Journal of Surface Engineered Materials and Advanced Technology
Vol.2 No.3(2012), Article ID:21323,4 pages DOI:10.4236/jsemat.2012.23026
Improved Corrosion Resisting Property of Magnetism Iron Fiber by SiO2 Coating
![]()
State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao, China.
Email: *yujinku@ysu.edu.cn
Received February 22nd, 2012; revised March 25th, 2012; accepted April 10th, 2012
Keywords: Corrosion Resistance; Anti-Oxidation; Sol-Gel Method; SiO2
ABSTRACT
To improve the oxidation resistance property of iron fibers, a SiO2 coated iron fiber was prepared by sol-gel method, and its microstructure, element and phase composition, antioxidation property, and crystallization were characterized by scanning electron microscopy, thermal gravimetric analysis, X-ray diffraction and 3% CuSO4 solution dripping. It was found that the surface of the iron fiber can be fully covered with SiO2 by using sol-gel method. Our results also indicated that the time of iron begin to be corrupted in 3% CuSO4 solution drip increased from 30 s to 240 s, and the temperature increased from 200˚C to 310˚C. In addition, the oxidation and antioxidation mechanisms of the SiO2 coated iron fiber have also been discussed in this work.
1. Introduction
Up to now, the most commonly used microwave absorbers are magnetic materials (such as ferrite particles) and dielectric materials (such as carbon black particles) [1-3]. However, the expansion of applications was limited due to their thickness and weight. Recently, some researchers has been devoted to the study of the electromagnetic properties of nonspherical magnetic materials [4-8], such as iron fibers (MIFs) etc. Owing to MIFs’ unique properties, lower cost and important applications as functional microwave absorbing materials, the preparation and properties of MIFs have been investigated by many researchers [9,10]. To date, MIFs can be produced by bundle-drawing, melt-extraction, and chatter-machining [7, 11-16] methods. For example, MIFs with diameter less than 2 μm have been prepared by using bundle-drawing method [7]. However, the MIFs easily oxidized in air, and this poor anti-corrosion ability prevents its wide applications. To overcome this disadvantage, many methods have been used to improve the anti-corrosion ability of the MIFs [17]. However, the anti-corrosion ability is not good enough. Therefore, a new technique is necessary to develop to improve the anti-corrosion ability of MIFs.
The sol-gel technique is a low-temperature route widely employed to prepare thin films for use in the different fields, because it can offer the homogeneous thin films at molecular scale and control of chemical purity. Recently, it has a rapid development in the field of machining electromagnetic materials and the interest to prepare the materials by sol-gel methods [18-19] has increased remarkably. Moreover, SiO2 has excellent insulation property and antioxidation property. To this end, a novel SiO2 coated MIFs has been developed by sol-gel technique in this paper. Our experimental results further demonstrated that the antioxidation property of the SiO2 coated MIFs has been improved evidently.
2. Experimental Methods
In this work, silicon dioxide sols were prepared by using tetraethoxysilane (TEOS) as precursor, ammonia water as activator and absolute alcohol as solvent. Firstly, 100 ml TEOS/alcohol solution was prepared by mixing TEOS with absolute ethanol at a molar ratio 3:10 of TEOS to C2H5OH, and then 50 ml ammonia water was added into the TEOS/alcohol solution smoothly under stirring simultaneously. After that, 10 g iron fibers were added into the above prepared solution, at the same time, the system was kept shaking and the temperature was kept at 25˚C in water shaker for 30 min. Finally, the iron fibers were dried in oven at 100˚C, and a SiO2 coated MIFs were obtained.
For passivation, a solution of chromiumtrioxide/ phosphoric acid/blackening agent/accelerant was prepared with molar ratio of 6:3:4:15 and the amount of the reagents in solution were varied in the range 20 - 35 g. Then put the above treated MIFs into the passivation solution and passivating for 4 hours at 25˚C. After that, the final passivated MIFs were washed with distilled water and then dried in air at 60˚C.
To characterize the final obtained MIFs, scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), X-ray diffraction (XRD) and 3% CuSO4 solution dripping were used. In detail, SEM was used to observe the morphology of the films. Elements of the passivated films are conformed by using energy dispersive spectrum (EDS). Phase composition of the passivated films after heat treatment was identified by XRD. The thermal behavior of the samples was determined by TGA. 3% CuSO4 solution drip was adopted to determine the anticorrosion property of the passivated films.
3. Results and Discussion
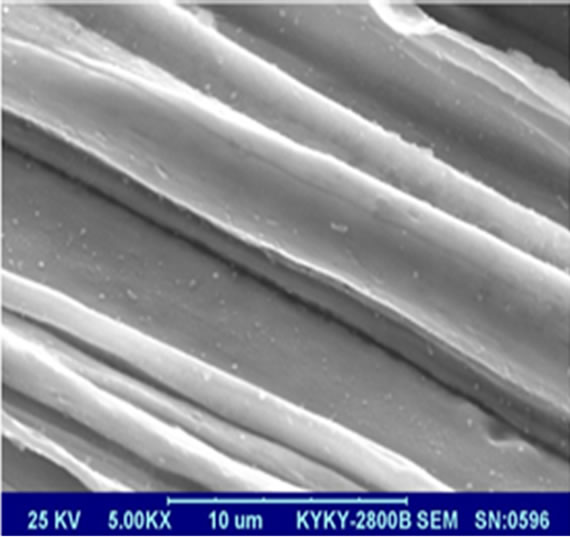
To study the morphology of MIFs before and after solgel processing, SEM is performed for the MIFs samples, and they are shown in Figure 1. The SEM images of the MIFs before and after treatment are shown in Figures 1(a) and (b), respectively. As can be seen from Figure 1, the surface of the MIFs without treatment was rough and full of drawing trace, which can improve the physics limitation and easily been corrupted. As shown in Figure 1(b), after sol-gel processing, the surface of the MIFs has been covered with SiO2 successfully.
In order to investigate the morphology of SiO2 coated MIFs after passivation, SEM is also perform for SiO2 coated MIFs after passivation, and shown in Figure 2. As can be seen from Figure 2, the surface of the MIFs has been covered with spherical SiO2 particles.
In this work, element distribution is also characterized by EDS for the SiO2 coated MIFs and the results are shown in Figure 3. It can be seen from Figure 3, besides of the peaks corresponding to Ni, C, O, Cu elements, there are several characteristic peaks of Fe element, which attributed to the iron fibers. The Si, O elements comes from the sol-gel method, and the Ni elements are obtained from the passivation. This result further indiated that the surface of the MIFs has been coated with SiO2 successfully, and this further confirms our previous SME results.
 (a)
(a)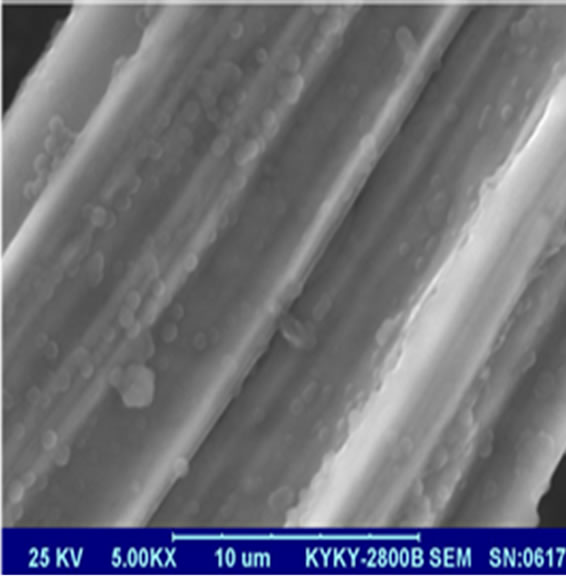 (b)
(b)
Figure 1. Surface appearance of the MIFs under SEM: (a) without treatment; (b) after treatment.

Figure 2. Surface appearance of the MIFs film after treatment under SEM.
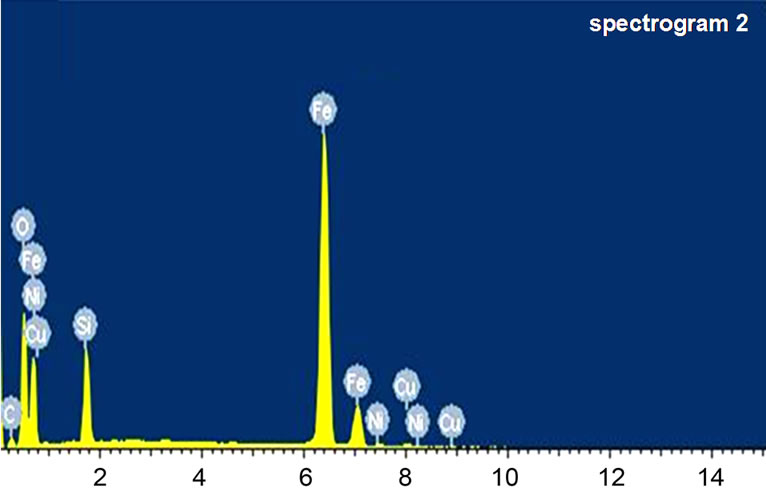
Figure 3. EDS analysis of the MIFs after treatment.
In order to study the oxidation resistance property of MIFs after sol-gel processing and passivation, TGA experiments are performed for MIFs before and after solgel processing and passivation, and they are shown in Figure 4. It is found that the weight begin to increase at 200˚C for MIFs without treatment, this means that the MIFs without treatment began to oxidize at this point. When the temperature reaches to 370˚C, the MIFs start to aggravate. Compared to the MIFs without treatment, the temperature for the treated MIFs begin to oxidize is 310˚C, and the aggravate temperature is 440˚C. Meanwhile, the increased weight of the treated MIFs is always less than the MIFs without treatment. These results indicate that the oxidation resistance of the MIFs is enhanced after sol-gel processing and passivation evidently.
To further study the oxidation resistance property and phase stability of MIFs after sol-gel processing and passivation, XRD experiments are performed for MIFs sample after heat treatment under 400˚C for 3 hours, and they are shown in Figure 5. In this work, two kinds of MIFs samples were heat treated, one is the MIFs sample just after sol-gel processing, and the other one is the MIFs sample after both sol-gel processing and passivation treatment. It can be concluded from Figure 5, after heat treatment under 400˚C for 3 hours, the XRD pattern of the MIFs sample just after sol-gel processing exists two main phases: pure iron and Fe3O4. But for the XRD pattern of the MIFs sample after both sol-gel processing and passivation treatment exist only one main phase: pure iron. These results imply that the oxidation resistance property of the sol-gel processing treated MIFs can be further improved by passivation processing. Our present results also consist with the previous TGA study, and further confirmed our previous experiments. The anticorrosion property of the MIFs was identified by 3% CuSO4 solution drip and the result turns up to be: the anti-corrosion time of the MIFs after treatment increased from 65 s to 240 s.
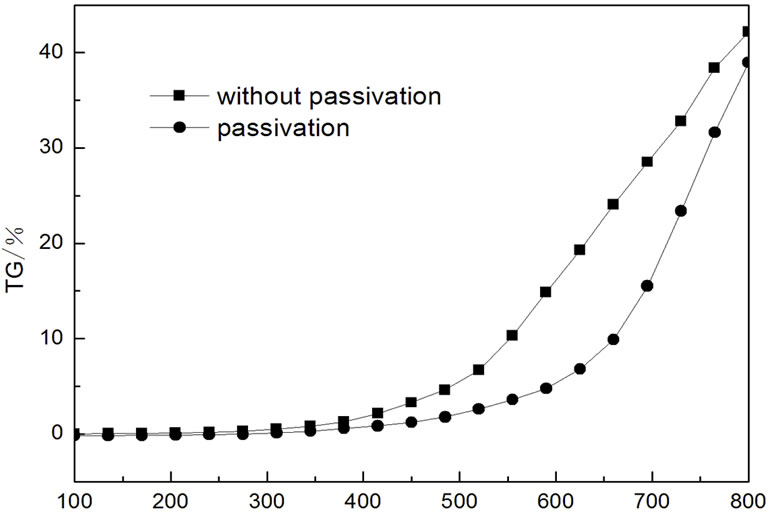
Figure 4. TG curve of the MIFs before and after treatment.
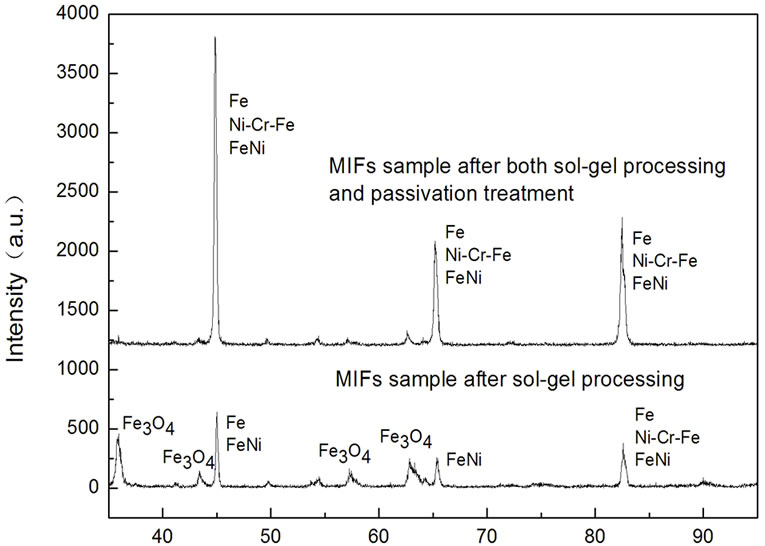
Figure 5. X-ray diffraction spectrum of the MIFs after heat treatment.
4. Conclusion
In order to improve the oxidation resistance property of MIFs, a novel SiO2 coated MIFs has been developed, and to further improve the oxidation resistance property of MIFs, a passivation processing was studied. The experimental results indicated that, the surface of the MIFs can be covered by SiO2 after sol-gel processing. The antioxidation property of SiO2 coated MIFs can be improved by further passivation treatment.
5. Acknowledgements
The authors gratefully acknowledge the support of the SIWEITE metal fibers company for providing the MIFs. Thanks are also made to Professor J. K. Yu and M. Z. Wang for help with experiments and valuable discussions.
REFERENCES
- X. D. Chen, G. Q. Wang, Y. P. Duan and S. H. Liu, “Microwave Absorption Properties of Barium Titanate/Epoxide Resin Composites,” Journal of Physics D: Applied Physics, Vol. 40, No. 6, 2007, pp. 1827-1830. doi:10.1088/0022-3727/40/6/035
- M. A. Bhuiyan, S. M. Hoque and S. Choudhury, “Effects of Sintering Temperature on Microstructure and Magnetic Properties of NiFe2O4 Prepared from Nano Size Power of NiO and Fe2O3,” Journal of Bangladesh Academy of Sciences, Vol. 34, No. 2, 2010, pp. 189-195.
- J. P. Zhao, Y. Li and P. L. Wu, “Recent Developments of Studies on New Electromagnetic Wave Absorbing Materials,” Materials Science and Technology, Vol. 10, No. 2, 2002, pp. 219-224.
- Y. C. Qing, W. C. Zhou, F. Luo and D. M. Zhu, “Electromagnetic and Microwave Absorption Properties of Carbonyl Iron and Carbon Fiber Filled Epoxy/Silicone Resin Coatings,” Applied Physics A, Vol. 321, No. 4, 2009, pp. 1177-1181.
- L. C. Cheng, S. K. Pan, C. F. Lu and X. T. Luo, “Microwave Absorbing Property of Fe-Si-Cr Alloy Micropowders,” Electronic Components and Materials, Vol. 30, No. 1, 2011, pp. 66-68.
- F. S. Wen, W. L. Zuo, H. B. Yi, N. Wang, L. Qiao and F. S. Li, “Microwave-Absorbing Properties of Shape-Optimized Carbonyl Iron Particles with Maximum Microwave Permeability,” Physics B, Vol. 404, No. 20, 2009, pp. 3567-3570.
- H. F. Cheng, W. Xie, Z. Y. Chu, Y. J. Zhou, G. P. Tang and Z. H. Chen, “Effect of Grinding Techniques on Absorbing Characteristics of Short Iron Fibers,” Journal of Wuhan University of Technology—Materials Science Edition, Vol. 22, No. 3, 2007, pp. 400-403.
- R. C. Pullar and A. K. Bhattacharya, “The Magnetic Properties of Aligned M Hexa-Ferrite Fibres,” Journal of Magnetism and Magnetic Materials, Vol. 300, No. 2, 2006, pp. 490-499. doi:10.1016/j.jmmm.2005.06.001
- X. A. Fan, J. G. Guan, W. Wang, Y. L. Wang, G. X. Tong and F. Z. Mou, “Preparation, Microstructure Control and Magnetic Properties of 1D Ferromagnetic Metal Nanomaterials,” Progress in Chemistry, Vol. 21, No. 1, 2009, pp. 143-151.
- A. Itou, O. Hashimoto, H. Yokokawa and K. Sumi, “A Fundamental Study of a Thin λ/4 Wave Absorber Using FSS Technology,” Electronics and Communications in Japan, Vol. 87, No. 11, 2004, pp. 77-86.
- M. Z. Wu, H. H. He, Z. S. Zhao and X. Yao, “Preparation of Magnetic Cobalt Fibres and Their Microwave Properties,” Journal of Physics D: Applied Physics, Vol. 33, No. 22, 2000, pp. 2927-2930. doi:10.1088/0022-3727/33/22/309
- A. S. Antonov, N. A. Buznikov, A. B. Granovsky and N. S. Perov, “Nonlinear Magnetoimpedance Effect in Soft Magnetic Amorphous Wires Extracted from Melt,” Sensors and Actuators A, Vol. 106, No. 1-3, 2003, pp. 208- 211. doi:10.1016/S0924-4247(03)00168-7
- E. A. Aguilar and R. A. L. Drew, “Melt Extraction Processing of Structural Y2O3-Al2O3 Fibers,” Journal of the European Ceramic Society, Vol. 20, No. 8, 2000, pp. 1091-1098. doi:10.1016/S0955-2219(99)00279-4
- X. Q. Shen, K. Cao, and J. X. Zhou, “Preparation of Ferromagnetic Binary Alloy Fine Fibers by Organic GelThermal Reduction Process,” Transactions of Nonferrous Metals Society of China, Vol. 16, No. 5, 2006, pp. 1003- 1008. doi:10.1016/S1003-6326(06)60368-3
- Y. J. Di, J. J. Jiang, D. U. Gang, B. Tian, S. W. Bie and H. H. He, “Magnetic and Microwave Properties of GlassCoated Amorphous Ferromagnetic Microwires,” Transactions of Nonferrous Metals Society of China, Vol. 17, No. 6, 2007, pp. 1352-1357.
- X. L. Yu, X. C. Zhang and H. H. Li, “Simulation and Design for Stratified Iron Fiber Absorbing Materials,” Materials & Design, Vol. 23, No. 1, 2002, pp. 51-57. doi:10.1016/S0261-3069(01)00042-5
- Y. J. Zhou and H. F. Cheng, “Preparation of SiO2 Coating on the Surface of Microwave Absorbent by Sol-Gel Method,” New Technology and New Process, No. 10, 2006, pp. 54-56.
- V. G. San and A. Morales, “Sol-Gel TiO Anti-Reflective Films for Textured Monocrystal-Line Silicon Solar Cells,” Thin Solid Films, Vol. 403-404, 2002, pp. 335-338.
- M. M. Yusuf, H. Imai and H. Hirashima, “Preparation of Mesoporous TiO2 Thin Films by Surfactant Tinplating,” Journal of Non-Crystalline Solids, Vol. 285, No. 1-3, 2001, pp. 90-95. doi:10.1016/S0022-3093(01)00437-9
NOTES
*Corresponding author.

