American Journal of Computational Mathematics
Vol.06 No.04(2016), Article ID:72912,21 pages
10.4236/ajcm.2016.64034
ADI Finite Element Method for 2D Nonlinear Time Fractional Reaction-Subdiffusion Equation
Peng Zhu1, Shenglan Xie2
1Department of Mathematics, Jiaxing University, Jiaxing, China
2Nanhu College, Jiaxing University, Jiaxing, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 18, 2016; Accepted: December 18, 2016; Published: December 21, 2016
ABSTRACT
In this paper, an alternating direction Galerkin finite element method is presented for solving 2D time fractional reaction sub-diffusion equation with nonlinear source term. Firstly, one order implicit-explicit method is used for time discretization, then Galerkin finite element method is adopted for spatial discretization and obtain a fully discrete linear system. Secondly, Galerkin alternating direction procedure for the system is derived by adding an extra term. Finally, the stability and convergence of the method are analyzed rigorously. Numerical results confirm the accuracy and efficiency of the proposed method.
Keywords:
Nonlinear Fractional Differential Equation, Alternating Direction Implicit Method, Finite Element Method, Riemann-Liouville Fractional Derivative

1. Introduction
In this paper, we consider the following two-dimensional nonlinear fractional reaction- subdiffusion equation
 (1)
(1)
with boundary and initial conditions
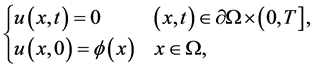 (2)
(2)
where ,
, 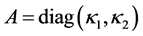 ,
, 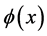 is sufficiently smooth function. For simplicity, we assume coefficients
is sufficiently smooth function. For simplicity, we assume coefficients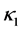 ,
, 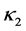 and
and 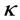 are positive constants in this paper. In fact, our method and its corresponding theoretic result are also valid for variable coefficients.
are positive constants in this paper. In fact, our method and its corresponding theoretic result are also valid for variable coefficients. 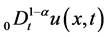 is the Riemann-Liouville time fractional derivative of order
is the Riemann-Liouville time fractional derivative of order  defined by [1]
defined by [1]
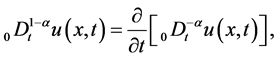 (3)
(3)
where 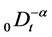 denotes the Riemann-Liouville fractional integral operator defined as [1]
denotes the Riemann-Liouville fractional integral operator defined as [1]
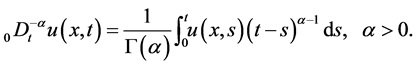 (4)
(4)
In addition, we assume that the nonlinear source term  satisfies the Lipschitz condition with respect to
satisfies the Lipschitz condition with respect to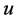 , i.e., there exists a positive constant
, i.e., there exists a positive constant 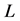 such that
such that
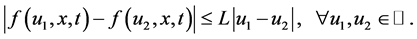
Problem (1) can be considered as a model for reaction-diffusion phenomena with anomalous diffusion, which has been widely applied in various fields of science and engineering. Generally, solutions of (1) can’t be obtained by analytical approach. So, there are various numerical methods developed for solving (1). Li and Ding [2] proposed higher order finite difference methods for solving 1D linear reaction and anomalous- diffusion equations. Zhuang, Liu and Anh, et al. [3] presented an implicit finite element method for solving 1D nonlinear fractional reaction-subdiffusion process. Dehghan, Abbaszadeh and Mohebbi [4] analyzed a meshless Galerkin method with radial basis functions of 2D linear fractional reaction-subdiffusion process. Yu, Jiang and Xu [5] derived an implicit compact finite difference scheme for solving 2D nonlinear fractional reaction-subdiffusion equation.
Alternating direction implicit (ADI) method was proposed by Peaceman, Rachford and Douglas [6] [7] [8] in 1950’s for multidimensional differential equations of integer order, which could reduce original multidimensional problem into a sequences of one- dimensional problems. Since the first ADI based finite difference (FD) scheme presented for 2D space fractional diffusion equation by Meerschaert, Scheffler and Tadjeran [9] , there are many literatures about various multidimensional fractional differential equations numerically solved by ADI technique. The following problem is always discussed:
 (5)
(5)
where 

In the case of



is also often studied. For instances, Cui [16] presented a compact ADI FD scheme, where the local truncation error was analyzed and the stability was discussed by the Fourier method. Furthermore, the author analyzed the cause of low time accuracy when 
There are also lots of ADI based numerical methods for multidimensional space fractional differential equations. Fast iterative ADI FD schemes [24] [25] are designed for 2D/3D linear space fractional diffusion equations, which are first order accuracy in both time and space and have the advantage of low computational work and low memory storage. High order accurate ADI FD schemes are proposed for 2D linear space fractional diffusion equations [26] [27] and two-sided space fractional convection-dif- fusion equations [28] , which are based on weighted and shifted Grünwald operators or Lubich operators approximating Riemann-Liouville fractional derivatives respectively. Spectral direction splitting methods [29] are derived for 2D space fractional differential equations. Semi-implicit alternating direction FD scheme [30] and ADI FE scheme [31] are used for solving 2D fractional Fitz Hugh-Nagumo monodomain model, which consists of a coupled 2D space fractional nonlinear reaction-diffusion equation and an ordinary differential equation, on irregular domain and rectangle domain respectively. ADI Galerkin-Legendre spectral method [32] is developed for 2D Riesz space fractional nonlinear reaction-diffusion equation.
Most of the above mentioned works contribute on linear fractional differential equations and finite difference method combined with ADI technique. A few work consider ADI FEM [14] [31] or nonlinear fractional differential equations [30] [31] [32] . Compared with FD method, FE method has the advantage of easily handling variable coefficients problem and boundary conditions. And many realistic problems involve nonlinear fractional differential equations. Based on these motivations, our attention in this paper will focus on developing ADI FE schemes for efficiently solving a class of nonlinear time fractional differential equations. This is the first time ADI FE scheme proposed and analyzed rigorously for nonlinear fractional differential equations. We will use problem (1.1) as a model problem to illustrate our approach.
The outline of this paper is organized as follows. In Section 2, we introduce some preliminaries and notations which will be used later. The formulation of ADI finite element method for nonlinear time fractional reaction-subdiffusion equation is presented in Section 3. The stability and error estimates of the proposed method are discussed in Section 4. In convenience of computation, we give the matrix form of ADI finite element scheme in Section 5. Some numerical experiments are displayed in Section 6. It aims to confirm our theoretical results. In the end, some concluding remarks are given in Section 5.
In the following, 




2. Preliminary and Notations
Let 


Recall that the Sobolev space 

Denote by 



If 

and
Denote


and
In convenience, the following notations will be used. For a positvie integer



Define linear operator 

and its variational form
and corresponding energy norm by
Obviously, we have
Some useful lemmas are given as follows.
Lemma 1. [3] Let
then 
1)
2)
Lemma 2. [3] If
where

We state here for convenience the discrete version of Gronwall’s inequality.
Lemma 3. [33] Suppose that 



where 
3. Formulation of ADI FEM
Integrating both sides of (1) with respect to the time variable 



Applying Lemma 2 and the following integration formula
Equation (8) is equivalent to

where the remainder term 

The weak form of Equation (9) is: find 


Then the finite element approximation to Equation (11) is: find 

or equivalently

The choice of the initial values 
Let


so that the alternating-direction Galerkin scheme of (1) can be defined as, for 

where

Remark 1. Numerical experiments in Section 6 demonstrate that the ADI Galerkin finite element scheme (14) has bad numerical performance for
in left hand side of (14) is extra added. Its effect on temporal accuracy cannot be ignored when

on the right hand side of (14). By the way, the similar remedy was also adopted by [11] [16] in finite difference framework.
In conclusion, when

where


In the following, we will focus our attention on the ADI Galerkin finite element scheme (14).
4. Stability and Error Estimate
Firstly, we introduce some notation and lemmas. Given a smooth function


or equivalently
The operator defined in (18) has the following approximate properties:
Lemma 4. [34] Let 






Lemma 5. [33] If 



where
Lemma 6. Let 




then it holds that
Proof. When


and
By direct algebraic calculation, and noticing the properties of 
The proof is completed.
Next, we consider the stability of the ADI Galerkin method (14). Define the following problem dependent norm for any

which will be used in stability analysis.
Assume the initial value 



Theorem 1. The ADI Galerkin method (14) is stable with respect to initial value 


holds for any
Proof. The equivalent form of (14) is:

Then the perturbation equation of (23) can be written as

where 

Taking 


Further, by Lipschitz property of 

Summing for 

For sufficiently small
Using discrete Gronwall inequality, we have
Let

Theorem 2. Let 

Assume that


provided the initial value 
Proof. Denote



Subtracting (28) from (23) leads to

Taking


Now, by Young inequality and Schwartz inequality, we can write
Substitute the above three inequalities into the right hand side of (30), and sum for
By discrete Gronwall inequality, if 
Consequently, we obtain

Using the triangle inequality and (31), we get

It remains to estimate terms on the right-hand side of (32). Firstly, by Lemma 3, we can conclude


By (10) and

Secondly, using calculus equality
and Hölder inequality, we have
Further, combined with Lemma 3, we can write

Since
similar as (36), we can prove
Additionally, according to Lemma 3 and Lemma 4, we have
As a result, we obtain

Similar as (37), we can write

Combining (33)-(38), and recalling 
provided 



Remark 2. Although in our theoretic analysis, we only obtain 



Note that we may choose the initial approximation as

This involves an elliptic problem to be solved. With this choice,
In practical computations, it is often sufficient to take 


5. Matrix Form of ADI FEM
Equations (14) define the ADI finite element method in inner product form. To describe the algebraic problem to which these equations lead, suppose
where 


be a tensor product basis for




so that
For convenience, we denote
If in (14) we choose

We define the matrices
and let
with 

where, from (14), the components of 
and 
which is equivalent to
where 




in the 

in the 



6. Numerical Experiments
In this section, two numerical examples are given to demonstrate the effectiveness and accuracy of the ADI Galerkin finite element methods. In all numerical examples, we take the linear tensor product basis
where



In our numerical simulation, we present the errors in 
and numerical convergence orders are computed by
Example 1. Consider the following problem
where
with initial and boundary conditions
where
Table 1 shows the L2 norm errors and the temporal convergence orders for 


To investigate the necessary of the correction term (16) in ADI scheme (17) if

for 


Table 1. L2 errors and temporal convergence orders, fixing h = π/64.
Table 2. Comparison between ADI scheme (14) and (17) for α = 0.1, h = π/64.
convergence order is 0.1, which also suggest our theoretical order 
scheme (14) if
Table 3 shows the L2 norm errors and the spatial convergence orders for 

Example 2. Consider the following problem
where
with initial and boundary conditions
where

The nonlinearity of Example 2 is stronger than Example 1. In the following simulation, we have to take much smaller temporal step 



Table 5 displays the comparison between the two ADI Galerkin finite element scheme (14) and (17) for



Table 3. L2 errors and spatial convergence orders, fixing τ = 1/5000.
Table 4. L2 errors and temporal convergence orders, fixing h = π/64.
Table 5. Comparison between ADI scheme (14) and (17) for α = 0.2, h = π/128.
Table 6. L2 errors and spatial convergence orders, fixing τ = 1/5000.
Table 6 shows the L2 norm errors and the spatial convergence orders for 

7. Conclusion
In this work, we proposed an alternating direction Galerkin finite element method for 2D nonlinear time fractional reaction sub-diffusion equation in the Riemann-Liouville type. The stability and convergence of the method are proved for

Acknowledgements
We thank the Editor and the referee for their comments. Research of P. Zhu is funded by the Natural Science Foundation of Zhejiang province, China (Grant No. LY15A010018). This support is greatly appreciated.
Cite this paper
Zhu, P. and Xie, S.L. (2016) ADI Finite Element Method for 2D Nonlinear Time Fractional Reaction- Subdiffusion Equation. American Journal of Computational Mathematics, 6, 336-356. http://dx.doi.org/10.4236/ajcm.2016.64034
References
- 1. Liu, Y., Song, S.J. and Xu, S.X. (2003) Advances in the Study on the Chemical Constituents and Biological Activities of Prunella vulgaris L. Shenyang Yike Daxue Xuebao, 20, 55-59.
- 2. Qin, J.H. (2008) Preparation of Prunella Vulgaris Extract Composition Used in Medicine. CN Patent No. 101274012 A.
- 3. Psotova, J., Svobodova, A., Kolarova, H. and Walterova, D. (2006) Photoprotective Properties of Prunella vulgaris and Rosmarinic Acid on Human Keratinocytes. Journal of Photochemistry and Photobiology B: Biology, 84, 167-174.
http://dx.doi.org/10.1016/j.jphotobiol.2006.02.012 - 4. Cheng, H.Y. and Zhang, Q.F. (2008) Enhanced Analysis of Triterpenes, Flavonoids and Phenolic Compounds in Prunella vulgaris L. by Capillary Zone Electrophoresis with the Addition of Running Buffer Modifiers. Journal of Chromatography A, 1213, 231-238.
http://dx.doi.org/10.1016/j.chroma.2008.10.033 - 5. Sun, W.G., Liao, H.L., Ye, Z.M. and He, G.X. (2003) Chemical and Pharmacological Studies Overview of Prunella vulgari. Zhongguo Zhongyiyao Xinxi Zazhi, 10, 86-88.
- 6. Wang, Z.J., Zhao, Y.Y., Chen, Y.Y., et al. (2000) Triterpenoid Compounds of Prunella Genus and Their Features of 13C NMR Spectroscopy. Chinese Materia Medica, 25, 583-588.
- 7. Cai, Z.Q., Ma, H.Y. and Zhang, M. (2009) Content Analysis of Oleanolic Acid and Ursolic Acid in Prunella vulgaris Based on Its Provenances and Appearances. Guangdong Yaoxueyuan Xuebao, 25, 256-258.
- 8. Sun, Y.M., Xu, J.Z., Wang, Z.A. and Yu, X.P. (2009) Determination of Ursolic Acid in Prunella vulgaris from Different Areas by HPLC. Shizhen Guoyi Guoyao, 20, 1125-1126.
- 9. Kojima, H. and Ogura, H. (1986) Triterpenoids from Prunella vulgaris. Phytochemistry, 25, 729-729.
http://dx.doi.org/10.1016/0031-9422(86)88033-5 - 10. Kojima, H., Tominaga, H., Sato, S., Ogura, H., et al. (1987) Pentacyclic Triterpenoids from Prunella vulgaris. Phytochemistry, 26, 1107-1111.
http://dx.doi.org/10.1016/S0031-9422(00)82359-6 - 11. Kojima, H., Tominaga, H. and Sato, S. (1988) Two Novel Hexacyclic Triterpenoids from Prunella vulgaris. Phytochemistry, 27, 2921-2925.
http://dx.doi.org/10.1016/0031-9422(88)80689-7 - 12. Ryu, S.Y., Oak, M.H., Yoon, S.-K., Cho, D.-I., Yoo, G.-S., Kim, T.-S. and Kim, K.-M. (2000) Anti-Allergic and Anti-Inflammatory Triterpenes from the Herb of Prunella vulgaris. Planta Medica, 66, 358-360.
http://dx.doi.org/10.1055/s-2000-8531 - 13. Gai, C.Y., Kong, D.Y., Wang, S.G., et al. (2010) Study on Chemical Constituents of Prunella vulgaris L. Chinese Journal of Pharm, 41, 8580-8582.
- 14. Zhang, L.Z., Guo, Y.J., Tu, G.Z., et al. (2008) A Novel Triterpenoid Saponin from Prunella vulgaris. Yaoxue Xuebao, 43, 169-172.
- 15. Lee, I.K., Kim, D.H., Lee, S.Y., Kim, K.R., Choi, S.U., Hong, J.K., Lee, J.H., Hyun, L.J. and Hyun, P.Y. and Ro, L.K. (2008) Triterpenoic Acids of Prunella vulgaris var. lilacina and Their Cytotoxic Activities in Vitro. Archives of Pharmacal Research, 31, 1578-1583.
http://dx.doi.org/10.1007/s12272-001-2154-6 - 16. Zhang, J.Y. and Yang, C.R. (1995) The Two New Ursane Glycosides from Prunella vulgaris in France. Yunnan Zhiwu Yanjiu, 17, 468-472.
- 17. Wang, Z.J., Zhao, Y.Y., Tu, G.Z., Hong, S.L. and Chen, Y.Y. (1999) Studies on Chemical Constituents from Prunella vulgaris. Huaxue Xuebao, 34, 679-681.
- 18. Hisashi, K., Noriko, S., Akiko, H. and Haruo, O. (1990) Constituents of the Labiatae Plants Part 5. Sterol Glucosides from Prunella vulgaris. Phytochemistry, 29, 2351-2355.
http://dx.doi.org/10.1016/0031-9422(90)83073-A - 19. Meng, Z.M. and He, L.W. (1995) Studies on Constituent of Prunella vulgaris L. Zhongguo Yaoke Daxue Xuebao, 26, 329-331.
- 20. Liu, B., Ma, G.P. and Shi, R.B. (2002) The Determination of Cyasterone in Prunella vulgaris L Var. Leucantha Schur Sec. Bailey Using RP-HPLC. Chinese Traditional and Herbal Drugs, 33, 898-899.
- 21. Tian, J., Xiao, Y.Y., Zhao, Y.Y., et al. (2000) Structure Identification of New Compound Vulgarsaponin a from Prunella vulgaris. Yaoxue Xuebao, 35, 29-31.
- 22. Psotova, J., Chlopcikova, S., Miketova, P. and Simánek, V. (2005) Cytoprotectivity of Prunella vulgaris on Doxorubicin-Treated Rat Cardiomyocytes. Fitoterapia, 76, 556-561.
http://dx.doi.org/10.1016/j.fitote.2005.04.019 - 23. Zhu, J.U., Wang, Z.J., Zhao, Y.Y., et al. (2001) Depsides from Prunella vulgaris. Zhongguo Shiyan Fangjixue Zazhi, S1, 157-161.
- 24. Hu, X., Cui, S., Chen, X. and Hu, Z.D. (2009) CE with Field-Enhanced Stacking for Rapid and Sensitive Determination of Umbelliferone, Rutin and Aesculetin in Prunella vulgaris. Chromatographia, 70, 1733-1736.
http://dx.doi.org/10.1365/s10337-009-1373-8 - 25. Jozef, S. (1963) Phytochemical Studies on Prunella vulgaris and Prunella Grandiflora Saponin and Triterpene Compounds. Dissertations Pharm, 15, 333-333.
- 26. Dmitruk, I.S., Dmitruk, S.E., Berezovskaya, T.P., et al. (1987) Flavonoids of Vulgaris. Khimiya Prirodnykh Soedinenii, 3, 449-449.
- 27. Dmitruk, I.S. (1987) Coumarins of Prunella vulgaris. Khimiya Prirodnykh Soedinenii, 4, 510-510.
- 28. Yang, L.J., Li, Z.Q., Pu, F., et al. (1988) The Chemical Composition of the Essential Oil of Prunella vulgaris Liun. Yaowu Fenxi Zazhi, 8, 264-265.
- 29. Wang, H.B., Zhang, Z.Y. and Su, Z.W. (1994) The Constituents of the Essential Oil from Three Plants of Prunella vulgari. Zhongguo Yaoxue Zazhi, 29, 652-653.
- 30. Xu, Z.X., Zhou, Q.Q. and Xu, S.L. (1996) Recent Research Progress on Chemical Constituents and Biological Activities of Prunella vulgaris. Zhongchengyao, 18, 42-42.
- 31. Tabba, H.D., Chang, R.S. and Smith, K.M. (1989) Isolation, Purification and Partial Characterization of Prunellin, an Anti-HIV Component from Aqueous Extracts of Prunella vulgaris. Antiviral Research, 11, 263-273.
http://dx.doi.org/10.1016/0166-3542(89)90036-3 - 32. Natherova, L., Leifertora, I. and Kunetkova, M. (1973) Evaluation of Flavonoids in the Domestic Specices of Genus Fragaria L. Ceskoslovenská Farmacie, 22, 441-443.
- 33. Feng, Y., Xue, M., Jiang, L.H., et al. (2008) Chemical Study on Water Soluble Polysaccharides from Prunella vulgaris L. Zhongguo Yiyuan Yaoxue Zazhi, 28, 431-434.
- 34. Rasool, R., Ganai, B.A., Akbar, S., Kamili, A.N. and Masood, A. (2010) Phytochemical Screening of Prunella vulgaris L.—An Important Medicinal Plant of Kashmir. Pakistan Journal of Pharmaceutical Sciences, 23, 399-402.
- 35. Wang, Q.X. (1998) Treatment of Hypertension by the Effective Remedy. Shizhen Guoyi Guoyao, 9, 342-343.
- 36. Jia, C.C., Wang, X.J. and Luan, Y.Y. (1999) External Usage of Traditional Chinese Medicine in the Treatment of Hypertension. Zhongguo Minjian Liaofa, 7, 14-15.
- 37. He, Y.Q., Li, R.Z., Feng, L.Z. and Li, Z.P. (1985) Studies on the Chemical Constituents of Prunella vulgaris (I). Beijing Yika Daxue Xuebao, 17, 297-299.
- 38. Li, Y.H., Niu, X. and Li, Y.G. (1996) Research on the Antihypertensive Activity of Sancaojiangyatang. Beijing Zhongyiyao Daxue Xuebao, 19, 45-46.
- 39. Wang, H.B., Zhang, Z.Y., Su, Z.W., et al. (1994) On the Effect of Total Saponins from Common Selfheal (Prunell vulgaris) on Experimental Myocardial Infarction and Hypertension of Anesthetized Rats. Chinese Traditional and Herbal Drugs, 25, 302-303.
- 40. Feng, P.F., Qin, N.P., Fang, R.Y., et al. (1998) Effect of Sanwu Hypertensive Decoction on Blood Pressure and Lymphokine-Activated Killer Cell in SHR. Zhongguo Xunhuan Zazhi, 13, 178-180.
- 41. Panza, J.A., Carcia, C.E., Kileoyne, C.M., Quyyumi, A.A. and Cannon III, R.O. (1995) Impaired Endochelium Dependent Vasodilation in Patients with EH Evidence that Nitric Oxide Abnormality Is Not Localized to a Angle Aignal Transduction Pathway. Circulation, 91, 1732-1738.
http://dx.doi.org/10.1161/01.CIR.91.6.1732 - 42. Kurmar, K.V. and Das, U.N. (1993) Are Free Radical Involved in the Pathobiology of Human Essential Hypertension. Free Radical Research, 19, 59-66.
http://dx.doi.org/10.3109/10715769309056499 - 43. Chen, W.H. and He, J.Y. (1997) Antagonistic Effects of Ephedra Sinica, Prunlla vulgaris and Cuttlefish Bone on the Model of Rat Acute Blood Stasis. Beijing Zhongyiyao Daxue Xuebao, 20, 39-41.
- 44. Liu, J.X., Qin, Y.J. and Zhang, J.Y. (1998) Experimental Study on Treatment of Endometriorsis in Rabbit Model with the Method of Removing Blood Stasis and Resolving Phlegm, Softening and Resolving the Lump. Shangdong Zhongyiyao Daxue Xuebao, 22, 227-229.
- 45. Li, S.C., Fan, J.F., Li, S.P., et al. (2001) Observation on Curative Effects of Supplemented Rhizoma Polygonati Sicao Decoction on the Patients with Ischemic Brain Damage. Zhongguo Zhongxiyi Jijiu Zazhi, 8, 376-377.
- 46. Liang, X.L. (1998) Study on the Effect of the Model with Antilipidemic Activity with Sian Capsule. Chengdu Zhongyiiyao Daxue Xuebao, 21, 39-41.
- 47. Psotova, J., Kolar, M., Sousek, J., Svagera, Z., Vicar, J. and Ulrichová, J. (2003) Biological Activities of Prunella vulgaris Extract. Phytotherapy Research, 17, 1082-1087.
http://dx.doi.org/10.1002/ptr.1324 - 48. Wang, Z.J., Zhao, Y.Y., Chen, Y.Y., et al. (2001) Chemical Constituents and Pharmacological Activities of Prunella Genus. World Phytomedicines, 16, 7-9.
- 49. Zheng, M. (1990) Experimental Study of 472 Herbs with Antiviral Action against the Herpes Simplex Virus. Zhongxiyi Jiehe Zazhi, 10, 39-41.
- 50. Zheng, M.S. and Li, W. (1991) Experimental Investigation into Antiviral Action of Prunella vulgaris L. on Type I Herpes Simplex Virus. Jiangxi Yixueyuan Xuebao, 31, 15-17.
- 51. Chen, Q., Ceng, Y.G. and Cao, M.C. (2002) Research on the Analgesic and Anti-Inflammatory Activity of the Oral Drench Made from Prunella Vulgaries L. Jiceng Zhongyao Zazhi, 16, 6-7.
- 52. Tang, C.K., Chen, G.J., Shen, Y.Q., et al. (1999) The Antipyretic and Anti-Inflammatory Activity of the Pills Made from Prunella vulgarites L. Zhongguo Shiyan Fangjixue Zazhi, 5, 54-55.
- 53. Xiao, L.Y. and Huang, C.P. (2001) A Discussion on Sensitivity of 23 Chinese Herbage Medicines against MRSA. Shizhen Guoyi Guoyao, 12, 878-879.
- 54. Zhang, F.X., Deng, W.D., Hu, Y.J. and Wu, X.X. (1999) Inhibitory Effect of Aikeqing on the Activity of Immunodeficiency Virus of Simian in Vitro. Guangzhou Zhongyiyao Daxue Xuebao, 16, 127-129.
- 55. Fang, X.Y., Chang, R.C.C., Yuen, W.H. and Zee, S.Y. (2005) Immune Modulatory Effects of Prunella vulgaris L. International Journal of Molecular Medicine, 15, 491-496.
- 56. Xu, H.X., Lee, S.H.S., Lee, S.F., White, R.L. and Blay, J. (1999) Isolation and Characterization of an Anti-HSV Polysaccharide from Prunella vulgaris. Antiviral Research, 44, 43-54.
http://dx.doi.org/10.1016/S0166-3542(99)00053-4 - 57. Lam, T.L., Lam, M.L., Au, T.K., Ip, D.T.M., Ng, T.B., Fong, W.P. and Wan, D.C.C. (2000) A Comparison of Human Immunodeficiency Virus Type-1 Protease Inhibition Activities by the Aqueous and Methanol Extracts of Chinese Medicinal Herbs. Life Sciences, 67, 2889-2896.
http://dx.doi.org/10.1016/S0024-3205(00)00864-X - 58. Kageyama, S., Kurokawa, M. and Shiraki, K. (2000) Extract of Prunella vulgaris Spikes Inhibits HIV Replication at Reverse Transcription in Vitro and Can Be Absorbed from Intestine in Vivo. Antiviral Chemistry & Chemotherapy, 11, 157-164.
- 59. Yamasaki, K., Nakano, M., Kawahata, T., Mori, H., Otake, T., Ueba, N., Oishi, I., Inami, R., Yamne, M., Nkamura, M., Murata, H. and Naknishi, T. (1998) Anti-HIV-1 Activity of Herbs in Labiatae. Biological & Pharmaceutical Bulletin, 21, 829-833.
http://dx.doi.org/10.1248/bpb.21.829 - 60. Yamasaki, K., Otake, T., Mori, H., Morimoto, M., Ueba, N., Kurokawa, Y., Shiota, K. and Yuge, T. (1993) Screening Test of Crude Drug Extract on Anti-HIV Activity. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan, 113, 818-824.
- 61. Au, T., Lam, T., Ng, T., Fong, W. and Wan, D. (2001) A Comparison of HIV-1 Integrase Inhibition by Aqueous and Methanol Extracts of Chinese Medicinal Herbs. Life Sciences, 68, 1687-1694.
http://dx.doi.org/10.1016/S0024-3205(01)00945-6 - 62. Zheng, M.S. and Zhang, Y.Z. (1990) Anti-HBsAg Herbs Employing ELISA Technique. Zhongxiyi Jihe Zazhi, 10, 560-562.
- 63. Zheng, J., He, J.G., Ji, B.P., Li, Y. and Zhang, X.F. (2007) Antihyperglycemic Activity of Prunella vulgaris L. in Streptozotocin-I Nduced Diabetic Mice. Asia Pacific Journal of Clinical Nutrition, 16, 427-431.
- 64. Huang, N., Hauck, C., Yum, M.Y., Rizshsky, L., Widrlechner, M.P., Mccoy, J.A., Murphy, P.A., Dixon, P.M., Nikolau, B.J. and Birt, D.F. (2009) Rosmarinic Acid in Prunella vulgaris Ethanol Extract Inhibits Lipopolysaccharide-Induced Prostaglandin E2 and Nitric Oxide in RAW 264.7 Mouse Macrophages. Journal of Agricultural and Food Chemistry, 57, 10579-10589.
http://dx.doi.org/10.1021/jf9023728 - 65. Zdarilová, A., Svobodová, A., Simánek, V. and Ulrichová, J. (2009) Prunella vulgaris Extract and Rosmarinic Acid Suppress Lipopolysaccharide-Induced Alteration in Human Gingival Fibroblasts. Toxicology in Vitro, 23, 386-392.
http://dx.doi.org/10.1016/j.tiv.2008.12.021 - 66. Jung, Y.B., Roh, K.J., Jung, J.A., Jung, K., Yoo, H., Cho, Y.B., Kwak, W.J., Kim, D.K., Kim, K.H. and Han, C.K. (2001) Effect of SKI 306X, a New Herbal Anti-Arthritic Agent, in Patients with Osteoarthritis of the Knee: A Double-Blind Placebo Controlled Study. The American Journal of Chinese Medicine, 29, 485-491.
http://dx.doi.org/10.1142/S0192415X01000502 - 67. Choi, J.H., Kim, D.Y., Yoon, J.H., Youn, H.Y., Yi, J., Rhee, H.I., Ryu, K., Jung, K. and Han, C.K. (2002) Effects of SKI 306X, a New Herbal Agent, on Proteoglycan Degradation in Cartilage Explant Culture and Collagenase-Induced Rabbit Osteoarthritis Model. Osteoarthritis and Cartilage, 10, 471-478.
http://dx.doi.org/10.1053/joca.2002.0526 - 68. Jiang, Y., Jun, J.H. and Sun, X.Y. (1988) The Effect of Prunella vulgaris L on the Thymus, Spleen and Adrenalin of Animal. Gansu Yiyao, 7, 4-7.
- 69. Fang, X., Yu, M.M.S., Yuen, W.H., Zee, S.Y. and Chang, R.C. (2005) Immune Modulatory Effects of Prunella vulgaris L. on Monocytes/Macrophages. International Journal of Molecular Medicine, 16, 1109-1116.
- 70. Ma, D.E., Wang, Z.M. and Ma, A.Y. (1983) The Anti-Inflammatory Activity and the Effect on the Immune Organs of Prunella vulgaris. Shanxi Yiyao Zazhi, 12, 4-7.
- 71. Xu, L.S. (1989) Pharmacological Research on the Hypoglycemic Effect of the Active Ingredienst in Prunella vulgaris. Chinese Traditional and Herbal Drugs, 20, 22-24.
- 72. Liu, B.L., Zhu, D.N. and Wang, G. (1995) Effects of the Alcohol Extract of Spica Pranellae on Bloodglucose in Mice. Zhongguo Yaoke Daxue Xuebao, 26, 44-46.
- 73. Feng, M.L., Jia, L.L., Wu, Y.P., et al. (2000) Effect of the Alcoholic Extraction of Spica Prunellae on Experimental Diabetic Nephropathy. Shanxi Zhongyi Xueyuan Xuebao, 1, 7-9.
- 74. Wang, J.G. (2001) Liver-Protective Effect of Prunella vulgaris Mixture. Chinese Traditional and Herb Drugs, 32, 625-627.
- 75. Zhang, J. and Tao, X.P. (2000) the Effect of Prunella vulgaris on Liver Fibrosis Indexes. Jiceng Zhongyao Zazhi, 14, 60-60.
- 76. Meng, G., Zhang, K.J. and Zhang, M.Z. (2007) Chemical Constituents of Prunella vulgaris L and Antitumor Activity. Northwest Pharmaceutical Journal, 22, 211-213.
- 77. Liu, L., Xu, D.S., Zhou, R.Y. and Zhong, Y. (2008) Effective Fraction of Prunella Vulgaris and Its Application in Preparation of Medicines for Treating Colon Cancer. CN Patent No. 101317883 A.
- 78. Wang, K., Dong, H.F., Zhang, X.Y., et al. (2000) The Effect of Prunella vulgaris L on SGC-7901 Cells. Shanghai Yixue Jianyan Zazhi, 15, 305-306.
- 79. Lee, K.H., Lin, Y.M., Wu, T.S., Zhang, D.C., Yamagishi, T., Hayashi, T., Hall, I.H., Chang, J.J., Wu, R.Y. and Yang, T.H. (1988) The Cytotoxic Principles of Prunella vulgaris, Psychotria serpens, and Hyptis capitata: Ursolic Acid and Related Derivatives. Planta Medica, 54, 308-311.
http://dx.doi.org/10.1055/s-2006-962441 - 80. Xu, Z.W., Zhou, R.Y., Wang, W.H., et al. (2001) Mechanism Study of “Prunella Injection” on Formation of Pleural Fibrosis. Zhejiang Zhongxiyi Jiehe Zazhi, 11, 5-8.
- 81. Zhou, R.Y., Xu, Z.W. and Ni, A.D. (2001) Clinical and Experimental Observations of Hydrothorax in Lung Cancer Cases Treated with Closed Drainage and Injection of Selfheal Spike Injection. Shanghai Daxue Xuebao, 15, 49-51.
- 82. Chen, C.Y., Wu, G. and Zhang, M.Z. (2009) The Effects and Mechanism of Action of Prunella vulgaris L Extract on Jurkat Human T Lymphoma Cell Proliferation. Chinese-German Journal of Clinical Oncology, 8, 426-429.
- 83. Zhang, M.Z. and Liu, H.M. (2009) Method for Extracting Anti-Tumor Effective Parts from Chinese Medicine Prunella. CN Patent No. 101461842 A.
- 84. Lee, H. and Lin, J.Y. (1988) Antimutagenic Activity of Extracts from Anticancer Drugs in Chinese Medicine. Mutation Research/Genetic Toxicology, 204, 229-234.
- 85. Lamaison, J., Petitjean-Freytet, C. and Carnat, A. (1990) Medicinal Lamiaceae with Antioxidant Properties, a Potential Source of Rosmarinic Acid. Pharmaceutica Acta Helvetiae, 66, 185-188.
- 86. Liu, J. (2005) Oleanolic Acid and Ursolic Acid: Research Perspectives. Journal of Ethnopharmacology, 100, 92-94.
http://dx.doi.org/10.1016/j.jep.2005.05.024 - 87. Wang, H.B., Zhang, Z.Y., Zai, Z.X., Su, Z. and Li, C.K. (1993) Qualitative Analysis of Chinese Drug Xiakucao (Prunella). Zhongguo Zhongyao Zazhi, 18, 655-657.
- 88. Muto, Y., Ninomiya, M. and Fujiki, H. (1990) Present Status of Research on Cancer Chemoprevention in Japan. Japanese Journal of Clinical Oncology, 20, 219-224.
- 89. Niikawa, M., Hayashi, H., Sato, T., Nagase, H. and Kito, H. (1993) Isolation of Substances from Glossy Privet (Ligustrum lucidum Ait.) Inhibiting the Mutagenicity of Benzo[A]Pyrene in Bacteria. Mutation Research/Genetic Toxicology, 319, 1-9.
- 90. Young, H., Chung, H., Lee, C., Park, K., Yokozawa, T. and Oura, H. (1994) Ursolic Acid Inhibits Aflatoxin B1-Induced Mutagenicity in a Salmonella Assay System. Biological & Pharmaceutical Bulletin, 17, 990-992.
http://dx.doi.org/10.1248/bpb.17.990 - 91. Huang, M.T., Ho, C.T., Wang, Z.Y., Ferraro, T., Lou, Y.R., Stauber, K., Ma, W., Georgiadis, C., Laskin, J.D. and Conney, A.H. (1994) Inhibition of Skin Tumorigenesis by Rosemary and Its Constituents Carnosol and Ursolic Acid. Cancer Research, 54, 701-708.
- 92. Wang, J.J., Wang, B., Guo, J., Gao, Y., Xu, Q. and Chen, W.S. (2000) Inhibitory Effect of Ursolic Acid on Angiogenesis in Vitro. Academic Journal of Second Military Medical University, 21, 1071-1073.
- 93. Balanehru, S. and Nagarajan, B. (1991) Protective Effect of Oleanolic Acid and Ursolic Acid against Lipid Peroxidation. Biochemistry International, 24, 981-990.
- 94. Ringbom, T., Segura, L., Noreen, Y., Perera, P. and Bohlin, L. (1998) Ursolic Acid from Plantago Major, a Selective Inhibitor of Cyclooxygenase-2 Catalyzed Prostaglandin Biosynthesis. Journal of Natural Products, 61, 1212-1215.
http://dx.doi.org/10.1021/np980088i - 95. Subbaramaiah, K., Michaluart, P., Sporn, M.B. and Dannenberg, A.J. (2000) Ursolic Acid Inhibits Cyclooxygenase-2 Transcription in Human Mammary Epithelial Cells. Cancer Research, 60, 2399-2404.
- 96. Zhao, G.Q. (1996) Antineoplastic Activity of Ursolic Acid in Loquat Leaves Guowai Yiyao. Zhiwuxue Fence, 11, 180-180.
- 97. Lee, H.Y., Chung, H.Y., Kim, K.H., Lee, J.J. and Kim, K.W. (1994) Induction of Differentiation in the Cultured F9 Teratocarcinoma Stem Cells by Triterpene Acids. Journal of Cancer Research and Clinical Oncology, 120, 513-518.
http://dx.doi.org/10.1007/BF01221027 - 98. Paik, K.J., Jeon, S.S., Chung, H.Y., Lee, K.H., Kim, K.W., Chung, J.K. and Kim, N.D. (1998) Induction of Differentiation of the Cultured Rat Mammary Epithelial Cells by Triterpene Acids. Archives of Pharmacal Research, 21, 398-405.
http://dx.doi.org/10.1007/BF02974633 - 99. Hanahan, D., Christofori, G., Naik, P. and Arbeit, J. (1996) Transgenic Mouse Models of Tumour Angiogenesis: The Angiogenic Switch, Its Molecular Controls, and Prospects for Preclinical Therapeutic Models. European Journal of Cancer, 32, 2386-2393.
http://dx.doi.org/10.1016/S0959-8049(96)00401-7 - 100. Sohn, K.H., Lee, H.Y., Chung, H.Y., Young, H.S., Yi, S.Y. and Kim, K.W. (1995) Anti-Angiogenic Activity of Triterpene Acids. Cancer Letters, 94, 213-218.
http://dx.doi.org/10.1016/0304-3835(95)03856-R - 101. Wang, B., Wang, J.J. and Xu, J. (2001) Inhibitory Effect of Ursolic Acid on Angiogenesis in Vitro. Zhongliu Fangzhi Zazhi, 8, 351-352.
- 102. Baek, J.H., Lee, Y.S., Kang, C.M., Kim, J., Kwon, K.S., Son, H.C. and Kim, K.W. (1997) Intracellular Ca2+ Release Mediates Ursolic Acid-Induced Apoptosis in Human Leukemic HL-60 Cells. International Journal of Cancer, 73, 725-728.
http://dx.doi.org/10.1002/(SICI)1097-0215(19971127)73:5<725::AID-IJC19>3.0.CO;2-4 - 103. Huang, J., Sun, Y., Lu, S.X., Su, T. and Jiao, S.C. (1999) Experimental Study on Apoptosis Induced by Ursolic Acid Isolated from Asparagus in HL260 Cells. Zhongguo Zhongxiyijiehe Zazhi, 19, 296-298.
- 104. Xu, G.F., Zhao, X.L. and Zhao, L. (2000) Apoptosis Inducde by Flavonoids Extract of Wheat Germ in a Human Breast Cancer Cell Line. Yingyang Xuebao, 22, 43-46.
- 105. Wang, C., Zhang, L.S. and He, T. (2002) Study on Antiproliferation Effects of Genistein in Vitro. Zhongguo Gonggong Weisheng, 18, 1056-1058.
- 106. Trochon, V., Blot, E., Cymbalista, F., Engelmann, C., Tang, R.P., Thomaïdis, A., Vasse, M., Soria, J., Lu, H. and Soria, C. (2000) Apigenin Inhibits Endothelial-Cell Proliferation in G2/M Phase Whereas It Stimulates Smooth-Muscle Cells by Inhibiting P21 and P27 Expression. International Journal of Cancer, 85, 691-696.
http://dx.doi.org/10.1002/(SICI)1097-0215(20000301)85:5<691::AID-IJC15>3.0.CO;2-Q - 107. Zi, X., Zhang, J., Agarwal, R. and Pollak, M. (2000) Silibinin Up-Regulates Insulin-Like Growth Factor-Binding Protein 3 Expression and Inhibits Proliferation of Androgen-Independent Prostate Cancer Cells. Cancer Research, 60, 5617-5620.
- 108. Senderowicz, A.M. (1999) Flavopiridol: The First Cyclin-Dependent Kinase Inhibitor in Human Clinical Trials. Investigational New Drugs, 17, 313-320.
http://dx.doi.org/10.1023/A:1006353008903 - 109. Huynh, H.T. and Teel, R.W. (1999) Selective Induction of Apoptosis in Human Mammary Cancer Cells (MCF-7) by Pycnogenol. Anticancer Research, 20, 2417-2420.
- 110. Wu, B., Xu, G.F., Zhao, X.L. and Ren, X. (2001) Antioxidation of Flavones of Wheat Germ on Mammary Tulnor of Rats. Weisheng Yanjiu, 30, 215-217.
- 111. Chen, J.W., Hu, T.X. and Zhu, D.Y. (2002) Study on Structure-Activity Relationship of Eleven Natural Flavonoids Scavenging Superoxide Anion. Zhongguo Yaoxue Zazhi, 37, 57-58.
- 112. Zhang, R.Q. and Han, Z.K. (1994) The Effect of Flavonoids on the Immune Function and the Level of Β-Endorphins in the Blood of Mouse. Zhongguo Mianyi Zazhi, 10, 91-92.
- 113. Rafi, M.M., Rosen, R.T., Vassil, A., Ho, C.T., Zhang, H., Ghai, G., Lambert, G. and Dipaola, R.S. (1999) Modulation of Bcl-2 and Cytotoxicity by Licochalcone-A, a Novel Estrogenic Flavonoid. Anticancer Research, 20, 2653-2658.
- 114. Mcvean, M., Xiao, H., Isobe, K.I. and Pelling, J.C. (2000) Increase in Wild-Type P53 Stability and Transactivational Activity by the Chemopreventive Agent Apigenin in Keratinocytes. Carcinogenesis, 21, 633-639.
http://dx.doi.org/10.1093/carcin/21.4.633 - 115. Chan, F.L., Choi, H., Chen, Z., Chan, P.S. and Huang, Y. (2000) Induction of Apoptosis in Prostate Cancer Cell Lines by a Flavonoid, Baicalin. Cancer Letters, 160, 219-228.
http://dx.doi.org/10.1016/S0304-3835(00)00591-7 - 116. Wenzel, U., Kuntz, S., Brendel, M.D. and Daniel, H. (2000) Dietary Flavone Is a Potent Apoptosis Inducer in Human Colon Carcinoma Cells. Cancer Research, 60, 3823-3831.
- 117. Ogata, S., Miyake, Y., Yamamoto, K., Okumura, K. and Taguchi, H. (2000) Apoptosis Induced by the Flavonoid from Lemon Fruit (Citrus limon BURM. F.) and Its Metabolites in HL-60 Cells. Bioscience, Biotechnology, and Biochemistry, 64, 1075-1078.
http://dx.doi.org/10.1271/bbb.64.1075 - 118. Blankson, H., Grotterød, E. and Seglen, P. (2000) Prevention of Toxin-Induced Cytoskeletal Disruption and Apoptotic Liver Cell Death by the Grapefruit Flavonoid, Naringin. Cell Death & Differentiation, 7, 739-746.
http://dx.doi.org/10.1038/sj.cdd.4400705 - 119. Jiang, C., Agarwal, R. and Lü, J. (2000) Anti-Angiogenic Potential of a Cancer Chemopreventive Flavonoid Antioxidant, Silymarin: Inhibition of Key Attributes of Vascular Endothelial Cells and Angiogenic Cytokine Secretion by Cancer Epithelial Cells. Biochemical and Biophysical Research Communications, 276, 371-378.
http://dx.doi.org/10.1006/bbrc.2000.3474 - 120. Ishiwa, J., Sato, T., Mimaki, Y., Sashida, Y., Yano, M. and Ito, A. (2000) A Citrus Flavonoid, Nobiletin, Suppresses Production and Gene Expression of Matrix Metalloproteinase 9/Gelatinase B in Rabbit Synovial Fibroblasts. The Journal of Rheumatology, 27, 20-25.
- 121. Nakahata, N., Kyo, R., Kutsuwa, M. and Ohizumi, Y. (1999) Inhibition of Mitogen-Activated Protein Kinase Cascade by Baicalein, a Flavonoid of Natural Origin. Nippon Yakurigaku Zasshi, 114, 215-219.
http://dx.doi.org/10.1254/fpj.114.supplement_215 - 122. Zhou, J., Purna, M. and Steven, K. (1998) Soybean Components Inhibit the Growth of Human Prostate Cancer Cell Line Lncap in SCID Mice via Alteration in Cells Apoptosis, Angiogenesis and Proliferation. FASEB Journal, 18, 3822.
- 123. Ranelletti, F.O., Maggiano, N., Serra, F.G., Ricci, R., Larocca, L.M., Lanza, P., Scambia, G., Fattorossi, A., Capelli, A. and Piantelli, M. (2000) Quercetin Inhibits P21-RAS Expression in Human Colon Cancer Cell Lines and in Primary Colorectal Tumors. International Journal of Cancer, 85, 438-445.
http://dx.doi.org/10.1002/(SICI)1097-0215(20000201)85:3<438::AID-IJC22>3.0.CO;2-F - 124. Fotsis, T., Pepper, M., Adlercreutz, H., Hase, T., Montesano, R. and Schweigerer, L. (1995) Genistein, a Dietary Ingested Isoflavonoid, Inhibits Cell Proliferation and in Vitro Angiogenesis. The Journal of Nutrition, 125, 790S-797S.
- 125. Shin, T.Y., Kim, Y.K. and Kim, H.M. (2001) Inhibition of Immediate-Type Allergic Reactions by Prunella vulgaris in a Murine Model. Immunopharmacology and Immunotoxicology, 23, 423-435.
http://dx.doi.org/10.1081/IPH-100107341 - 126. Kim, S.Y., Kim, S.H., Shin, H.Y., Lim, J.P., Chae, B.S., Park, J.S., Hong, S.G., Kim, M.S., Jo, D.G., Park, W.H. and Shin, T.Y. (2007) Effects of Prunella vulgaris on Mast Cell-Mediated Allergic Reaction and Inflammatory Cytokine Production. Experimental Biology and Medicine, 232, 921-926.
- 127. Podlubny, I. (1999) Fractional Differential Equations. Academic Press, San Diego.
- 128. Li, C. and Ding, H. (2014) Higher Order Finite Difference Method for the Reaction and Anomalous-Diffusion Equation. Applied Mathematical Modelling, 38, 3802-3821.
https://doi.org/10.1016/j.apm.2013.12.002 - 129. Zhuang, P., Liu, F., Anh, V., et al. (2009) Stability and Convergence of an Implicit Numerical Method for the Nonlinear Fractional Reaction-Subdiffusion Process. SIAM Journal on Mathematical Analysis, 74, 645-667.
- 130. Dehghan, M., Abbaszadeh, M. and Mohebbi, A. (2015) Error Estimate for the Numerical Solution of Fractional Reaction-Subdiffusion Process Based on a Meshless Method. Journal of Computational and Applied Mathematics, 280, 14-36.
https://doi.org/10.1016/j.cam.2014.11.020 - 131. Yu, B., Jiang, X. and Xu, H. (2015) A Novel Compact Numerical Method for Solving the Two-Dimensional Non-Linear Fractional Reaction-Subdiffusion Equation. Numerical Algorithms, 68, 923-950.
https://doi.org/10.1007/s11075-014-9877-1 - 132. Douglas, J. (1955) On the Numerical Integration of Uxx+Uyy=Ut by Implicit Methods. Journal of the Society for Industrial and Applied Mathematics, 3, 42-65.
- 133. Douglas, J. and Gunn, J.E. (1964) A General Formulation of Alternating Direction Method, Part I. Parabolic and Hyperbolic Problem. Numerische Mathematik, 6, 428-453.
https://doi.org/10.1007/BF01386093 - 134. Peaceman, D.W. and Rachford, H.H. (1955) The Numerical Solutions of Parabolic and Elliptic Differential Equations. Journal of the Society for Industrial and Applied Mathematics, 3, 28-41.
https://doi.org/10.1137/0103003 - 135. Meerschaert, M.M., Scheffler, H.P. and Tadjeran, C. (2006) Finite Difference Methods for Two-Dimensional Fractional Dispersion Equation. Journal of Computational Physics, 211, 249-261.
https://doi.org/10.1016/j.jcp.2005.05.017 - 136. Zhang, Y. and Sun, Z. (2011) Alternating Direction Implicit Schemes for the Two-Dimensional Fractional Sub-Diffusion Equation. Journal of Computational Physics, 230, 8713-8728.
https://doi.org/10.1016/j.jcp.2011.08.020 - 137. Cui, M. (2013) Convergence Analysis of High-Order Compact Alternating Direction Implicit Schemes for the Two-Dimensional Time Fractional Diffusion Equation. Numerical Algorithms, 62, 383-409.
https://doi.org/10.1007/s11075-012-9589-3 - 138. Zhang, Y., Sun, Z. and Zhao, X. (2012) Compact Alternating Direction Implicit Scheme for the Two-Dimensional Fractional Diffusion-Wave Equation. SIAM Journal on Numerical Analysis, 50, 1535-1555.
https://doi.org/10.1137/110840959 - 139. Wang, Z. and Vong, S. (2015) A High-Order ADI Scheme for the Two-Dimensional Time Fractional Diffusion-Wave Equation. International Journal of Computer Mathematics, 92, 970-979.
https://doi.org/10.1080/00207160.2014.915960 - 140. Li, L., Xu, D. and Luo, M. (2013) Alternating Direction Implicit Galerkin Finite Element Method for the Two-Dimensional Fractional Diffusion-Wave Equation. Journal of Computational Physics, 255, 471-485.
https://doi.org/10.1016/j.jcp.2013.08.031 - 141. Fairweather, G., Yang, X., Xu, D. and Zhang, H. (2015) An ADI Crank-Nicolson Orthogonal Spline Collocation Method for the Two Dimensional Fractional Diffusion-Wave Equation. Journal of Scientific Computing, 65, 1217-1239.
https://doi.org/10.1007/s10915-015-0003-x - 142. Cui, M. (2012) Compact Alternating Direction Implicit Method for Two-Dimensional Time Fractional Diffusion Equation. Journal of Computational Physics, 231, 2621-2633.
https://doi.org/10.1016/j.jcp.2011.12.010 - 143. Zhang, Y. and Sun, Z. (2014) Error Analysis of a Compact ADI Scheme for the 2D Fractional Subdiffusion Equation. Journal of Scientific Computing, 59, 104-128.
https://doi.org/10.1007/s10915-013-9756-2 - 144. Yao, W., Sun, J., Wu, B. and Shi, S. (2015) Numerical Simulation of a Class of Fractional Subdiffusion Equations via the Alternating Direction Implicit Method. Numerical Methods for Partial Differential Equations, 32, 531-547.
https://doi.org/10.1002/num.22004 - 145. Wang, Y. and Wang, T. (2016) Error Analysis of a High-Order Compact ADI Method for Two-Dimensional Fractional Convection-Subdiffusion Equations. Calcolo, 53, 301-330.
https://doi.org/10.1007/s10092-015-0150-3 - 146. Wang, Z. and Vong, S. (2014) A High-Order Exponential ADI Scheme for Two Dimensional Time Fractional Convection-Diffusion Equations. Computers & Mathematics with Applications, 68, 185-196.
https://doi.org/10.1016/j.camwa.2014.05.016 - 147. Zhai, S., Feng, X. and He, Y. (2014) An Unconditionally Stable Compact ADI Method for Three-Dimensional Time-Fractional Convection-Diffusion Equation. Journal of Computational Physics, 269, 138-155.
https://doi.org/10.1016/j.jcp.2014.03.020 - 148. Gao, G. and Sun, Z. (2015) Two Alternating Direction Implicit Difference Schemes with the Extrapolation Method for the Two-Dimensional Distributed-Order Differential Equations. Computers & Mathematics with Applications, 69, 926-948.
https://doi.org/10.1016/j.camwa.2015.02.023 - 149. Gao, G. and Sun, Z. (2016) Two alternating Direction Implicit Difference Schems for Two-Dimensional Distributed-Order Fractional Diffusion Equations. Journal of Scientific Computing, 69, 506-531.
https://doi.org/10.1007/s10915-016-0208-7 - 150. Wang, H. and Wang, K. (2011) An O(Nlog2N) Alternating-Direction Finite Difference Method for Two-Dimensional Fractional Diffusion Equations. Journal of Computational Physics, 230, 7830-7839.
https://doi.org/10.1016/j.jcp.2011.07.003 - 151. Wang, H. and Du, N. (2014) Fast Alternating-Direction Finite Difference Methods for Three-Dimensional Space-Fractional Diffusion Equations. Journal of Computational Physics, 258, 305-318.
https://doi.org/10.1016/j.jcp.2013.10.040 - 152. Tian, W., Zhou, H. and Deng, W. (2015) A Class of Second Order Difference Approximations for Solving Space Fractional Diffusion Equations. Mathematics of Computation, 294, 1703-1727.
https://doi.org/10.1090/S0025-5718-2015-02917-2 - 153. Chen, M. and Deng, W. (2014) Fourth Order Accuarate Scheme for the Space Fractional Diffusion Equations. SIAM Journal on Numerical Analysis, 52, 1418-1438.
https://doi.org/10.1137/130933447 - 154. Chen, M. and Deng, W. (2014) A Second-Order Numerical Method for Two-Dimensional Two-Sided Space Fractional Convection Diffusion Equation. Applied Mathematical Modelling, 38, 3244-3259.
https://doi.org/10.1016/j.apm.2013.11.043 - 155. Song, F. and Xu, C. (2015) Spectral Direction Splitting Methods for Two-Dimensional Space Fractional Diffusion Equations. Journal of Computational Physics, 299, 196-214.
https://doi.org/10.1016/j.jcp.2015.07.011 - 156. Liu, F., Zhuang, P., Turner, I., Anh, V. and Burrage, K. (2015) A Semi-Alternating Direction Method for a 2-D Fractional Fitz Hugh-Nagumo Monodomain Model on an Approximate Irregular Domain. Journal of Computational Physics, 293, 252-263.
https://doi.org/10.1016/j.jcp.2014.06.001 - 157. Bu, W., Tang, Y., Wu, Y. and Yang, J. (2015) Crank-Nicolson ADI Galerkin Finite Element Method for Two-Dimensional Fractional FitzHugh-Nagumo Mondomain Model. Applied Mathematics and Computation, 257, 355-364.
https://doi.org/10.1016/j.amc.2014.09.034 - 158. Zeng, F., Liu, F., Li, C., et al. (2014) A Crank-Nicolson ADI Spectral Method for a Two-Dimensional Riesz Space Fractional Nonlinear Reaction-Diffusion Equation. SIAM Journal on Numerical Analysis, 52, 2599-2622.
https://doi.org/10.1137/130934192 - 159. Dendy, J.E. (1975) An Anlysis of Some Galerkin Schemes for the Solution of Nonlinear Time Problems. SIAM Journal on Numerical Analysis, 12, 541-565.
https://doi.org/10.1137/0712042 - 160. Fernandes, R.I. and Fairweather, G. (1991) An Alternating Direction Galerkin Method for a Class of Second-Order Hyperbolic Equations in Two Space Variables. SIAM Journal on Numerical Analysis, 28, 1265-1281.
https://doi.org/10.1137/0728067









































































