World Journal of Neuroscience
Vol.3 No.2(2013), Article ID:31617,7 pages DOI:10.4236/wjns.2013.32011
Molecular signaling of 5-HT1A and presence of serotonergic cells in the fetal cerebral cortex
![]()
1Laboratorio de Neurontogenia, Departamento de Fisiología, Biofísica y Neurociencias, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Ciudad de México, México
2Unidad de Investigación Biomolecular, Hospital de Cardiología, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Ciudad de México, México
Email: *aboyzo@fisio.cinvestav.mx
Copyright © 2013 Alfonso Boyzo Montes de Oca et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 19 January 2013; revised 20 February 2013; accepted 17 March 2013
Keywords: Serotonergic System; Serotonin Receptor 5-HT1A; Molecular Signaling Paths
ABSTRACT
Early appearance of the serotonergic system in the fetal brain and the various effects of serotonin (5-HT) on brain morphogenesis, have given support to a neurotrophic role of serotonin. This function of serotonin is accomplished through a system of serotonin nerve terminals in the target regions that involves various 5-HT receptors. In visual, auditory and somatosensory cortex an early and intense serotonergic innervation is particularly important. The neuronal somata of these terminals are normally located in the mesencephalon and they have not been observed in the maturing cerebral cortex, neither in the adult brain. By using immunolabeling techniques, fluorescence and confocal microscopy, we observe the presence of both, 5-HT terminals and 5-HT cells in mesencephalon (Me, E17) and in the neopallium (Np, E13-E16) cocultures. Cells immunopositive to 5-HT and to tryptophan-5-hydroxilase are also observed in the Np on day 12 of culture. These results concerning the unexpected presence of serotonergic cells in the fetal cerebral cortex are interesting and may be of importance in corticogenesis. As it happens with other elements of the serotonergic system, the presence of these phenotypically serotonergic cells in the early cerebral cortex may be transitory and probably supporting cortex maturation processes. The molecular signaling path of the 5-HT1A receptor has also been identified.
1. INTRODUCTION
The early appearance of the serotonergic system in the fetal brain [1-3] together with the well known effects of serotonin (5-HT) during the morphogenesis of the central nervous system (CNS) [4] support that these early functions of 5-HT are exerted through a system of axon terminals in the target regions of the fetal brain, derived of neuronal somata exclusively located in the brainstem. A series of studies support 5-HT as an early appearing molecule (E11) during CNS development in the laboratory rat which has a neurotrophic role in advance to its neurotransmitter and neuromodulator role in the adult brain. Both 5-HT and its specific receptors bound to ionic channels or to metabotropic cascades have, at these early periods of brain development, various functions [5, 6]. We obtained in our laboratory complementary information on the functional role of 5-HT in neuroblastic structures like the axonal growth cones from rat E17 encephala, and the presence of the 5-HT1A and 5-HT1B receptors [3,7]; the 5-HT synthetic path as a well as a 5-HT releasing system K+ and Ca++ dependent and a Na+ dependent uptake system [3,8].
A transitory increase in the expression of SERT and 5-HT nerve terminals in sensory areas of the cerebral cortex, has also been described [9,10]. The early appearance of serotonergic terminals in the maturating cerebral cortex is particularly intense especially in the sensory areas: visual, somatosensory and auditory [9,11,12]. Mazer et al., [13] reported that experimental decrease of serotonergic signals alters the normal development and maturation of the cerebral cortex, decreasing the synaptic density with a consecutive damage of cognitive development. It is also known that 5-HT has a regulatory role on various cortical areas through axonic terminals of serotonergic neurons located in the mesencephalic raphe nuclei, and not observed, to our knowledge, in the developing cerebral cortex. Lebrand [14] proposed that regulation of corticogenesis by 5-HT, takes place through thalamo-cortical fibers, supported by the presence of 5-HT1A and 5-HT1B receptors and the serotonin transporter (SERT) in this system, also shown the presence of serotonergic receptors in fetal rat brain [15].
There are other types of early appearance neurons in the growing cerebral cortex like Cajal-Retzius in the marginal zone, related to cell migration and to the insideout cell development pattern of the cortex [16]. As well as gabaergic and tyrosine hydroxylase (TH) immunopositive neurons in the rat dorsolateral and medial cortex [17] prenatally and posnatally [15]. Probably their expression is epigenetically regulated, like in the peripheral nervous system [19,20], so that cortical neurons may transitorily express some enzyme molecules like TH [18]. Here we propose that the same may happen with the serotonin synthesizing enzyme Tryptophan-5-hidroxylase (T5-H), and other key 5-HT markers. So, in the present work we describe the presence of 5-HT cells in the fetal neopallium in coculture with the mesencephalon, and in also not cultured cortices in the same developmental stage, and besides the molecular signaling path of the 5-HT1A receptor, which is presented in the fetal cortical target area.
2. METHODS
Experimental Animals: Nuliparous female Wistar rats, 200 - 300 g body weight (bw), were used. Kept during two weeks before experiments under standard conditions: 12 hours light periods; 20˚C ± 1.5˚C temperature and 55% - 65%, humidity; fed water and Purina Chaw pellets ad-libitum. 250 g bw males were employed for mating and the presence of a vaginal plug was taken as the beginning of gestation. Crown-rump length was assessed to confirm gestational age. At E13, E16 or E17, a cesarean section was practiced under pentobarbital anesthesia (40 mg/Kg, ip), then the fetal encephala were dissected out and kept in a Petri dish in PBS at 4˚C. The experimental protocol was approved by The Internal Committee for the Use of Experimental Animals, according to the Official Mexican Norm (NPM-062-ZOO-1999) and to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publ. No. 8023, 1978).
Organotypic Cultures: The Stoppini method was generally followed, briefly: the E-17 encephala were included in low melting point agarose (8˚C - 17˚C, Sigma, 6% in PBS) [21]. 300 μm slices were obtained with a Vibratome (Pellco101, series 1000) in the area of the mesencephalon (Me), following the coordinates of Foster and Lavdas, where serotonergic cells are located [22,23] (Figures 1(a) and (b)). Neopallium (Np) fragments (0.5
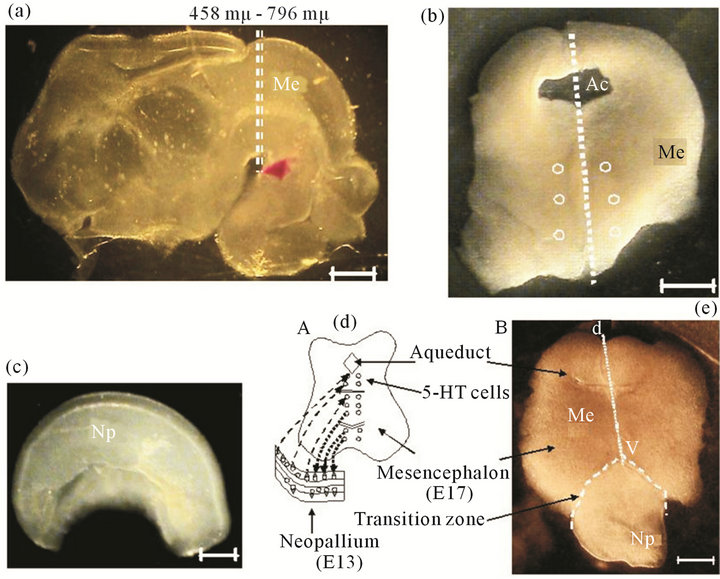
Figure 1. Microdisection procedure to obtain fetal encephalon (E17). (a) Lateral view of fetal rat encephalon. h, Hemispheres; Me, mesencephalon (E17). Dotted lines indicate the cross section to obtain a 300 μm mesencephalic slices (Foster 1998); (b) A 300 μm thick slice of mesencephalon that is used for the culture. Ac, aqueduct; dotted line, dorso-ventral axis (d-v); open circles, location of 5-HT cells. Scale bar = 1 mm; (c) Lateral view of a fetal hemisphere, the neopallium (Np, E13 or E16) is dissected and separated in sections of 0.5 to 1 mm that later are used in coculture. Scale bar = 1 mm; (d) Model proposed for the organotypic culture. Shows mesencephalon in contact to the neopallium, fiber communication paths (......) from Me to Np; or (- - -) from Np to Me; (e) Actual Me section and Np (E13 or E16), after 3 days in culture. (- - -) transition zone between both regions; Dorso-ventral axis of Me (d-v). Scale bar = 1 mm.
to 1.0 mm) from E13 or E16 encephala were dissected out (Figure 1(c)). Slices were set on culture wells on a permeable membrane (0.4 μm pore, Falcon), close to each other with 1.2 ml culture medium: Sigma MEMEagle, added with 24% of inactivated horse serum (By Products); 0.2 mM Hepes (Sigma); 2% glutamax (Gibco); 0.07% gentamicin (Sigma). Both regions were put in close contact (Figures 1(d) and (e)). Cultures were incubated at 37˚C in 5% CO2 (Hotpack, Phil., PA, USA) during 12 days and 50% of medium was changed every 48 hours.
Immunolabeling and Microscopic Fluorescence: Tissues were washed in cold phosphate buffer saline (PBS), pH 7.4 and fixed with 4% paraformaldehyde. Immunolabeling was carried out with polyclonal antibodies for 5-HT1A, 5-HT, serotonin transporter (SERT), MAPK, ERK1 or Vimentin (1:1000), or with monoclonal antibodies for T5-H, GFAP, Giα-1, MAPKK, Enolase or NeuN. Fluorescent secondary antibodies (1:500) were used for FITC (Jackson IR), Fluorescein (Pierce), Alexa 568 (Molecular Probes) and Lisamine-Rhodamine (LRSC, Jackson IR). Epifluorometry (Carl Zeiss, axioscope) or Confocal microscopy (Leica, TCS SP2) were performed to identify and locate the various labeled molecules. Appropiate negative controls omitting the primary antibodies were included in each experiment.
3. RESULTS
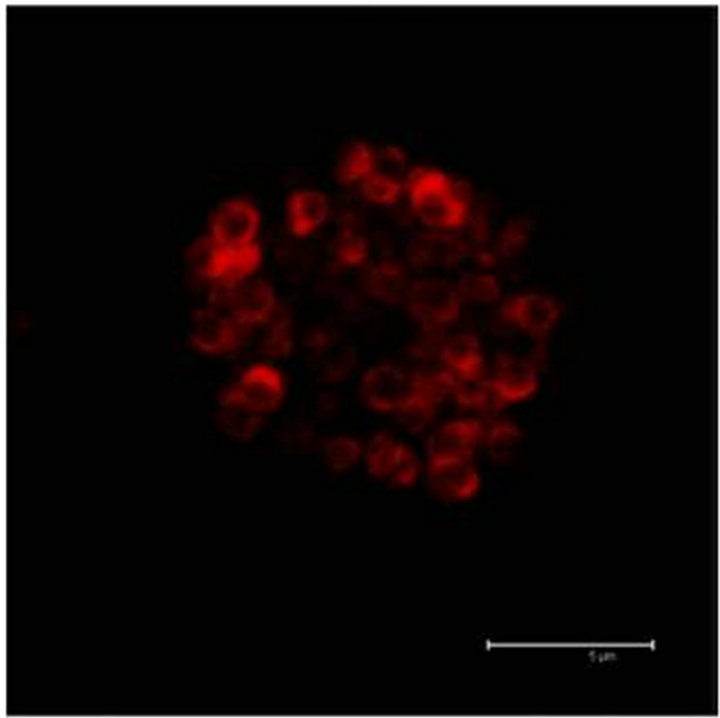
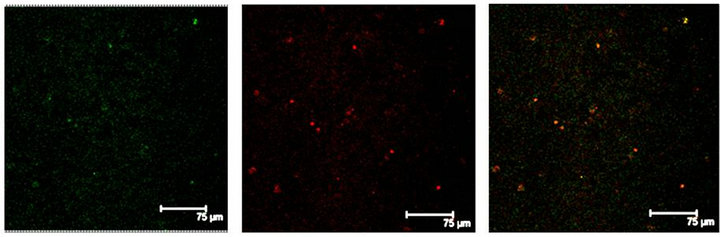
In Figure 2(a) there is a control that confirms the presence of 5-HT cells in the Me area in coculture. Figure 2(b) shows immunopositive cells for 5-HT in the Np. The rate limiting enzyme T5H, (Figure 2(c)) is in a not cultured Np. In Figure 2(d), 5HT1A Immunopositive cells also in the Np are shown.
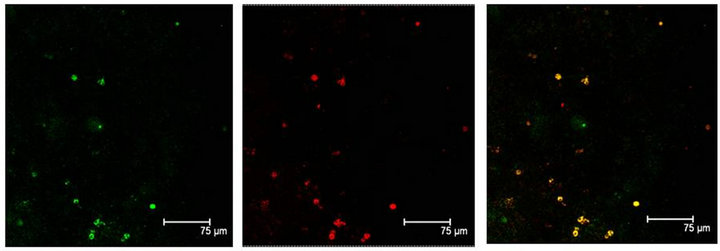
Immunopositive cells to T5H and 5-HT coexisting in the same cell were also observed in coculture or in not cultured Np (Figures 3(a) and (b)). Neuronal markers Enoalse or NeuN were positive together with 5-HT, in culture or in not cultured Np (Figures 4 (a) and (b)), SERT colocalizes with T5H or 5-HT in cells of the Np (Figures 5(a) and (b)), GFAP label also did coexist with the serotonin marker (T5H) in cells of cultured or in not cultured Np (Figures 6(a) and (b)), GFAP coexist with 5-HT in cells of not cultured Np (Figure 6(c)). The signaling molecules (Giα-1, MAPK, MAPKK and ERK1) also do colocalize with their receptor 5-HT1A, in the cocultured as well as in not cultured Np (Figures 7-11).
 (a)
(a)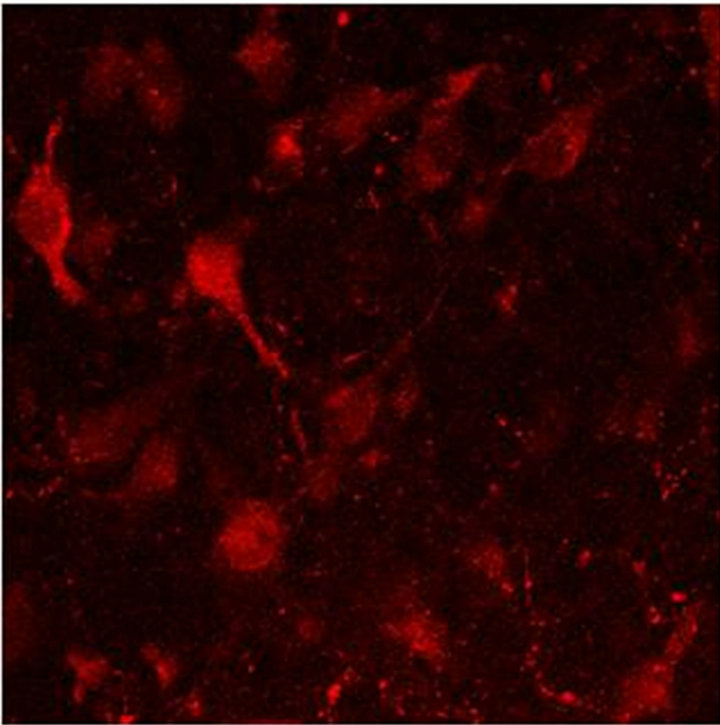 (b)
(b)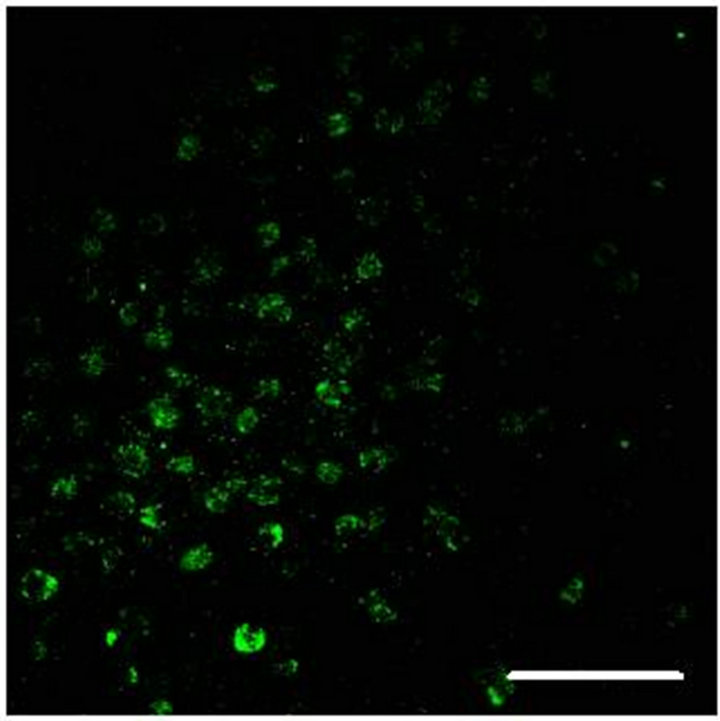 (c)
(c)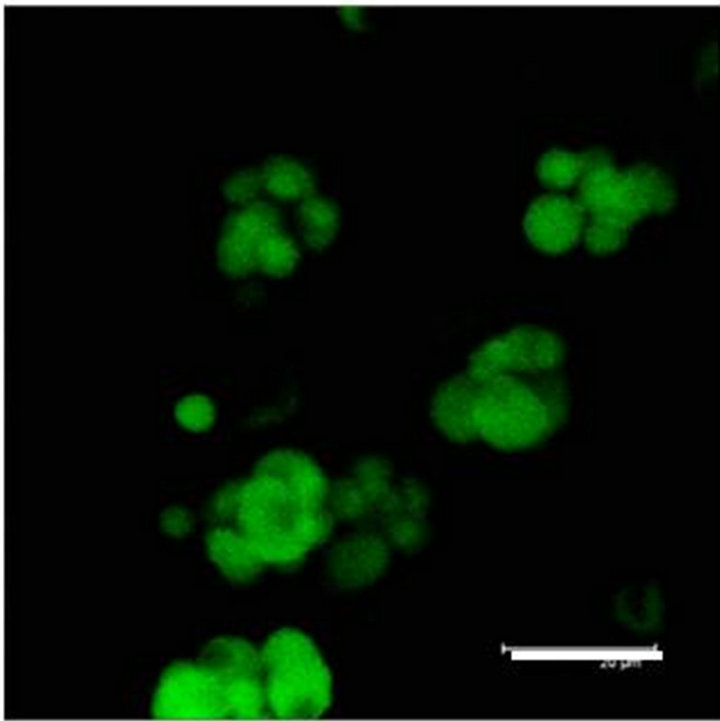 (d)
(d)
Figure 2. (a) Control preparation that shows the presence in the Me of 5-HT immunopositive cells in coculture. Scale bar = 75 μm; (b) 5-HT Immunopositive cells in the Np in coculture. Scale bar = 5 μm; (c) T5H positive cells in a not cultured Np. Scale bar = 20 μm; (d) 5-HT1A positive cells in the Np in coculture. Scale bar = 75 μm. Confocal microscopy.
4. DISCUSSION
There are cells with a neurochemical phenotype that appears early in the primitive cerebral cortex, like gabaergic neurons probably implicated in the processes of corticogenesis [17,24]. Catecholaminergic cells also appear in the prenatal period probably implied too in neurogenesis [18,25,26]. Very early in the rombencephalon area (E11-E12) serotonergic neurons are already presented, they will form later the mesencephalic raphe nuclei, also observed in some early hypothalamic nuclei
 (a)
(a)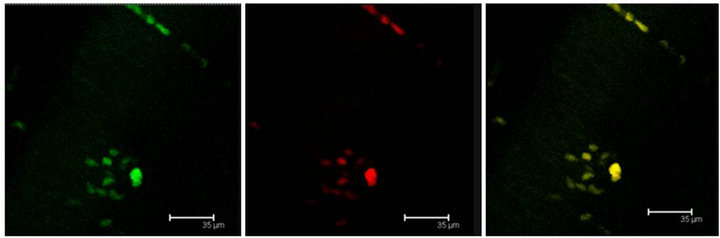 (b)
(b)
Figure 3. (a) Immunopositive 5-HT label (red) coexist with T5H label (green) and both labels in the same cell (yellow) in the Np in coculture. Scale bar = 75 μm; (b) Coexistence of T5H (green) and 5-HT (red) antibody labels in the same cells (yellow) in a not cultured Np. Scale bar = 35 μm. Confocal microscopy.
 (a)
(a)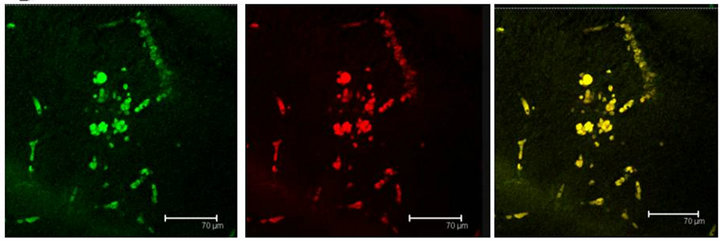 (b)
(b)
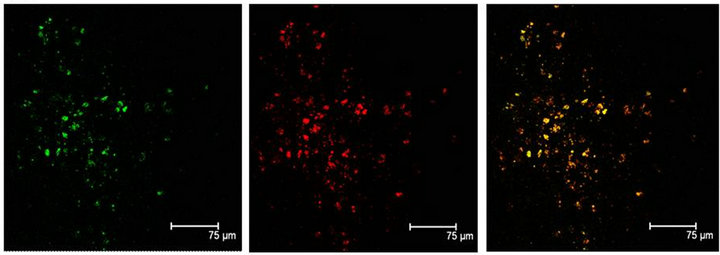
Figure 4. (a) 5-HT immunopositive cells (red) also positive to NeuN (green) label, in the Np in coculture. Both labels coexisting in the same cells, are shown in yellow. Scale bar = 75 μm; (b) Enolase (green) and 5-HT (red) coexist (yellow) in a not cultured Np. Scale bar = 70 μm. Confocal microscopy.
 (a)
(a)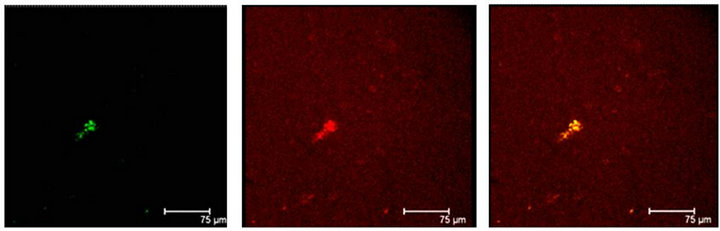 (b)
(b)
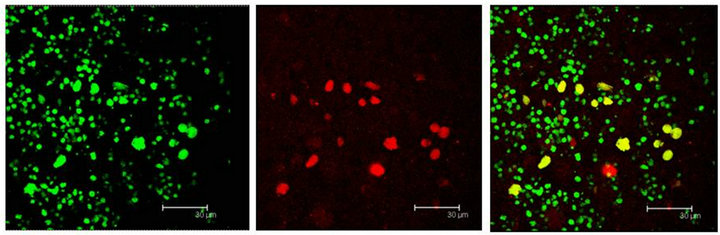
Figure 5. (a) T5H (green) or SERT (red) antibody labels do coexist in cells (yellow) of the Np in coculture. Scale bar = 75 μm; (b) 5-HT (green); antibody label does also coexist with SERT (red) in the same cells (yellow), in the Np in coculture. Scale bar = 75 μm. Confocal microscopy.
 (a)
(a)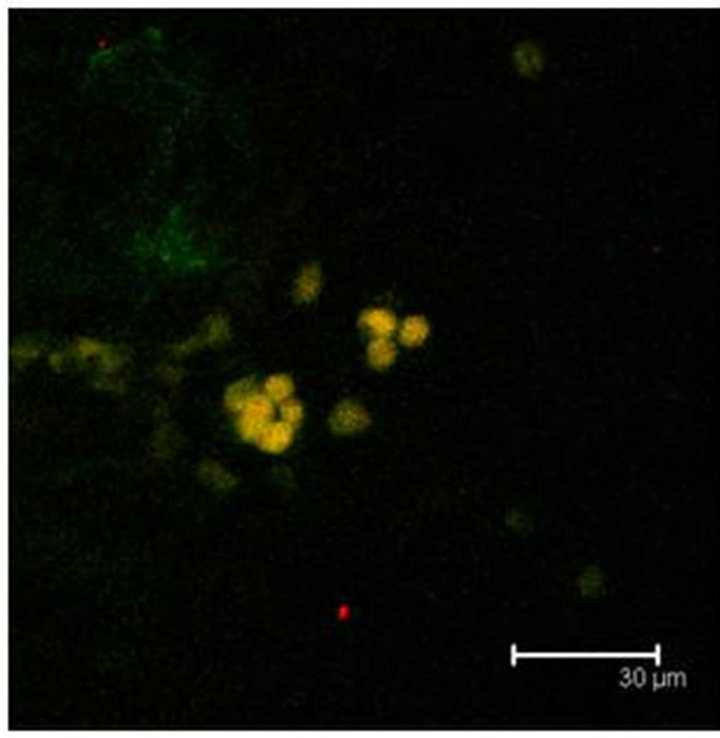 (b)
(b)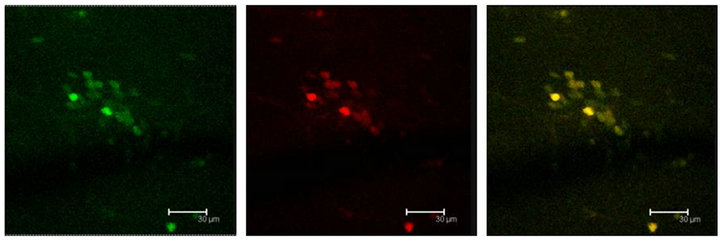 (c)
(c)
Figure 6. (a) GFAP positive cells (green) show also the T5H antibody labels (red) and coexistence of both (yellow) in the Np in coculture. Scale bar = 75 μm; (b) GFAP antibody labels (green) and T5H (red), can be seen coexisting (yellow) in cells of not cultured Np. Scale bar = 30 μm; (c) Glial cells, with GFAP positive mark (green), show also the 5-HT antibody labels (red), in a not cultured Np. Scale bar = 30 μm. Confocal microscopy.
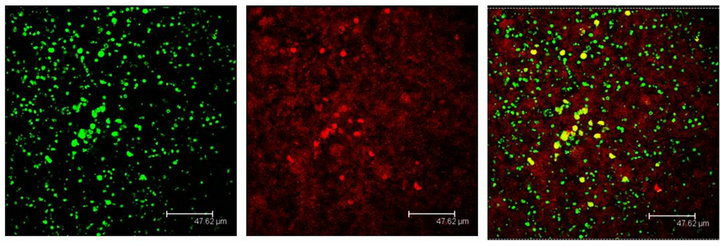
Figure 7. Cells immunopositive to the 5HT1A antibody (green), show the Giα-1 antibody labels (red) coexisting in cells of the Np in coculture (yellow). Scale bar = 47 μm. Confocal microscopy.
[14]. However, the early serotonergic cells do differ with
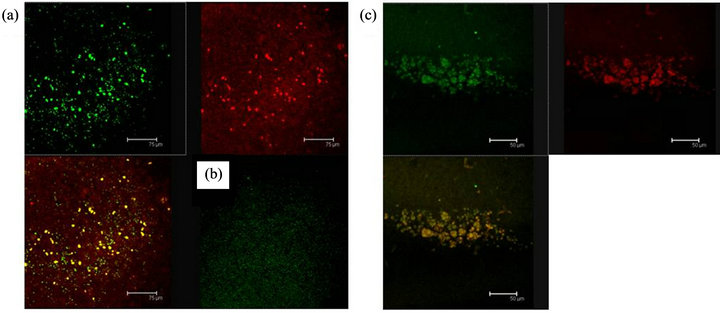
Figure 8. Cells in (a), shows the antibody labels for the 5HT1A receptor (green) and MAPK (red) coexisting (yellow) in cells of the Np in coculture. Scale bar = 75 μm; In (b) a representative negative control, without the primary antibody is shown; (c) 5HT1A labelled cells (green) and MAPK (red) coexisting in cells of a not cultured Np (yellow). Scale bar = 50 μm. Confocal microscopy.
 (a)
(a) (b)
(b)
Figure 9. (a) MAPKK immunopositive cells (red) and 5HT1A receptor labelled cells (green) show coexistence in cells of the Np in coculture (yellow). Scale bar = 30 μm; (b) The same sequence in a not cultured Np. Scale bar = 75 μm. Confocal microscopy.
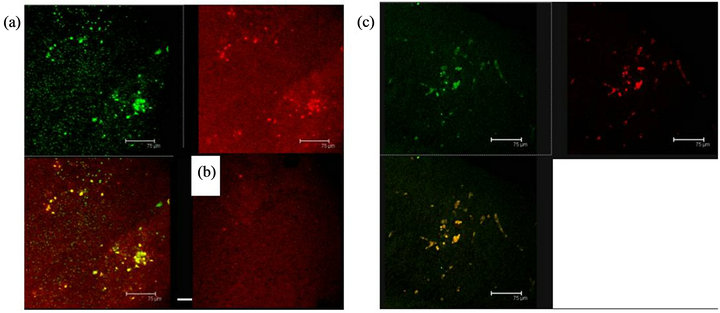
Figure 10. (a) 5HT1A labelled cells (green) and ERK-1 (red) coexistence of both labels in cells of the Np in coculture. Scale bar = 75 μm; (b) A representative Negative control without primary antibody; (c) 5HT1A positive cells (green) and ERK-1 (red) show coexistence of both labels (yellow) in cells of not cultured Np. Scale bar = 75 μm. Confocal microscopy.
the catecholaminergic cells in that they have not been observed in the early cerebral cortex (neopallium) of the fetal brain, neither in the adult cortex.

Figure 11. 5-HT1A serotonin receptor signaling path is shown in this scheme in a theoretical fetal Np target cell, observed at 12 days of coculture with the fetal Me. Within squares the signaling molecules, coexisting with the 5-HT1A receptor label, are shown.
Early serotonergic cells appearing at the mesencephalic area, are the sources of a wide axonal system afferent to multiple brain regions including the maturing cerebral cortex, particularly the sensory cortex, or areas like the marginal zone, septal, piriform and the cortical plate. Up to now it is accepted that regulatory serotonergic messages are displayed through this neuronal axon fiber system, widely distributed [13,27-29]. These elements of the serotonergic system can increase their expression and function during some periods of cortical development [11] as it is the case of SERT, the serotonin transporter, in the visual and in the somatosensory cortices [7,12,30].
Considering the preceding reference framework, the observations made in the present work may take relevance concerning the unexpected presence of serotonergic cells in the rat fetal neopallium in the culture conditions here described, with the 5-HT label coexisting in the same cells with T5-H, the main enzyme in the serotonin synthesizing path. Their presence has been also found in the fetal Np (E16) not cultivated (in situ), suggesting that they appear earlier. It is also interesting that serotonin markers did also coexist with Vimentin and with GFAP, strongly suggesting that young and mature neuroglia are also expressing the serotonergic phenotype in the maturing cortex. The morphology of the cells, excludes the possibility of serotonergic terminals.
In conclusion it seems possible that as it happens with serotonergic fibers that augment significantly during certain periods of corticogénesis and after some time they decrease to normal, the serotonergic cells here described in the fetal neopallium in culture or in situ, could transiently express the serotonergic phenotype that would possibly allow them to participate directly in the structuration processes of the cerebral cortex, to disappear afterwards, as it happens with the mentioned TH positive cells [18,31].
Results of the present study also show the presence of a molecular signaling system that coexists with 5-HT1A receptors in cells of the Np after 12 days in cocultures and also in situ. So it may well be that these molecules (Giα-1, ERK1, MAPK and MAPKK) are involved in a signaling cascade initiated by the serotonergic input on the 5-HT1A receptor.
It is also known that elements of the serotonergic system may increase their expression during some periods of cortical development [11] as it is the case of SERT in the visual and somatosensory cortex [7,12,30,32]. The presence of SERT in the Np both in-situ or cultured, would reinforce the hypothesis that there are serotonin recognizing target cells in the fetal Np. We are currently examining how long their presence could last and how could we study their possible functional role.
5. ACKNOWLEDGEMENTS
We are grateful for excellent technical assistance to T. I. Ignacio Vargas Martínez and T. I. Marisol Bautista Flores, Lab. de Neurontogenia, Departamento de Fisiología, Biofísica y Neurociencias, and M.V.Z. Rafael Leyva Muñoz, Unidad de Producción y Experimentación de Animales de Laboratorio, Cinvestav-IPN.
![]()
![]()
REFERENCES
- Tecott, L., Shtrom, S. and Julius, D. (1995) Expression of a serotonin-gated ion chanel in embryonic neural and nonneural tissues. Molecular and Cellular Neuroscience, 6, 43-55.
- Wallace, J.A. and Lauder, J.M. (1983) Development of the serotonergic system in the rat embryo: An immunocytochemical study. Brain Research Bulletin, 10, 459-479. doi:10.1016/0361-9230(83)90144-2
- Mercado, C.R. and Hernández, R.J. (1992) A molecular recognition system of serotonin in rat fetal axonal growth cones: Uptake and high affinity binding. Developmental Brain Research, 69, 133-137. doi:10.1016/0165-3806(92)90130-O
- Whitaker-Azmitia, P.M. and Azmitia, E.C. (1986) Autoregulation of fetal serotonergic neuronal development: Role of high affinity serotonin receptors. Neuroscience Letters, 67, 307-312.
- Nguyen, L., Rigo, J.M., Rocher, V., Belachew, S., Malgrange, B., Rogister, B., Leprince, P. and Moonen, G.L. (2001) Neurotransmitters as early signals for central nervous system development. Cell and Tissue Research, 305, 187-202. doi:10.1007/s004410000343
- Kojic, L., Dick, R.H., Gu, Q., Douglas, R.M., Matsubara, J. and Cynader, M.S. (2000) Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proceedings of the National Academy of Sciences, 97, 1841-1844. doi:10.1073/pnas.97.4.1841
- Cases, O., Vitalis, T., Seif, I., De Maeyer, E., Sotelo, C. and Gaspar, P. (1996) Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: Role of a serotonin excess during the critical period. Neuron, 16, 97-307. doi:10.1016/S0896-6273(00)80048-3
- Mercado, R., Florán, B. and Hernández, R.J. (1998) Regulated release of serotonin from axonal growth cones isolated from the fetal rat brain. Neurochemistry International, 32, 103-106. doi:10.1016/S0197-0186(97)00039-9
- D’amato, R.J., Blue, M.E., Larget, B.L., Ledbetter, D.J., Molliver, M.E. and Snyder, S.H. (1987) Ontogeny of the serotonergic projection to rat neocortex: Transient expression of a dense innervation to primary sensory areas. Proceedings of the National Academy of Sciences of the United States, 84, 4322-4326. doi:10.1073/pnas.84.12.4322
- Zhou, F.C., Sari, Y. and Zhang, J.K. (2000) Expression of serotonin transporter protein in developing brain. Developmental Brain Research, 119, 33-45. doi:10.1016/S0165-3806(99)00152-2
- Nakazawa, M., Koh, T., Kani, K. and Maeda, T. (1992) Transient patterns of serotonergic innervation in the rat visual cortex: Normal development and effects of neonatal enucleation. Developmental Brain Research, 66, 77-90. doi:10.1016/0165-3806(92)90143-K
- Chubakov, A.R., Gromova, E.A., Konovalov, G.V., Chumasov, E.I. and Sarkisova, E.F. (1985) Effect of serotonin on the development of a rat cerebral cortex tissue culture. Neuroscience and Behavioral Physiology, 35, 926-934.
- Mazer, C., Muneyyirci, J., Taheny, K., Raio, N., Borella, A. and Whitaker-Azmitia, P. (1997) Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Research, 760, 68-73. doi:10.1016/S0006-8993(97)00297-7
- Lebrand, C., Cases, O., Adelbretch, C., Doye, A., Alvarez, C., El Mestikawy, S., Seif, I. and Gaspar, P. (1996) Transient uptake and storage of serotonin in developing thalamic neurons. Neuron, 17, 823-835. doi:10.1016/S0896-6273(00)80215-9
- Manjarrez, G.G., Manuel, A.L., Mercado, C.R. and Hernández, R.J. (2003) Serotonergic receptors in the brain of in útero undernourished rats. International Journal of Developmental Neuroscience, 21, 283-289. doi:10.1016/S0736-5748(03)00034-0
- Super, H., Soriano, E. and Uylings, H.B.M. (1998) The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Research Reviews, 27, 40-64. doi:10.1016/S0165-0173(98)00005-8
- Secevic, N. and Milosevic, A. (1985) Initial development of gamma-aminobutyric acid immunoreactivity in the human cerebral cortex. Developmental Brain Research, 23, 1-159.
- Berger, B., Verney, C., Gaspar, P. and Febvret, A. (1985) Transient expresión of tyrosine-hydroxylase immunoreactivity in some neurons of the rat neocortex turing posnatal development. Developmental Brain Research, 23, 141-144. doi:10.1016/0165-3806(85)90013-6
- Le Douarin, N.M. and Teillet, M.A. (1973) The migration of neural crest cells to the wall of the digestive tract in avian embryo. Journal of Embryology and Experimental Morphology, 30, 31-48.
- Patterson, P.H. (1978) Environmental determination of autonomic neurotransmitter functions. Annual Review of Neuroscience, 1, 1-17. doi:10.1146/annurev.ne.01.030178.000245
- Stoppini, L., Buchs, P.A. and Muller, D. (1991) A Simple Method for Organotypic Cultures of Nervous-Tissue. Journal of Neuroscience Methods, 7, 173-182. doi:10.1016/0165-0270(91)90128-M
- Foster, G.A. (1998) Chemical neuroanatomy of the prenatal rat brain. A developmental atlas. Oxford University Press, Oxford, 1998, 260 Pages.
- Lavdas, A.A., Blue, M.E., Lincoln, J. and Parnavelas, J.G. (1997) Serotonin promotes the differentiation of glutamate neurons in organotypic slice cultures of the developing cerebral cortex. The Journal of Neuroscience, 17, 7872-7880.
- Gordon-Weeks, P.R. and Lockerbie, R.O. (1984) Isolation and partial characterization of neuronal growth cones from neonatal rat forebrain. Neuroscience, 13, 119-136. doi:10.1016/0306-4522(84)90264-1
- Haynes, L.W., Rushton, J.A., Perrins, M.F., Dyer, J.K., Jones, R. and Howell, R. (1994) Diploid and hyperdiploid rat Schwann cell strains displaying negative autoregulation of growth in vitro and myelin sheath-formation in vivo. Journal of Neuroscience Methods, 52, 119-127. doi:10.1016/0165-0270(94)90120-1
- Verney, C., Lebrand, C. and Gaspar, P. (2002) Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. The Anatomical Record, 267, 87-93. doi:10.1002/ar.10089
- Lidov, H.G.W. and Molliver, M.E. (1982) An immunohistochemical study of serotonin neuron development in the rat: Ascending pathways and terminal fields. Brain Research Bulletin, 8, 389-430. doi:10.1016/0361-9230(82)90077-6
- Lidov, H.G.W. and Molliver, M.E. (1982) Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Research Bulletin, 9, 559-604. doi:10.1016/0361-9230(82)90164-2
- Papadopoulos, G.C., Parnavelas, J.G. and Buijs, R.M. (1987) Light and electron microscopic immunocytochemical analysis of the serotonin innervation of the rat visual cortex. Journal of Neurocytology, 16, 883-892. doi:10.1007/BF01611992
- Hansson, S.R., Mezey, E. and Hoffman, B.J. (1998) Serotonin transporter messenger rna in the developing rat brain: Early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience, 83, 1185-1201. doi:10.1016/S0306-4522(97)00444-2
- Gaspar, P., Berger, B., Febvret, A., Vigny, A., KriegerPoulet, M. and Borri-Voltattorni, C. (1987) Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: A novel catecholaminergic group? Neuroscience Letters, 80, 257-262. doi:10.1016/0304-3940(87)90464-2
- Chubakov, A.R., Gromova, E.A., Konovalov, G.V., Sarkisova, E.F. and Chumasov, E.I. (1986) The effect of serotonin on the morpho-functional development of rat cerebral neocortex in tissue culture. Brain Research, 369, 285- 297. doi:10.1016/0006-8993(86)90537-8
NOTES
*Corresponding author.

