-called “production line hypothesis”, oocyte quality is established during fetal life, and oocytes that are less susceptible to nondisjunction are ovulated first, leaving poor quality oocytes to be ovulated later in life. Another theory assumes an age-dependent accumulation of damage due to several proposed mechanisms: for instance, a gradual increase in intracellular oxidative stress [25].
Ovarian aging is characterized by lowered pregnancy rates and decreased ovarian responsiveness to gonadotropin administration, due to a decreased quantitative and qualitative pool of oocytes. Early follicular phase (basal) FSH is a widely used endocrine marker to predict ovarian reserve in women presenting for fertility evaluation and treatment [26]. Elevation in basal FSH levels are thought to be a reflection of ovarian aging, and the low pregnancy rates in these patients is related to the natural age dependent decline in oocyte quality [27]. Substantial data has shown that basal FSH can be an independent predictor of IVF outcome [26,28]. It has been demonstrated that elevation in this parameter is strongly associated with poor ovarian response, low peak estradiol levels achieved in response to gonadotropin stimulation and low pregnancy rates in patients undergoing assisted reproductive technologies, independent of age [29]. Authors have also suggested low conception rates and high fetal loss rates in a group of infertile patients with elevated FSH levels [30]. Our data demonstrated a significant negative association between day 3 FSH and the number of oocytes retrieved (p < 0.001), the number of oocytes cryopreserved (p < 0.001) and the number of mature oocytes (p < 0.001).
The number of antral follicles in the early follicular phase correlates with ovarian reserve [31]. Low numbers of antral follicles are a sign of ovarian aging, and are observable earlier than a rise in FSH serum level [32]. Problems with antral follicle count (AFC) include observed cycle-cycle differences, biological variation and intra-observer differences. However, it has been proposed that AFC is possibly a better prognostic indicator than age or endocrine markers [33]. We found that BAFC was positively associated with the number of oocytes retrieved, cryopreserved and the number of mature oocytes (p < 0.001). Thus, BAFC is considered a predictor of ovarian reserve and was directly indicative of a successful oocyte cryopreservation cycle.
Overall our findings demonstrate that the best predictors of a successful oocyte cryopreservation cycle, number of mature oocytes cryopreserved, are BAFC (r = 0.36), FSH (r = –0.25) and finally age (r = –0.18). Our findings suggest that patients are presenting after the reproductive age at which this elective procedure could most optimally serve and would most greatly be of benefit to them. Currently, the ASRM recommends oocyte cryopreservation be performed only under an IRB approved study protocol and that significant attention should be paid to the medical and psychological health of the patients. We previously suggested that further large studies should be carried out by individual centers before the application of this new technology becomes widespread [19].
Education and counseling of the female population is vital for their understanding of the significant potential that oocyte cryopreservation carries. As chronological age is not the most important indicator of a successful cryopreservation cycle, the application of early testing for physiological aging (day 3 FSH and BAFC) provides the most important information to practitioners and patients to determine the need for fertility preservation as early as possible. Hopefully, scientific advances will result in lowering the physical, emotional and financial costs of oocyte cryopreservation. Stimulation protocols involving novel oral medications or advances in in vitro maturation may make the process more accessible to a greater number of women. Furthermore, continued work is needed to determine optimal techniques and methodology for cryopreservation of human oocytes. The technology of oocyte cryopreservation has advanced greatly in the last several years and review of our experience documents the significant need for improved public awareness regarding new information and for continued scientific investment to be made in this field.
5. ACKNOWLEDGEMENTS
The authors wish to thank Embryologist Lora Valluzzo, B.S., Research Assistants Lesley Chuang, M.S. and Casey McDonald, M.S, and Drs. Tanmoy Mukherjee and Lawrence Grunfeld, for their significant contribution to this manuscript.
REFERENCES
- Chen, C. (1986) Pregnancy after human oocyte cryopreservation. Lancet, 1, 884-886. doi:10.1016/S0140-6736(86)90989-X
- Gook, D.A., Osborn, S.M. and Johnston, W.I. (1993) Cryopreservation of mouse and human oocytes using 1, 2-propanediol and the configuration of the meiotic spindle. Human Reproduction, 8, 1101-1109.
- Gook, D.A., Osborn, S.M., Bourne, H. and Johnston, W.I. (1994) Fertilization of human oocytes following cryopreservation; normal karyotypes and absence of stray chromosomes. Human Reproduction, 9, 684-691.
- Gook, D.A., Schiewe, M.C., Osborn, S.M., Asch, R.H., Jansen, R.P. and Johnston, W.I. (1995) Intracytoplasmic sperm injection and embryo development of human oocytes cryopreserved using 1,2-propanediol. Human Reproduction, 10, 2637-2641.
- Fineschi, V., Neri, M. and Turillazzi, E. (2005) The new Italian law on assisted reproduction technology (Law 40/2004). Journal of Medical Ethics, 31, 536-539. doi:10.1136/jme.2004.010231
- Porcu, E., Fabbri, R., Seracchioli, R., Ciotti, P.M., Magrini, O. and Flamigni, C. (1997) Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertility and Sterility, 68, 724-726. doi:10.1016/S0015-0282(97)00268-9
- Porcu, E., Fabbri, R., Damiano, G., Giunchi, S., Fratto, R., Ciotti, P.M., et al. (2000) Clinical experience and applications of oocyte cryopreservation. Molecular and Cellular Endocrinology, 169, 33-37. doi:10.1016/S0303-7207(00)00348-8
- Fosas, N., Marina, F., Torres, P.J., Jové, I., Martín, P., Pérez, N., et al. (2003) The births of five Spanish babies from cryopreserved donated oocytes. Human Reproduction, 18, 1417-1421. doi:10.1093/humrep/deg297
- Bianchi, V., Coticchio, G., Fava, L., Flamigni, C. and Borini, A. (2005) Meiotic spindle imaging in human oocytes frozen with a slow freezing procedure involving high sucrose concentration. Human Reproduction, 20, 1078-1083. doi:10.1093/humrep/deh736
- Nottola, S.A., Coticchio, G., De Santis, L., Macchiarelli, G., Maione, M., Bianchi, S., et al. (2008) Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reproductive BioMedicine Online, 17, 368-377. doi:10.1016/S1472-6483(10)60220-9
- Gook, D.A. and Edgar, D.H. (2007) Human oocyte cryopreservation. Human Reproduction Update, 13, 591-605. doi:10.1093/humupd/dmm028
- Stachecki, J.J., Cohen, J. and Willadsen, S. (1998) Detrimental effects of sodium during mouse oocyte cryopreservation. Biology of Reproduction, 59, 395-400. doi:10.1095/biolreprod59.2.395
- Boldt, J., Cline, D. and McLaughlin, D. (2003) Human oocyte cryopreservation as an adjunct to IVF-embryo transfer cycles. Human Reproduction, 18, 1250-1255. doi:10.1093/humrep/deg242
- Katayama, K.P., Stehlik, J., Kuwayama, M., Kato, O. and Stehlik, E. (2003) High survival rate of vitrified human oocytes results in clinical pregnancy. Fertility and Sterility, 80, 223-224. doi:10.1016/S0015-0282(03)00551-X
- Noyes, N., Porcu, E. and Borini, A. (2009) Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reproductive BioMedicine Online, 18, 769-776. doi:10.1016/S1472-6483(10)60025-9
- Noyes, N., Knopman, J., Labella, P., McCaffrey, C., Clark-Williams, M. and Grifo, J. (2010) Oocyte cryopreservation outcomes including pre-cryo and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertility and Sterility, 94, 2078-2082. doi:10.1016/j.fertnstert.2010.01.019
- Oktay, K., Cil, A.P. and Bang, H. (2006) Efficiency of oocyte cryopreservation: A meta-analysis. Fertility and Sterility, 86, 70-80. doi:10.1016/j.fertnstert.2006.03.017
- De Melo-Martin, I. and Cholst, I.N. (2008) Researching human oocyte cryopreservation: Ethical issues. Fertility and Sterility, 89, 523-528. doi:10.1016/j.fertnstert.2007.03.039
- Barritt, J., Luna, M., Duke, M. and Copperman, A.B. (2007) Ethical issues surrounding the cryopreservation of human oocytes. Fertility and Sterility, 88, 1016. doi:10.1016/j.fertnstert.2007.07.1365
- Barritt, J., Luna, M., Duke, M., Grunfeld, L., Mukherjee, T., Sandler, B., et al. (2007) Report of four donor-recipient oocyte cryopreservation cycles resulting in high pregnancy and implantation rates. Fertility and Sterility, 87, 13-17. doi:10.1016/j.fertnstert.2006.04.052
- Practice Committee of the Society for Assisted Reproductive Technology, Practice Committee of the American Society for Reproductive Medicine (2007) Essential elements of informed consent for elective oocyte cryopreservation: A practice committee opinion. Fertility and Sterility, 88, 1495-1496. doi:10.1016/j.fertnstert.2007.10.009
- Ezcurra, D., Rangnow, J., Craigm M. and Schertzm J. (2008) The HOPE Registry: First US registry for oocyte cryopreservation. Reproductive BioMedicine Online, 17, 743-744. doi:10.1016/S1472-6483(10)60399-9
- Martinuzzi, K. and Copperman, A.B. (2007) Oocyte cryopreservation for elective preservation of reproductive potential. Current Women’s Health Reviews, 3, 209-215. doi:10.2174/157340407781387663
- Eichenlaub-Ritter, U. (1998) Genetics of oocyte ageing. Maturitas, 12, 143-169. doi:10.1016/S0378-5122(98)00070-X
- Tarín, J.J. (1995) Aetiology of age-associated aneuploidy: A mechanism based on the “free radical theory of ageing”. Human Reproduction, 10, 1563-1565.
- Muasher, S.J., Oehninger, S. and Sinometti, S. (1988) The value of basal and/or stimulated serum gonadotrophin levels in prediction of stimulation response and in vitro fertilization outcome. Fertility and Sterility, 50, 298-307.
- Sherman, B.M. and Korenman, S.G. (1975) Hormonal characteristics of the human menstrual cycle throughout reproductive life. Journal of Clinical Investigation, 55, 699-706. doi:10.1172/JCI107979
- Toner, J.P., Philput, C.B., Jones, G.S. and Muasher, S.J. (1991) Basal follicle stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertility and Sterility, 55, 784-791.
- Scott, R.T. and Hofmann, G.E. (1995) Prognostic assessment of ovarian reserve. Fertility and Sterility, 63, 1-11.
- Levi, A.J., Raynault, M.F., Bergh, P.A., Drews, M.R., Miller, B.T. and Scott, R.T. Jr. (2001) Reproductive outcome in patients with diminished ovarian reserve. Fertility and Sterility, 76, 666-669. doi:10.1016/S0015-0282(01)02017-9
- Frattarelli, J.L., Lauria-Costab, D.F., Miller, B.T., Bergh, P.A. and Scott, R.T. (2001) Basal antral follicle number and mean ovarian diameter predict cycle cancellation and ovarian responsiveness in assisted reproductive technology cycles. Fertility and Sterility, 75, 834-835.
- Scheffer, G.J., Broekmans, F.J., Dorland, M., Habbema, J.D., Looman, C.W. and Te Velde, E.R. (1999) Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertility and Sterility, 72, 845-851. doi:10.1016/S0015-0282(99)00396-9
- Nahum, R., Shifren, J.L., Chang, Y., Leykin, L., Isaacson, K. and Toth, T.L. (2001) Antral follicle assessment as a tool for predicting outcome in IVF—Is it a better predictor than age and FSH? Journal of Assisted Reproduction and Genetics, 18, 151-155. doi:10.1023/A:1009424407082
NOTES
*Corresponding author.

