Advances in Biological Chemistry
Vol.2 No.4(2012), Article ID:24566,6 pages DOI:10.4236/abc.2012.24044
Measuring free tissue sulfide
![]()
1Deptartment of Pathology, University of Otago, Christchurch, New Zealand
2Deptartment of Pharmacology & Toxicology, University of Otago, Dunedin, New Zealand
Email: Madhav.bhatia@otago.ac.nz
Received 5 August 2012; revised 9 September 2012; accepted 19 September 2012
Keywords: Free Sulfide; Measuring; Tissue Sulfide; H2S
ABSTRACT
Hydrogen sulfide is synthesized endogenously in mammals and has been shown to have both physiological and pathological functions. So far there has been little agreement as to the actual levels of endogenous sulfide under physiological or pathological conditions; this is partly due to the complexity involved in measuring free sulfides due to H2S volatility, oxidation, reactivity and the presence of bound labile sulfur in tissues. In this report we describe a method of measuring free tissue sulfides using a zinc sulfide precipitation and wash method. It is an indirect method that measures the sulfide difference between samples prepared at pH 9 and pH 6, assuming that at pH 9 free sulfides would be retained in solution, while at pH 6 free sulfides would volatilize during sample preparation. Using this approach we were able to measure appreciable amounts of free sulfides in mouse: lung, pancreas, liver and kidney at 0.036 + 0.006, 0.082 + 0.009, 0.215 + 0.016 and 0.323 + 0.031 nmole per mg of tissue respectively (n = 6).
1. INTRODUCTION
Hydrogen sulfide (H2S) is a colorless, flammable gas with a characteristic odor of rotten eggs. In addition to environmental and bacterial sources, mammals are capable of H2S biosynthesis. As such endogenous H2S is increasingly being recognized as a third gaseous signaling molecule after carbon monoxide and nitric oxide. It has been shown to be involved in both physiological events such as neuromodulation [1] pancreatic b-cell function [2], cardiovascular function [3] and pathological events such as inflammation [4] and nociception [5].
Endogenous H2S can be synthesized via the desulfuration of cystine/cysteine by three enzymes; cystathionine beta synthase (CBS; EC 4.2.1.22), cystathionine gamma lyase (CSE; EC 4.4.1.1) and mercaptopyruvate sulfurtransferase (MST; EC 2.8.1.2). H2S has a pKa of 6.84 at 37˚C, therefore at physiological pH, about 30% is present as H2S and the remainder as the HS− anion [6]. It is still unknown as to which of these (or if both) free sulfide species confer its biological role. Efforts to measure sulfide content in biological samples have so far yielded a wide range, from non-detectable to micromolar levels, based on the different methods employed [7-11]. Added complexities to the determination of sulfide levels include the volatility of H2S and its reactivity with other molecules such as S-nitrosothiols [12]. Furthermore, sulfides are also present in biological systems in the form of free and bound labile sulfides [13,14].
An ideal method for measuring free sulfides in biological samples should be able to distinguish between free and bound labile sulfides, account for H2S volatility and possibly distinguish between free sulfide species. Currently, the use of a polarograhic electrode specific for H2S [15,16] represents the most direct, sensitive and specific method of detecting free H2S in a biological sample, particularly for real-time measurements. However, the sampling and turnover rate is low and the method does not take into account the free HS− present [16]. Other methods to detect free sulfides in biological samples include a direct method using gas chromatography [11] and HPLC which requires derivatisation with monobromobimane [17]. Although these methods are sensitive, they require specialised equipment which may not be readily available in a typical laboratory setting. Also, these methods are not suitable when working with a large number of samples at the same time.
The methylene blue assay has been widely employed to quantitatively measure sulfide by spectrophotometry. The method is based on the modified version [18] of the originally published method by Fischer et al. [19] used to determine sulfide concentration in water and other nonbiological samples, but has been recently been adapted to determine sulfide content and synthesis in biological samples [20-23]; however there are limitations in doing so. The acidic nature of the assay could potentially liberate bound acid labile sulfur [13,14], thus causing artefacts, while coloured substances could interfere with the assay [24]. Also, the lower detection limit of the assay is in the micromolar range, which may be insensitive to detect free sulfides in biological material. In this paper, we describe an indirect method of measuring free tissue sulfide based on a modified form of the methylene blue assay which involves a zinc precipitation step. These modifications removes the acidic condition of the assay and adds an additional protein solubilization and wash step to reduce potential artefacts and interference that may arise from the presence of biological material in the assay.
The advantages of the modified assay include: its simplicity (it is suitable for high throughput sampling), its sensitivity (it can detect approximately 4 fold lower levels of sulfide than the methylene blue assay) and it can quantify free sulfides from a range of tissues with a lower detection limit of 0.4 nmole. It also incorporates a reference that serves as a control for the background signal that may be generated from bound labile sulfur.
2. MATERIALS & METHODS
2.1. The Methylene Blue Assay for Determination of Sulfide Levels
The methylene blue assay has previously been used to determine sulfide levels in a range of solutions [22]. Briefly, 500 µL of a Na2S solution was mixed with 350 µL of zinc acetate (1% w/v), 133 µL of 20 mM N,Ndimethyl-p-phenylenediamine dihydrochloride (NNDP) in 7.2 M HCl, 133 µL iron reagent (30 mM FeCl3 in 1.2 M HCl) and 250 µL of trichloroacetic acid (10% w/v). The solution was then incubated for 10 min to allow the color to develop, before the absorbance was read on a spectrophotometer at 670 nm (Molecular Devices, Spectramax M5).
2.2. The Zinc Precipitation Assay for the Determination of Sulfide Levels
The zinc precipitation methodology was based upon a modified protocol that was originally developed by Gilboa-Garber [25]. 500 µL of a sample was added to 400 µL of a pre-mixed 1% w/v zinc acetate (350 µL) solution and 50 µL of 1.5 M sodium hydroxide. This was followed by centrifugation at 1200 g for 5 min to pellet the zinc sulfide that had been formed. The supernatant was then aspirated off and the pellet washed with 1.5 mL of Milli-Q water by vortexing thoroughly, followed by a centrifugation at 1200 g for 5 min. The supernatant was then aspirated off and the pellet reconstituted with 160 µL of Milli-Q water and mixed with 40 µL of pre-mixed dye (20 µL of 20 mM NNDP in 7.2 M HCl & 20 µl of 30 mM FeCl3 in 1.2 M HCl). The solution was then incubated for 10 min to allow the color to develop, before the absorbance was read on a spectrophotometer at 670 nm.
2.3. Determination of the Effects of pH on the Stability of Sulfide Solutions
Commercially available Na2S (Sigma) contains impurities which can interfere with sulfide measurement. Therefore, before use, Na2S crystals were washed with water and dried under vacuum. These crystals were then dissolved and diluted into buffer in polypropylene microfuge tubes. For the pH stability studies, a 100 µM Na2S standard solution was prepared in 50 mM sodium phosphate buffer at pH 6, 7, 7.5, 8, 8.5, and in 50 mM sodium carbonate buffer at pH 9 and 10. The tubes were then incubated for the designated time intervals at room temperature. To determine sulfide concentrations at each timepoint, 100 µL aliquots of the incubation mixtures were transferred to individual tubes and sulfide levels fixed by the addition of 60 µL zinc acetate (1% w/v). After the final time point, 40 µL of pre-mixed dye (20 µL of 20 mM NNDP in 7.2 M HCl & 20 µL of 30 mM FeCl3 in 1.2 M HCl) was added to each tube and incubated for 10 min for the color to develop before reading on a spectrophotometer at 670 nm.
2.4. Comparison of Methylene Blue and Zinc Precipitation Methods for Detecting Sulfides
A series of standard Na2S solutions were prepared using serially diluted Na2S dissolved in 50 mM sodium carbonate buffer at pH 9. The sulfide content of each standard was then independently evaluated using both the methylene blue and zinc precipitation assays. Additionally, to determine if zinc precipitation was effective in detecting sulfide in biological material, a standard curve was prepared using Na2S added to liver homogenate (40 mg/mL). The levels of spiked sulfide in the incubation were then measured using the zinc precipitation method. Briefly, a set of Na2S standards were prepared in 25 µL serial dilutions and precipitated with 400 µL of the zinc acetate and NaOH mixture, followed by addition of 500 µL liver homogenate and processed as described above.
2.5. Measuring Tissue Sulfide Using Zinc Precipitation Method
Use of animal tissues for this work was approved by University of Otago Animal Ethics committee. Tissues were harvested from 6 BALB/c mice anaesthetized with pentobarbital and immediately frozen on dry ice and stored at –80˚C. On the day of experiment, 40 mg of tissue was weighed out and immediately immersed in 1 mL of buffer (described below). Samples were then homogenized and sonicated on ice followed by centrifugation at 15,000 g for 10 min at 4˚C. 500 µL of the clear supernatant was then used for the zinc precipitation assay as described. To determine free sulfide levels, each tissue was prepared in a 50 mM sodium phosphate buffer pH 6 and a 50 mM sodium carbonate buffer pH 9. At pH 6, >90% of free sulfides should exist as the neutral H2S species which is highly volatile and easily lost by dissipation into the atmosphere. At pH 9, >90% of free sulfides should exist as the anionic HS– which is retained in solution. Free tissue sulfide was then calculated by deducting the value of total sulfides measured at pH 6 from pH 9.
In one set of experiments, a pH titration was performed to determine if the amount of measured sulfides in tissue adhered to the expected dissociation of H2S. This experiment was carried out with mice livers processed in 50 mM sodium phosphate buffer at pH 6, 6.5, 7, 7.5, 8 and 50 mM sodium carbonate buffer pH 9 using the method described above.
3. RESULTS
3.1. Effect of pH on Stability of Sulfides Measured by Methylene Blue Assay
The data showed that sulfides generated from Na2S were more stable when prepared in an alkaline buffer where HS- is the predominant species (Figure 1). This would be in agreement with the volatility of the neutral H2S species and the stability of the anionic HS− species in solution. The relationship between pH also showed that the methylene blue assay was only able to detect the HS− species of free sulfides. The observed sulfide stability at pH 9 and pH 10 was similar, which follows the predicted percentage of HS– to be at 99% at pH 9 and 99.9% at pH 10. It would therefore be beneficial to prepare samples in buffers no less than pH 9 to retain the maximal amount of free sulfides in the stable form of HS–. There was also a noticeable difference in absorbance at 0 mins. These differences could be attributed to the volatility of predominant sulfide specie being the gaseous form (H2S) as the pH decreases with respect to the pKa. This, coupled with the time it was taken to dissolve the sodium sulfide salt and pipette the replicates into the zinc acetate solution may result in some sulfide loss reflected in Figure 1.
3.2. Zinc Precipitation Method of Detecting Sulfides
The zinc sulfide precipitation step was first postulated by Pomeroy [26] and put into practice by Gilboa-Garber [25]. In our attempt to replicate the work by GilboaGarber, using H2S spiked tissue homogenates, we found that the ratio of zinc acetate, NaOH and sample was not optimal for the removal of all biological material while still retaining the ZnS pellet. We therefore optimized the ratio to 7 parts of zinc acetate (1% w/v) to 1 part of NaOH (1.5 M), and this solution was thoroughly mixed and allowed to stand until a granular precipitate formed. This mixture was then used in a 4:5 ratio with the supernatant of a tissue homogenate (~40 mg/mL). Compared to the methylene blue assay, the precipitation method yielded a more robust standard curve, with a discernible lower limit of 0.4 nmole (1.56 nmole for methylene blue) (Figure 2). The standard curve constructed with liver homogenate was able to yield a linear regression value of 0.9996 that was slightly elevated compared to the standard curve constructed with buffer only. This could be attributed to the endogenous sulfide from the tissue itself.
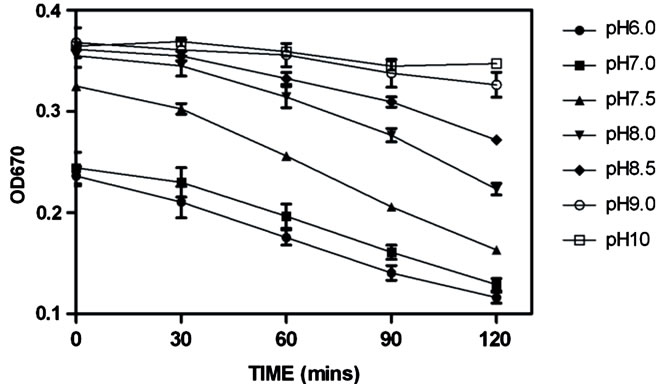
Figure 1. The effect of pH on the stability of sulfide in solution. 100 µM Na2S was incubated in 50 mM sodium phosphate buffer (pH 6 to 8.5) & 50 mM sodium carbonate buffer (pH 9 and 10) over time. Sulfide levels were then determined by the methylene blue assay (error bars represent S.E.M., n = 3). The immediate decrease in OD670 that was observed for solutions between pH 6 - 7.5 was due to the rapid diffusion of volatile H2S into which occurs rapidly upon mixing [30].
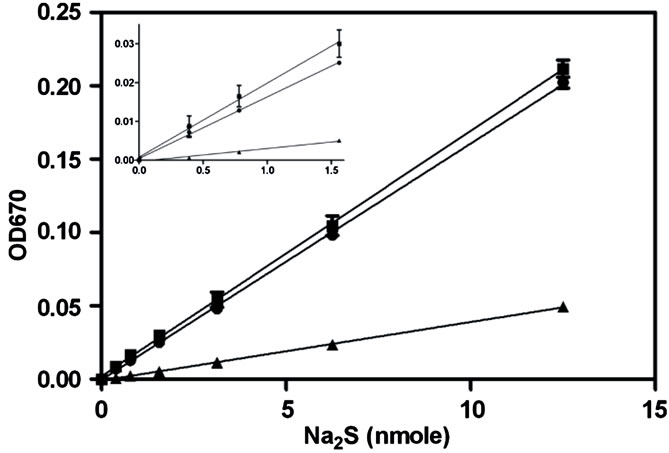
Figure 2. Analysis of the capacity of the methylene blue and zinc precipitation assays to quantify solution sulfide levels. A standard curve of serially diluted Na2S was prepared in 50 mM sodium carbonate buffer pH 9, sulfide levels were then determined using either the methylene blue assay (triangle) or the zinc precipitation and wash method (circles). Additionally serially diluted Na2S was spiked into liver homogenate (40 mg/ml) prepared in 50 mM sodium carbonate buffer (pH 9) and detected using the zinc precipitation method (boxes). The inset is a scaled-up representation of the 4 lowest points of the curves (error bars represent S.E.M., n = 3).
3.3. Measuring Tissue Sulfide
The zinc precipitation and wash method usually measures sulfides within the tissue homogenates at pH 9, under which alkaline conditions all sulfides should be present in the anionic form (HS–). We predicted that homogenates prepared at pH 6 would contain free sulfides predominantly as the neutral species (H2S), and that these species should readily volatalize during the process of sample preparation (Figure 1) and therefore not be detected by the assay. However homogenates prepared at pH 9 should contain free sulfides predominantly as the anionic species, and these should remain in solution, allowing their detection. To confirm this pH dependency, a titration was used to measure sulfides present in liver homogenates as a function of pH (Figure 3).
The results showed a curve that closely represented the expected dissociation of H2S with a measured pK of about 6.7 (pKa of H2S at 37˚C - 6.84˚C, [6]). These results allowed us to use the assay to quantify free and bound sulfides based upon their differential detection over the pH range. As the assay conducted at pH 9 measured both free and bound sulfide, while the assay conducted at pH 6 only measured bound sulfide, the amount of free tissue sulfides could be determined by deducting the measured sulfides in homogenates at pH 6 from pH 9. Using this approach we analyzed a range of tissues and found appreciable amounts of free sulfide in the: lung, liver, kidney and pancreas of BALB/c mice (Figure 4). The kidney had the highest amount of 0.323
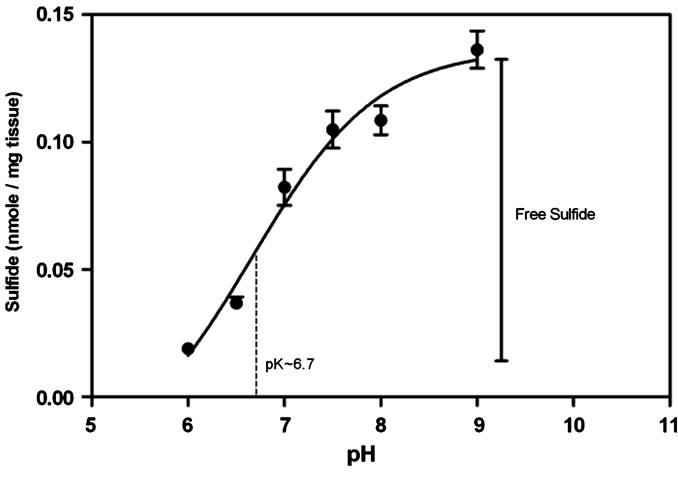
Figure 3. A pH titration curve of estimated tissue sulfides in liver homogenate (40 mg/ml) prepared in 50 mM sodium phosphate buffer pH 6, 6.5, 7, 7.5, 8, 8.5 and 50 mM sodium carbonate buffer pH 9 using the zinc precipitation method. The curve is concordant with the dissociation of H2S with a measured pK value of 6.7. Based on these findings, we assumed the difference between sulfides measured at pH 9 and pH 6 as the estimated free sulfides present in the tissue (Error bars represent S.E.M., n = 3) (pK value was determined using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA).
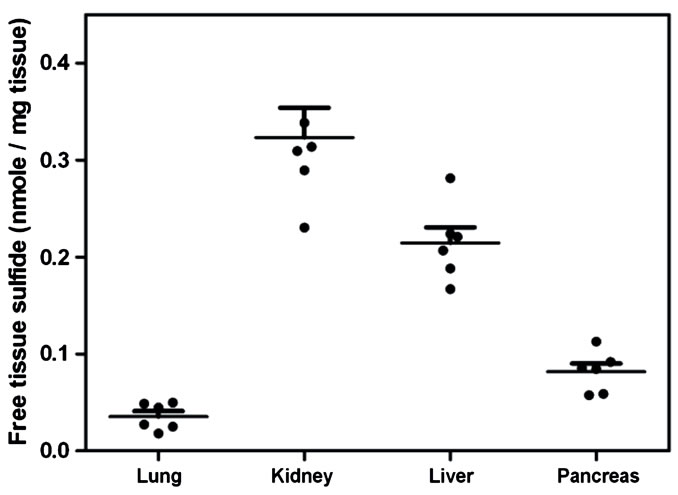
Figure 4. Estimation of tissue sulfides from murine tissue. Free sulfides were calculated by measuring the difference between levels measured in tissue homogenates (40 mg/ml) at pH6 and pH9 using the zinc precipitation method (Error bars represent S.E.M., n = 6).
+ 0.031 nmole per mg of tissue weight, while the lung had the lowest amount at 0.036 + 0.006 nmole per mg of tissue weight.
4. DISCUSSION
The methylene blue assay was first developed to determine sulfide content in water [19]. This method was refined by adapting the assay for modern spectrophotometry [18] and more recently has been used for detection of sulfides in biological samples [20-23]. However there has been much debate if the current direct method is accurate and sensitive enough to measure free sulfides in tissue and plasma [13,14,27]. The question of accuracy was mainly due to the possibility of artefacts being generated by the liberation of acid labile sulfur during the assay, as well as potential interference from coloured substances.
In this paper, we have described a method based on a zinc precipitation step that was first postulated by Pomeroy [26] and put into practice by Gilboa-Garber [25]. The zinc precipitation of sulfides was under strong alkali condition which would break down proteins and keep them in the soluble form to facilitate its subsequent removal. By including an initial precipitation step, we were able to remove the acidic conditions of the assay, which could potentially liberate acid labile sulfide from the tissue. Additionally it also allowed us to increase the sensitivity of the assay by concentrating the sulfide. Lastly, washing the ZnS pellet removed biological material that could potentially interfere with the color development of the assay. In this instance we then employed the methylene blue method for detecting sulfide in the final ZnS precipitate.
During the course of our assay development, we found that the protocol for tissue homogenization was equally important as the detection methodology for free sulfides. We have shown that at pH 9 and above, free sulfides remain stable in solution, due to the shift in balance towards the anionic species (pKa = 6.98 at 25˚C). Therefore, to retain the free sulfides in solution during sample preparation, we used a buffer at pH 9, which is in agreement with previous findings [11,17]. By controlling the pH at which we prepared the tissue homogenate, we were able to measure the change in free sulfide concentration by deducting the value measured at each pH from that at pH 9 (where >99% of free sulfide should exist as the soluble HS– species). A pH titration assay with liver homogenate showed that the amount of free sulfide measured adhered closely to the expected pKa value of H2S thus supporting our method of measuring free tissue sulfide.
For the purpose of measuring free tissue sulfides, we measured the difference between sulfide levels in homogenates at pH 9 and pH 6. The rationale being that theoretical calculations predict >90% of free sulfide to exist as the volatile H2S species at pH 6, based on the dissociation constant of sulfide. This, coupled with the physically vigorous sample preparation steps of homogenization and sonication, should ensure that most, if not all, of the free sulfide was present as the volatile H2S species, which can dissipate into the atmosphere during sample preparation at pH 6. Using this approach we have detected appreciable amounts of free sulfide in mice kidney, liver, pancreas and lungs, with the kidney having the most H2S and lungs the least.
Substantial sulfide was also measured in reference samples prepared at pH 6. The most likely source would be sulfur derived from iron-sulfur proteins. This bound sulfur has been shown to be liberated under alkaline-zinc conditions [28,29]. The same studies have also shown cysteinyl residues to be the unlikely source of sulfur release under alkaline-zinc conditions when incubated for short periods. Only a prolonged incubation of 20 hours yielded detectable sulfur release from BSA (containing 35 cysteine residues per mole) [28].
5. CONCLUSION
In summary, while there have been recent reports of direct and sensitive methods of measuring H2S [15] and free sulfides [11] in biological material, these methods require specialized equipment. We have developed a simple indirect method that involves the precipitation of free sulfides from tissue homogenates followed by a conventional detection assay. The major advantage of this approach is that it that is capable of quantifying free sulfide in murine tissue in a high-throughput manner without the need of specialized equipment.
6. ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support of the Lottery Health Research of New Zealand, who funded this work.
![]()
![]()
REFERENCES
- Rong, W., Kimura, H. and Grundy, D. (2011) The neurophysiology of hydrogen sulfide. Inflammation & Allergy-Drug Targets, 10, 109-117. doi:10.2174/187152811794776295
- Kaneko, Y., Kimura, Y., Kimura, H. and Niki, I. (2006) L-cysteine inhibits insulin release from the pancreatic beta-cell: Possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes, 55, 1391-1397. doi:10.2174/187152811794776295
- Bucci, M. and Cirino, G. (2011) Hydrogen sulphide in heart and systemic circulation. Inflammation & AllergyDrug Targets, 10, 103-108. doi:10.2174/187152811794776204
- Hegde, A. and Bhatia, M. (2011) Hydrogen sulfide in inflammation: Friend or foe? Inflammation & AllergyDrug Targets, 10, 118-122.
- Nishimura, S., Fukushima, O., Ishikura, H., Takahashi, T., Matsunami, M., Tsujiuchi, T., Sekiguchi, F., Naruse, M., Kamanaka, Y. and Kawabata, A. (2009) Hydrogen sulfide as a novel mediator for pancreatic pain in rodents. Gut, 58, 762-770. doi:10.1136/gut.2008.151910
- Hershey, J.P., Plese, T. and Miller, F.J. (1988) The pK1 for the dissociation of H2S in various ionic media. Geochimica et Cosmochimica Acta, 52, 2047-2051. doi:10.1016/0016-7037(88)90183-4
- Lindell, H., Jäppinen, P. and Savolainen, H. (1988) Determination of sulphide in blood with an ion-selective electrode by pre-concentration of trapped sulphide in sodium hydroxide solution. Analyst, 113, 839-840. doi:10.1039/an9881300839
- Goodwin, L.R., Francom, D., Dieken, F.P., Taylor, J.D., Warenycia, N.W., Reiffenstein, R.J. and Dowling, G. (1989) Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Post-mortem studies and two case reports. Journal of Analytical Toxicology, 13, 105- 109.
- Ogasawara, Y., Ishii, K., Togawa, T. and Tanabe, S. (1993) Determination of bound sulfur in serum by gas dialysis/high-performance liquid chromatography. Analytical Biochemistry, 215, 73-81. doi:10.1006/abio.1993.1556
- Furne, J., Saeed, A. and Levitt, M.D. (2008) Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. American Journal of Physiology: Regulatory, Integrative and Comparative Physiolog, 295, R1479-R1485. doi:10.1152/ajpregu.90566.2008
- Levitt, M.D., Abdel-Rehim, M.S. and Furne, J. (2011) Free and acid-labile hydrogen sulfide concentrations in mouse tissues: Anomalously high free hydrogen sulfide in aortic tissue. Antioxidants & Redox Signaling, 15, 373- 378. doi:10.1089/ars.2010.3525
- Teng, X., Scott Isbell, T., Crawford, J.H., Bosworth, C.A., Giles, G.I., Koenitzer, J.R., Lancaster, J.R., Doeller, J.E., W., Kraus, D. and Patel, R. (2008) Novel method for measuring S-nitrosothiols using hydrogen sulfide. Methods in Enzymology, 441, 161-172. doi:10.1016/S0076-6879(08)01209-3
- Ubuka, T. (2002) Assay methods and biological roles of labile sulfur in animal tissues. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences, 781, 227-249. doi:10.1016/S1570-0232(02)00623-2
- Tangerman, A. (2009) Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences, 877, 3366-3377. doi:10.1016/j.jchromb.2009.05.026
- Doeller, J.E., Isbell, T.S., Benavides, G., Koenitzer, J., Patel, H., Patel, R.P., Lancaster Jr., J.R., Darley-Usmar, V.M. and Kraus, D.W. (2005) Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Analytical Biochemistry, 341, 40-51. doi:10.1016/j.ab.2005.03.024
- Whitfield, N.L., Kreimier, E.L., Verdial, F.C., Skovgaard, N. and Olson, K.R. (2008) Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 294, R1930-R1937. doi:10.1152/ajpregu.00025.2008
- Shen, X., Pattillo, C.B., Pardue, S., Bir, S.C., Wang, R. and Kevil, C.G. (2011) Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radical Biology and Medicine, 50, 1021-1031. doi:10.1016/j.freeradbiomed.2011.01.025
- Fogo, J.K. and Popowsky, M. (1949) Spectrophotometric determination of hydrogen sulfide. Analytical Chemistry, 21, 732-734. doi:10.1021/ac60030a028
- Fisher, E. (1883) Bildung von Methylenblau als Reaction auf Schwefelwasserstoff. Chem Ber, 26, 2234-2236.
- Stipanuk, M.H. and Beck, P.W. (1982) Characterization of the enzymatic capacity for cysteine desulphydration in the liver and kidney of the rat. The Biochemical Journal, 206, 267-277.
- Zhao, W., Zhang, J., Lu, Y. and Wang, R. (2001) The vasorelaxant effect of H2S as a novel endogenous KATP channel opener. European Molecular Biology Organization, 20, 6008-6016.
- Bhatia, M., Wong, F.L., Fu, D., Lau, H.Y., Moochhala, S.M. and Moore, P.K. (2005) Role of hydrogen sulfide in acute pancreatitis and associated lung injury. Journal of Federation of American Societies for Experimental Biology, 19, 623-625.
- Ekundi-Valentim, E., Santos, K.T., Camargo, E.A., Denadai-Souza, A., Teixeira, S.A., Zanoni, C.I., Grant, A.D., Wallace, J., Muscará, M.N. and Costa, SK. (2010) Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. British Journal of Pharmacology, 159, 1463-1474. doi:10.1111/j.1476-5381.2010.00640.x
- Siegel, L.M. (1965) A direct microdetermination for sulfide. Analytical Biochemistry, 11, 126-132. doi:10.1016/0003-2697(65)90051-5
- Gilboa-Garber, N. (1971) Direct spectrophotometric determination of inorganic sulfide in biological materials and in other complex mixtures. Analytical Biochemistry, 43, 129-133. doi:10.1016/0003-2697(71)90116-3
- Pomeroy, R.P. (1954) Auxiliary pretreatment by zinc acetate in sulfide analyses. Analytical Chemistry, 26, 571- 572. doi:10.1021/ac60087a047
- Kolluru, G.K., Shen, X. and Kevil, C.G. (2011) Detection of hydrogen sulfide in biological samples: Current and future. Expert Review of Clinical Pharmacology, 4, 9-12. doi:10.1586/ecp.10.132
- Siegel, L.M., Murphy, M.J. and Kamin, H. (1973) Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. Journal of Biological Chemistry, 248, 251-264.
- Suhara, K., Takemori, S., Katagiri, M., Wada, K. and Kobayashi, H. (1975) Estimation of labile sulfide in iron-sulfur proteins. Analytical Biochemistry, 68, 632-636. doi:10.1016/0003-2697(75)90659-4
- Whiteman, M., Li, L., Rose, P., Tan, C.H., Parkinson, D.B. and Moore, P.K. (2010) The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxidants & Redox Signaling, 12, 1147-1154. doi:10.1089/ars.2009.2899

