Advances in Biological Chemistry
Vol. 2 No. 2 (2012) , Article ID: 18972 , 8 pages DOI:10.4236/abc.2012.22014
Characterization and biological activities of lectin isolated from Pinellia ternata
![]()
Institute of Bioengineering, Zhejiang Sci-Tech University, Hangzhou, China
Email: *fruijuan05@yahoo.com.cn
Received 16 February 2012; revised 15 March 2012; accepted 25 March 2012
Keywords: Pinellia ternata lectin; Mannose-Sepharose 4B affinity chromatography; Glycoprotein; Hela cell; MTT method
ABSTRACT
In this paper, Pinellia ternata lectin (PTL) purified from 95% saturated ammonium sulfate precipitation by applying mannose-Sepharose 4B affinity column chromatography was first described. The mannoseSepharose 4B affinity adsorption gel has already been designed and prepared before the experiments according the combinative characterization between lectin and mannose. Mass spectrometry analysis result shows that PTL is a glycoprotein with the molecular weight of 12.165 kD. It was conclude that PTL had strong agglutination effects on mouse red blood cells, and the minimum reaction concentration was 25 μg/ml according the Hemagglutination. Results of automatic amino acid analysis indicated that PTL mainly contained 15 varieties of amino acids, of which the minimum content was cysteine and aspartate was the maximum. Cell experiment results suggested that PTL of low concentrations (0.004 mg/ml, 0.02 mg/ml and 0.1 mg/ml) promoted HeLa cell proliferation, but the effect weakened with the concentrations and treated-time increasing. However, the HeLa cells proliferation was intensively inhibited by higher PTL concentrations (0.5 mg/ml and 1 mg/ml), and the effect increased in a dose and time dependent manner.
1. INTRODUCTION
Plant lectins or agglutinins, widely distributed in higher plant, are considered as an extended group of proteins, which according to a recently updated definition, comprise all plant proteins possessing at least one noncatalytic domain that binds reversibly to specific monoor oligosaccharides (glycoconjugate) [1,2]. One group of lectins is the so-called monocot mannose-binding lectin which are from the major plant lectin families that have been reported. Up to now,no standard methods for plant lectins extraction has been established. Generally, the aqueous buffer method is used for the extration of it, and the buffer including distilled water, normal saline, dilute acid, PBS and so on. The lowest effect extraction method is the distilled water method [3]. None fixed method for plant agglutinin purification and the costless and the most convenient method is salting out method, the principal is that different protein has different solubility in different concentrations of saline solution and proteins can be precipitated in different saturation saline solution, thus proteins was separated. (NH4)2SO4 fractional precipitation method was often used in the extraction of plant lectins, because of its large solubility and small temperature coefficient in water, but the purified protein cannot be obtained. The last method is most commonly used in biological chemical separation, purification and identification of biological macromolecules: chromatography. According to the principles of chromatography, there are three methods are used commonly: affinity chromatography (specific ligand-Sepharose, porcine thyroid globulin affinity chromatography), ion exchange chromatography (anion exchange chromatography DEAE-Sepharose, cation exchange chromatography CM-Sepharose) and molecular sieve chromatography (Sephadex, Sephacry). In order to achieve better separation effect, two or three kinds of different methods were often used together [4-8]. Separation and purification effect of the samples are also assessed by methods such as SDSPAGE or HPLC.
Pinellia ternata (Thunb.) Breit., a traditional Chinese medicinal herb belongings to family Araceae [9], has been characterized as pungent, warm in nature, and toxic and was firstly recorded in Shen Nong Ben Cao Jing. The main ingredients of which are starch, protein, pinellin, alkaloids, polysaccharides, amino acids, volatile oil, organic acids, sterols, flavonoids, tannins and other chemical compositions [10]. Pinellia ternata (Thunb.) Breit has pharmacological functions of terminating early pregnancy, anti-tumor, anti-arrhythmic, anti-ulcer, lowering blood pressure, sedative-hypnotic, antitussive and anti-inflammatory role in detoxification [11,12]. P. ternata lectin (PTL) was a monocot mannose-bingding lectin and was demonstrated as the major contributor for its antitumor activity [13,14]. PTL has the ability of control fungal pathogens through transgenic expression preferable alternative to toxic chemicals having serious ecological and social consequences has been proved.
PTL was first isolated with (NH4)2SO4 precipitation and crystallization method by Tao, Z. J. etc. in nineteen eighties [15]. One PTL which was speculated to be the same as Tao’s was attained by porcine thyroglobulin immobilized affinity column chromatography [16] and the molecular weight was speculated to be about 44 kD from the Sephadex G100 chromatography column filtration behavior. SDS-PAGE gel electrophoresis result showed that there was only one protein band with the molecular weight of about 12 kD, so it can be infered that the PTL consisted of four subunits. Gel filtration results of PTL measured by SPHER OGELTM-TSK 2000 SW showed its molecular weight was 33 kD, proving that PTL and molecular sieve column with hydrophobic interaction.
PTL is a glycoprotein, having agglutination activity on red blood cells and the nature of specific binding of mannose. Based on the nature of the mannose specific binding with PTL, the mannose-Sepharose 4B affinity gel was designed and the PTL was isolated successfully. Mass spectrometry was used to determine the precise molecular weight and purity of the isolated PTL. Amino acid composition of the PTL was detected with a the automatic amino acid analyzer L-8800 AAA system and the results was statisticed and classified. Detecting and hemagglutinating experiments of the glycoprotein were performed to identify the separated product. Meanwhile, its effect on the growth of Hela cells was studied preliminarily. These experiments were the groundwork for the further study of PTL isolation and its pharmacological mechanism.
The PTL subunits isolated and purificated by mannose-Sepharose 4B affinity chromatography was first reported in this paper and its coagulation activity, glycoprotein nature and amino acid composition were detected. Further more, the anti-tumor activity of it on Hela cells was also studied.
2. MATHERIALS AND METHODS
2.1. Materials
Fresh Pinellia tuber was obtained from the Conservatory of life of Biological Engineering Research Institute of Zhejiang Sci-Tech University and identified by associate professor Xu T. Experimental rats were purchased from Animal Center of Hangzhou Normal University. Hela cells were reserved in our lab.
2.2. Mannose-Sepharose 4B Affinity Gel Preparation
Cleaning: Washing DMSO away from alkylene oxide activated agarose gel preserved in 50% DMSO with deionized water 10 to 20 times the volume of the gel, then drained vacuum.
Coupling: 25 mg, 50 mg and 100 mg mannose was dissolved in 10 ml 0.1 M sodium carbonate buffer solution (pH 9.0), separately. Then put them into the drained activated gel, respectively. Couple 12 to 24 hr in a shaker oscillates at 37˚C. After the reaction, the solution was transferred to the funnel, drained (collect filtrate for unconjugated mannose analysis and coupling ligand density calculation), activated gel solution was washed by deionized water and then drained, three gradients of ligand density (H), (M), (L) gel were obtained.
Closing: Put the dry coupling gel into a 100 ml triangle bottle, added 30 ml 1 M ethanol amine solution, then reacted 4 hr at 37˚C stirring 120 × g. Then the solution was transferred to the funnel, drained. Finally, the gel was washed with deionized water.
Cleaning: The medium coupled mannose was washed by 5 times deionized water of the volum of the medium (0.5 M NaCl acetate buffer solution (pH 4.0), deionized water, 0.5 M NaCl boric acid-four sodium borate (pH 8.0) and deionized water)sufficiently, then drained.
Preservation: The product was preserved in 20% ethanol solution at 4˚C.
2.3. Isolation of PTL from P. ternata
Pinellia tuber was well grinded and soaked in 20% NaCl for 24 hr. The aqueous solution was centrifuged at 12,000 × g, 4˚C for 30 min. The precipitation was dissolved in 20% NaCl and dialyzed to remove (NH4)2SO4. Centrifuging and discarding the insoluble substance, then the supernatant was the rough product.
Mannose-Sepharose 4B affinity chromatographic coumn saturated with 20% NaCl was used to isolate PTL from the rough product. Adsorbed PTL was eluted with elution buffer (0.2 M mannose solution and 0.9% NaCl solution, respectively). The eluting sample was concentrated with Minitan Ultra Filtration System (Millipore, Molsheim, France), with a tangential flow decice on an ultra filtration membrane (cut-off 10,000 Da). The concentrating sample was dialyzed against PBS buffer (pH 7.4). After dialysising, the sample was assessed by polyacrylamide gel electrophoresis (SDS-PAGE) in a slab gel system from Bio-Rad. SDS-PAGE was performed on 15% acrylamide gels. Also, we studied the effect on the separating efficiency of different equilibrium buffer (20% NaCl solution, 10% NaCl solution and PBS buffer) and affinity gel of different ligand density.
2.4. Partial Properties and Biological Activities of Isolated PTL
The molecular weight of PTL was determined with a PBSIIC mass spectrometer. NP20 chip calibration instrument adding All-in-One standard protein was used to calibrate the data and the error range of the molecular weight was controlled in 0.1%. Chip reading instrument parameter settings were as follows: the laser intensity was 220, the detection sensitivity was 8, the optimization ranged from 1000 to 15,000 and the highest molecular weight was 5000 Da. Each point on the chip was collected 90 times per sec. The data were collected by Ciphergen Protein Chip 3.2.1 software.
The agglutination activity on mouse erythrocyte of PTL and total protein from P. ternata was assessed with coagulation plate. The specific protocol referred to the literature [16]. Periodate-Schiff staining method [17] was used to detect whether PTL was a glycoprotein or not.
The isolated PTL was hydrolyzed in 110˚C azeotropic hydrochloric acid for 24 hr and the hydrolysate was analyzed by the automatic amino acid analyzer L-8800 AAA system.
2.5. Research on Hela Cells Treated by Isolated PTL
Human cervical cancer HeLa cells preserved in our lab was cultured in DMEM medium supplemented with 10% fetal calf serum, 100 U/ml penicillin and streptomycin in an atmosphere of 5% CO2 of air at 37˚C. Cells were routinely trypsinized and passaged every 3 to 5 days.
HeLa cells of logarithmic growth phase, after digested by 0.25% trypsin and blowed gently into single cell suspension (1 to 2 × 104/ml), were seeded in 90 μl of medium in a 96-well plate in the presence or absence of PTL. After cells adherent on the plate, isolated PTL (dissolved in PBS) of different concentrations were added to the medium. PBS was used for control, the medium for blank, 3 multiple holes each concentration. After cultivated 24 hr, 48 hr and 72 hr, separately, 20 μl 5 mg/ml MTT was added to each hole. Subsequently, cells were incubated for four more hours at 37˚C in an atmosphere of 5% CO2 in air. Then the medium was discarded and 150 μl DMSO was added. Oscillate for about 20 min at room temperature to make the purple crystal dissolved. Then the purple solution was determined at 490 nm using a plate reader apparatus. This experiment was repeated 3 times.
HeLa cells of logarithmic growth phase, after digested by 0.25% trypsin and blowed gently into single cell suspension (1 to 2 × 104/ml), were seeded in 6-well plate equivalently. Low concentrations of PTL (0.004 mg/ml and 1 mg/ml) was added to the medium after cells adherent on the flask. After 24 hr, 48 hr and 72 hr cultivation, took photographs of cells treated by different concentrations of PTL in different time under an inverted microscope.
3. RESULTS
Results of isolated PTL assessed by SDS-PAGE gel electrophoresis showed that there was only one protein band with the apparent molecular weight about 12 kD (Figure 1). Proteins eluted by 0.2 M mannose solution and 0.9% NaCl solution were the same and sample eluted by 0.2 M mannose solution was 0.9 mg/ml, while 3 mg/ml eluted by 0.9% NaCl solution. Obviously, the elution efficiency of 0.9% NaCl solution was higher than 0.2 M mannose solution. It was speculated that hydrophobic groups of PTL were in action with its mannose binding specificity characteristic.
Results of the SDS-PAGE electrophoresis showed that the separation efficiency of the three different ligand density affinity gel had no significant difference (Figure 2). The separation efficiency of the equilibrium buffer 20% NaCl solution was 20 times more than 10% NaCl solution, while PBS had no separation effect.
Mass spectrometry was used for further detection of the purity and molecular weight of the isolated PTL. It showed one single peak in the mass chromatogram, indicating that it was a single strand protein, and its relative molecular weight was 12.165 kD (Figure 3).
Both total protein from Pinellia tuber and PTL had
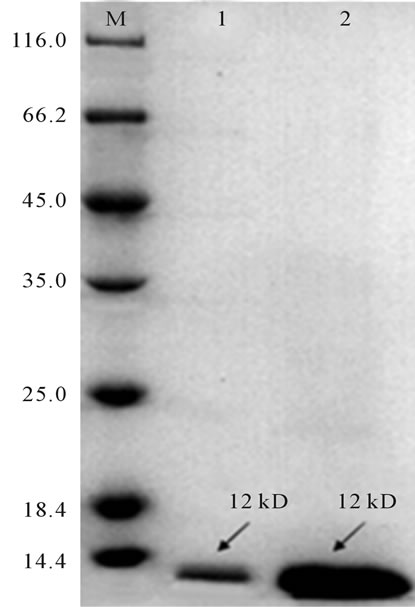
Figure 1. Results of SDS-PAGE electrophoresis of samples eluted from mannose affinity chromatographic column. M: 116 kD protein MW marker; 1: Sample eluted by 0.2 M Mannose solution; 2: Sample eluted by of 0.9% NaCl solution.
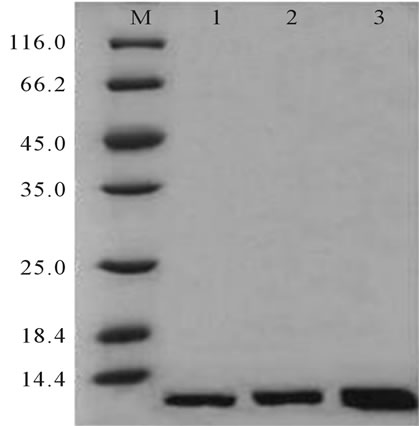
Figure 2. SDS-PAGE electrophoresis results of different ligand density. M: 116 kD protein MW marker; 1: Affinity gel of low ligand density; 2: Affinity gel of medium ligand density; 3: Affinity gel of high ligand density.
agglutinative activity on mouse erythrocytes (Table 1). The agglutinative activity of PTL was better than that of total protein from Pinellia tuber. It was infered that the mutual influence between different ingredients of total protein from Pinellia tuber may weaken its agglutinative effect.
Two SDS-PAGE gel strips of PTL obtained were stained with coomassie brilliant blue and periodate schiff reagent, respectively. The result showed that there was a distinct glycoprotein red staining bands in the correspongding position with the coomassie brilliant blue staining protein band, indicating that the PTL was a glycoprotein (Figure 4).
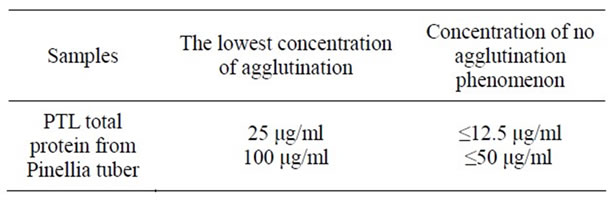
Table 1. The results of agglutinative effect of PTL and total protein from Pinellia tuber.
Fifteen kinds of amino acids which were similar to the amino acid composition of the general plant lectin were detected in PTL, among which aspartate content was the highest, while cysteine content was the lowest (Table 2) (Figure 5).
Datas (Figures 6 and 7) showed that low concentrations of PTL (0.004 mg/ml, 0.2 mg/ml and 0.1 mg/ml) promoted the proliferation of HeLa cells. Under the same concentration with the time-lapse the proliferation promoting effect of the three concentrations were 48 hr > 72 hr > 24 hr. For example, the inhibition ratio to HeLa cells was 23.36% (48 hr) > 18.42% (72 hr) > 1.26% (24 hr) when the PTL concentration is 0.004 mg/ml. The reason could be that it needs enough time to play a significant effect. When the time reached to 48 hr, the effect reached to the maximum, however, the proliferation promoting effect weakened as the time increasing, and still stronger than the effect of 24 hr. At the same time, the promoting proliferation effect of the three concentrations was 0.004 mg/ml > 0.002 mg/ml > 0.1 mg/ml. It was supposed that lower concentrations of PTL had significant role in promoting proliferation of HeLa cells. While high concentrations of PTL (0.5 mg/ml and 1 mg/ml) inhibited the
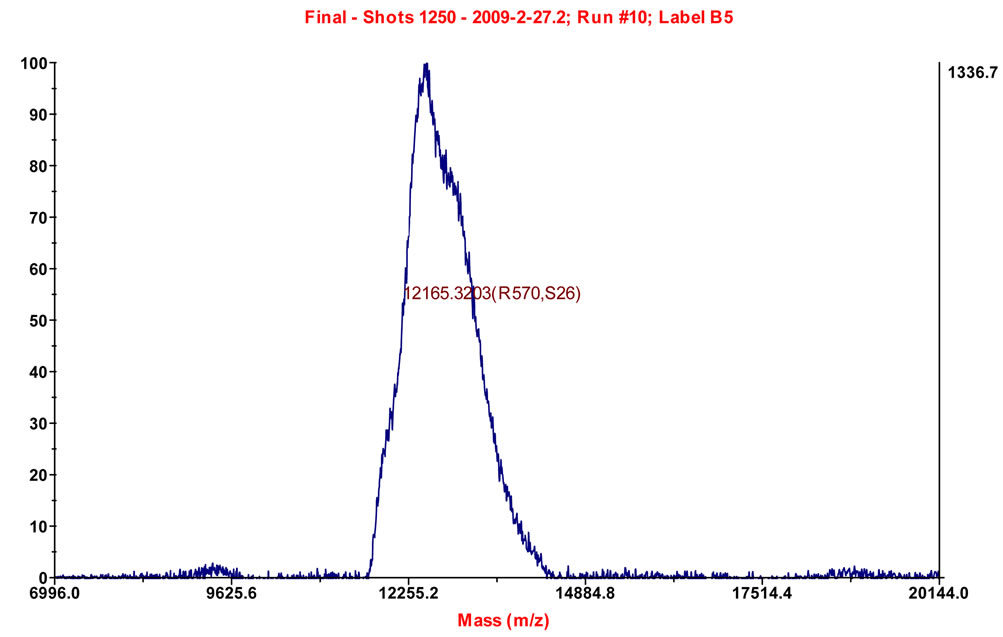
Figure 3. Mass spectrometric analysis of PTL.
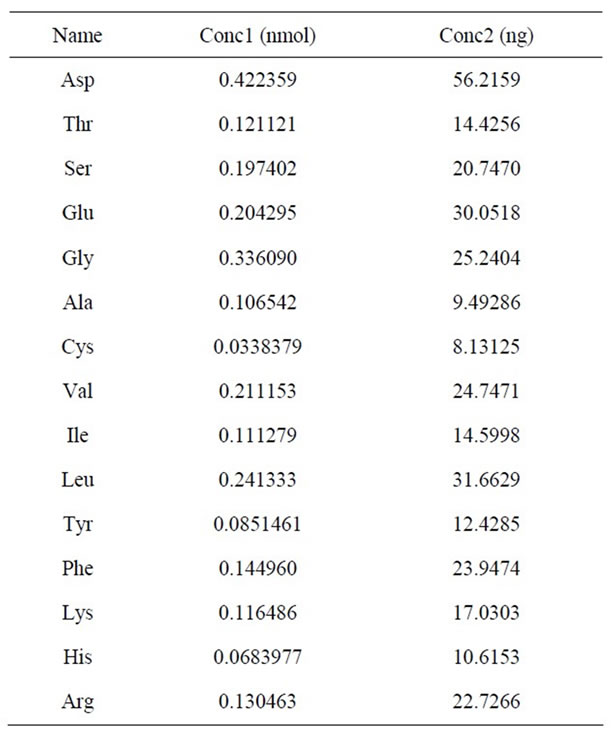
Table 2. Amino acids compositions of the PTL.
proliferation of HeLa cells significantly in a time-dosedependent manner. The maximum inhibition ratio of them was 62.3% and 71.89% at 72 hr, respectively.
The effect on HeLa cells treated by PTL of five different concentrations was that the prompting proliferation decreased with the concentration increasing and the suppression proliferation effect enhanced, and the turning point was at 0.1 mg/ml, indicating that low concen-
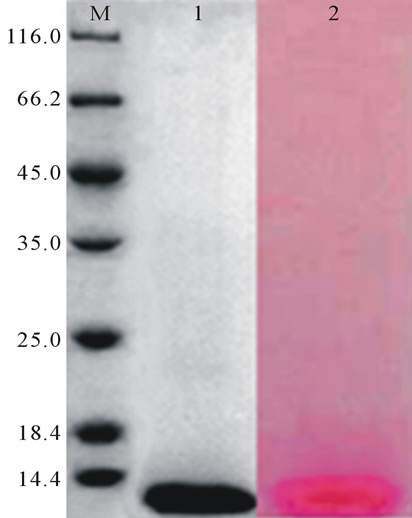
Figure 4. Detection of glycoprotein. M: 116 KD protein MW marker; 1: The coossie brilliant blue staining protein band; 2: The glycoprotein red staining band.
trations of PTL had the effect of promoting cells division. However, higher concentrations of PTL could inhibit HeLa cells proliferation.
Two concentrations (0.004 mg/ml and 1 mg/ml) of PTL were selected to treat HeLa cells. When cultivated for 24 hr, 48 hr and 72 hr, cells morphology were observed under the inverted phase contrast microscope and photographed (Figure 8).
Growth state of cells under the control group (PBS): When cultivated 24 hr, shapes of the cell were polygonal and all cells were adherent to the bottom of flasks and in the dividing state. Cell spaces were wide and there were
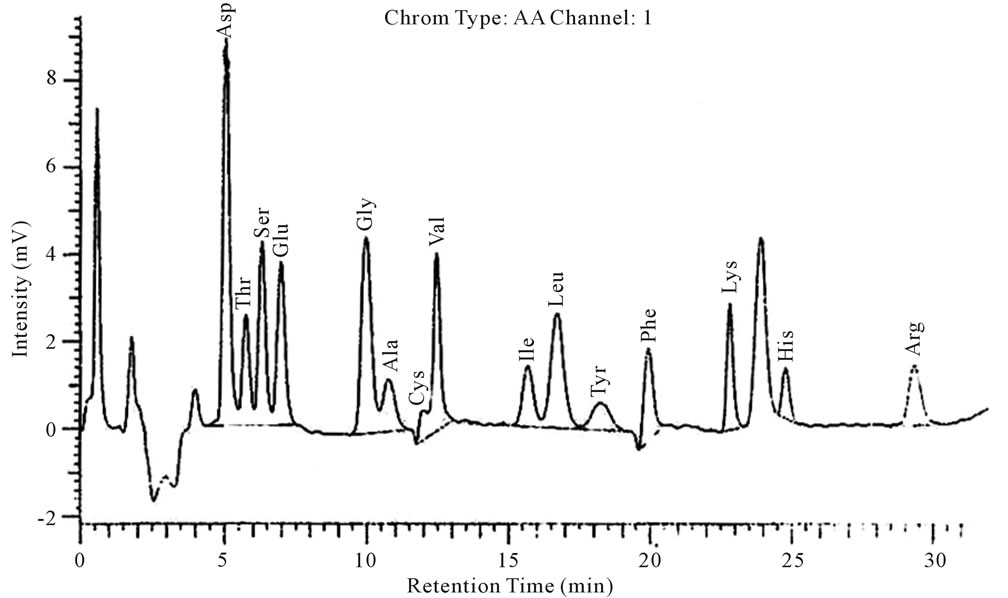
Figure 5. Amino acids composition analysis.
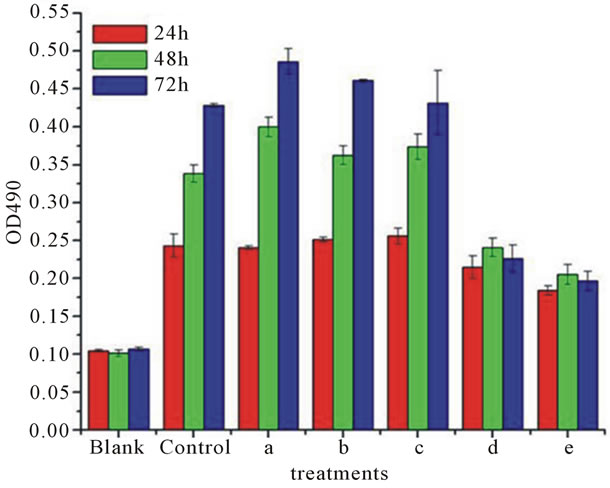
Figure 6. A 490 value of HeLa cells treated by PTL of different concentrations. Blank: medium; Control: PBS buffer; (a) 0.004 mg/ml; (b) 0.02 mg/ml; (c) 0.1 mg/ml; (d) 0.5 mg/ml; (e) 1 mg/ml.
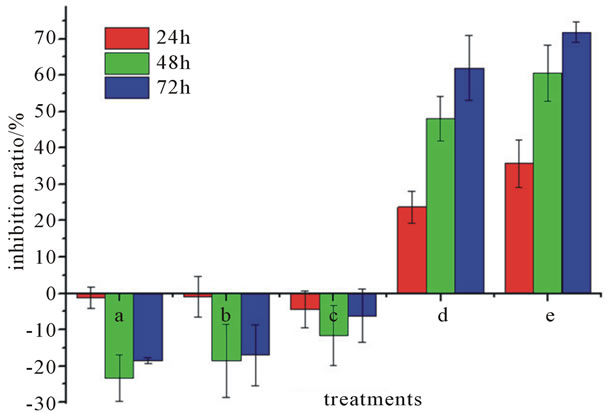
Figure 7. Inhibition ratio of Pinellia ternata lectin of different concentrations on HeLa cells. (a) 0.004 mg/mL; (b) 0.02 mg/mL; (c) 0.1 mg/mL; (d) 0.5 mg/mL; (e) 1 mg/mL.
a few dead cells suspended in culture medium, which were due to the intensity of dispersing cells in the cells digestion procedure. When cultivated 48 hr, compared with the cultivation of 24 hr, the cell spaces became smaller and the number of cells increased significantly. When cultivated 72 hr, compared with the above, the cell culture flask was covered with cells.
Growth state of Cells under the 0.004 mg/ml PTL treated group: Compared with the control group cells were grew well, cell density was higher than the control group at the same time. When cultivated for 72 hr, the cell culture flask was covered with cells appearing contact and density inhibition.
Growth state of cells under the 1 mg/ml PTL treated group: When cultivated 24 hr, there were a few cells adherent to the flask. Some cells became slim, and others
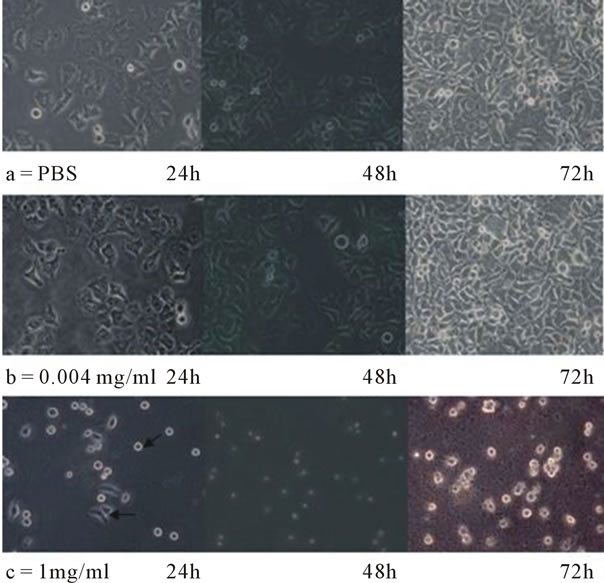
Figure 8. Microscopic observation results of HeLa cells treated by PTL. (a) PBS; (b) 0.004 mg/ml PTL; (c) 1 mg/ml PTL.
shrinked to death suspending in culture medium with a little cell debris. Cultivation of 48 hr, compared with 24 hr, all cells suspended in culture medium with more cell debris. When cultured 72 hr, all cells were dead suspending in culture medium with a large number of cell debris.
In Sum, it was found that cell growth state treated by 0.004 mg/ml PTL was similar to that of the control group, but a little better than the control group. Cells treated by 1 mg/ml PTL had greatly changes in cell morphology. When cultured 72 hr, cells proliferation was completely inhibited.
4. DISCUSSION
In this paper, the mannose-Sepharose 4B affinity gel was designed and a kind of PTL was successfully isolated by application of the designed affinity gel with the molecular weight of 12.165 kD detected by Mass spectrometry, which was similar to the pinellin with a inhibitory activity isolated from Pinellia ternata by Wang Houwei, and supposed to be the same protein [18]. However, compared with Wang Houwei’s separation method, our approach was easy to operate and had a good repeatability. In addition, a kind of PTL with its molecular weight of 44 kD had been isolated from Pinellia ternata by Wang Keyi, et al. by application of pig thyroid immobilized affinity column chromatography, and it was speculated that the PTL isolated in this study may be its subunit [4].
Results of the research on the PTL showed that it was a glycoprotein which can agglutinate mouse red blood cells with the minimum agglutinative concentration 25 μg/ml, and the aggregative effect was stronger than total protein of Pinellia ternata. The reason may be that different components in total protein of Pinellia ternata interact with each other affecting the aggregation reaction. Amino acid analysis results showed that it mainly contained 15 kinds of amino acids, content of aspartic acid was maximum while cysteine was the minimum.
Results of the studies on the binding percentage of PTL to mannose-ligand showed that there was no linear relationship between binding percentage and mannoseligand density. But the salt concentration of the equilibrium buffer has a greater impact on binding efficiency and reasons may be that high salt concentration makes the hydrophobic groups inside the PTL exposed to mannose-ligand. The elution yield of 0.9% NaCl solution was better than 0.2 M mannose solution, indicating that the hydrophobic groups in PTL played a significant role in binding with the affinity chromatography column.
Current studies on total protein of Pinellia ternata have shown that it has significant anti-tumor activity, such as the effect of total protein of Pinellia ternata on S-180 cell lines in mice, ovarian cancer lines, hematopoietic cells in human umbilical, human hepatoma carcinoma cells Bel-7402, and on human ovarian cancer cells SKOV3 [14,19-21]. However, total protein of Pinellia ternata contains a variety of ingredients, and so it is not clear that which components are the mainly active components. In this paper, we found that low concentrations of PTL (0.004 mg/ml, 0.02 mg/ml and 0.1 mg/ml) could promote HeLa cells proliferation, which was consistent with the conclusions of Sun Ce indicating that pinellin also had the activity of promoting cell division [22,23], and the promoting proliferation was negatively correlated with the concentration and time. Instead, high concentrations of PTL (0.5 mg/ml and 1 mg/ml) had obvious effectiveness in inhibiting HeLa cells proliferation. However, the mechanisms of promoting and inhibiting tumor cell proliferation of PTL needs further study.
From the above data, it can be concluded that the product isolated by mannose-Sepharose 4B affinity chromatography is PTL subunit with the molecular weight of 12.165 kD and has significant anti-tumor features.
These paper provide basic data for PTL isolation and its anti-tumor activity research. In addition, Pinellia ternata is one of the poisonous plants in the Chinese plant indexed, toxic for the whole plant, tubers toxicity for the maximum. Do not attempt to take, Eaten raw 0.1 to 0.8 g can lead to poisoning, suffocating to death, these should be noted in using and research.
![]()
![]()
REFERENCES
- Peumans, W.J. and Van Damme, E.J.M. (1995) Lectins as plant defense proteins. Plant Physiology, 109, 347-352. doi:10.1104/pp.109.2.347
- Van Damme, E.J.M., Peumans, W.J., Barre, A. and Rougé, P. (1998) Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Critical Reviews in Plant Sciences, 17, 575-692. doi:10.1080/07352689891304276
- Guo, C.S., Yu, L.Y., Ge, W., Zhang, L., Tang, C., et al. (2008) Research on extraction and hemagglutination effect of kidney bean phytohem agglutinin. Chinese Journal of Veterinary Drug, 42, 49-50.
- Wang, K.Y. and Guo, M. (1993) Purification of lectin from Pinellia ternata. Chinese Biochemical Journal, 9, 544-547.
- Zeng, Z.K., Wu, Q.Q. and Bao, J.K. (1995) Purification and characterization of cotton lectin. Acta Botanica Sinica, 37, 204-211.
- Lin, Y.M. and Su, A.H. (2003) Isolation, purification and characterization of lectin from dictyophora indusiata fisch. Chinese Journal of Biochemistry and Molecular Biology, 19, 261-263.
- Pan, K., Huang, B.Q. and Hou, X.W. (2007) Purification of a lectin from alocasia macrorrhiza and its toxic effect on four cultures insect cell line. Journal of Anhui Agricultural, 35, 5484-5485.
- Li, J., Xu, X.C. and Bao, J.K. (2008) Effects of denaturation and chemical modification on lectin from phaseolus multiflorus. Willd. Natural Product Research and Development, 20, 787-792.
- Gu, D.X. and Guo, Q.S. (1990) Study on biological characteristics in groups of Pinellia ternata (Thunb.) Breit. Journal of Nanjing Agricultural University, 13, 11-16.
- Ge, X.Y. and Wu, H. (2009) Phytochemical properties and quality evaluation methods of Pinellia ternata. China Pharmaceuticals, 18, 3-5.
- Li, B., Cheng, X.M., Zhou, Y.Y. and Lou, H.X. (2010) Progress on research of Pinellia ternata. Chinese Journal of Ethnomedicine and Ethnopharmacy, 19, 47-48.
- Wang, Z.Q. and Li, B.C. (2009) Progress on the research of pharmacology of Pinellia ternata. Shanxi Medical Journal, 38, 65-67.
- Xia, L.N. and Li, C.J. (1985) Study on the mechanism of anti-fertility effect and anti-early pregnancy of pinellin on mice. Journal of Fudan University (Medical Science), 12, 193-198.
- Sun, G.X., Ding, S.S. and Qian, Y.J. (1992) Extraction, chemical analysis and effect on S-180 cell lines in mice of total protein of Pinellia pedatisecta schott. Journal of Fudan University (Medical Science), 1, 17-19.
- Tao, Z.J., Xu, Q.Y., Wu, K.Z. and Lian, S.H. (1981) Isolation and crystallization and biological activities and some chemical characteristics of pinellin. Acta Biochimica et Biophysica Sinica, 13, 77-82.
- Wang, K.Y. and Guo M. (1993) Purification of lectin from Pinellia ternata. Chinese Biochemical Journal, 9, 544-547.
- Wang Y.Q., Wu G.H., Lin X.F., He, L.P. and Huang, Z.L. (2009) Study on improving of polyacrylamide gel electrophoresis staining of polysaccharides and glycoproteins. Plant Physiology Communications, 45, 169-172.
- Wang, H.W. (2008) Purification and N-terminal amino acid sequence analysis of one kind of protein with trypsin inhibitory activity in Pinellia ternata. Chinese Traditional Drug, 39, 1320-1323.
- Zhu, M.W., Zhou, K.M. and Ding, S.S. (1999) Study on the effect of total protein of Pinellia pedatisecta schott on ovarian cancer cell lines and hematopoietic cells in human umbilical. Shanghai Medical University, 26, 455-456.
- Huang, B.S. (2007) Study on apoptosis inducing effect of pinellin on human hepatoma carcinoma cells Bel-7402. Lishizhen Medicine and Materia Medica Research, 18, 1506.
- Zhou, L., Wang, H.C., Zheng, Y.F. and Shi, M.R. (2010) Research on protein expression profile of human ovarian cancer cells SKOV3 treated by total protein of Pinellia pedatisecta schott by application of two-dimensional gel electrophoresis. Chinese Archives of Traditional Chinese Medicine, 28, 789-791.
- Sun, C. (1981) Some biological properties of pinellin. Acta Biochimica et Biophysica Sinica, 15, 1.
- Tachibana, Y. and Kawanishi, K. (1992) Mitogenic activities in the protein fractions of crude drugs. Planta Medica, 58, 250-254.
NOTES
*Corresponding author.

