 J. Biomedical Science and Engineering, 2011, 4, 383-390 doi:10.4236/jbise.2011.45048 Published Online May 2011 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online May 2011 in SciRes. http://www.scirp.org/journal/JBiSE In vitro degradation behavior of chitosan based hybrid microparticles Ambalangodage C. Jayasuriya, Kristalyn J. Mauch Department of Orthopaedics, College of Medicine, University of Toledo Health Science Campus, Toledo, USA. Email: a.jayasuriya@utoledo.edu Received 9 February 2011; revised 9 March 2011; accepted 21 March 2011. ABSTRACT The degradation properties of the MPs is important to the long-term benefits of the use of the chitosan (CS) based hybrid MPs in bone tissue-engineering, because the degradation kinetics could affect a mul- titude of processes within the cell, such as cell growth, tissue regeneration, and host response. The aim of this study was to investigate the degradation of solid, hybrid CS microparticles (MPs), CS-10% calcium phosphate (CaHPO4, w/w), and CS-10% calcium carbonate (CaCO3, w/w) MPs in phosphate buffered solution (PBS) over a 30-week period. The hybrid MPs were synthesized by emulsification technique, cross-linked with 64% sodium tripolyphosphate (TPP), purified and air dried overnight. Each sample had 30 mg of MPs was placed in a glass vial with 9 ml of PBS added and then the vial was closed to prevent evapo- ration. Every week 4 ml of the incubated solution was removed for sample measurement and all samples were replaced with an equivalent amount of fresh medium. The samples were maintained at 37˚C under continuous shaking. The hybrid MPs were measured for pH and calcium release, every week in triplicate. At 0, 5, 10, 15, 20, 25, and 30 weeks, surface and bulk morphology were analyzed with a scanning electron microscope (SEM). The degradation data suggested that the hybrid MPs were stable at least up to 25 week and maintain the physiologically relevant pH. Therefore, we can use these hybrid MPs to apply in the bone tissue engineering applications since they do not degrade within a short period. Keywords: Degradation; In Vitr o; Microparticles; pH; Chitosan; Phosphate Buffered Saline 1. INTRODUCTION Scaffolds are typically used in bone tissue engineering, acting as a delivery system for the cells, genetic material, and growth factors to the site of interest or defect [1]. Bone tissue engineering involves combining bone- forming cells with a suitable scaffold. The scaffold is needed to function as a material for support, and a sub- strate for cell attachment and bone deposition [2,3]. The ultimate aim of bone tissue engineering is to help create a healing response in the bony area designated so that newly formed tissue would integrate with the surround- ing skeleton to be useful and resilient. Ideally, the engi- neered scaffold will slowly degrade in a controllable manner. Controllable in the sense that degradation would match extracellular matrix formation, transfer structural and functional roles progressively to the newly formed bone while maintaining mechanical strength until tissue regeneration is almost completed, and finally be reab- sorbed and metabolized by the body. The degradability of the biomaterial plays an important role in the long-term function of the engineered scaffold because it can affect many cellular process, including cell growth, tissue regeneration, and host response [1,4,5]. To find a suitable candidate that encompasses these aims, a variety of biomaterials have been researched for bone tissue engineering purposes, including biomaterials that can be synthetic or natural [3,5]. As natural components of living structures, naturally derived polymers are of particular interest as a scaffold material for bone tissue engineering applications due to the biological and chemical similarities that they have to natural tissues. The supposition being that natural mate- rials would have better acceptance by the human system because of its natural origins and having a closer relation to the body’s original components. Chitin and its deriva- tive chitosan (CS) are such natural biomaterials that can meet a variety of needs in the biomedical fields because their biological, physical, and chemical properties can be controlled and engineered under even mild processing conditions [4,6]. Mild processing conditions can avoid growth factor inactivation, allowing growth factors and other biomolecules to be incorporated into various com- posite forms for different applications [5]. Another fa- vorable property of the derivative CS is that it can be  A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 384 molded into different forms and shapes; for example, powders, pastes, films, fibers, sponges, scaffolds, mi- croparticles (MPs), and more have been made for vari- ous uses [7,8]. All these factors therefore lend credence to why chitin and CS are being increasingly studied for diverse applications as biomaterials in biomedical and pharmaceutical research, ranging from wound dressings to drug delivery carriers, as well as being used for cell encapsulation, or cartilage and bone tissue engineering [6,9]. CS is a linear copolymer that is formed by the random distribution of D-glucosamine and N-acetyl-D-gluco- samine residues that are linked by (1-4) glycosidic bonds. CS is not native to animal sources, however it is easily obtained from crustacean’s exoskeletons, such as shrimp and crab, by alkaline deacetylation of chitin [6,8,10,11]. Removing acetyl groups from the molecular chain of chitin leaves an amino group on the chain spe- cifically at the C-2 carbon of the glucopyranose ring in the deacetylation process of chitin to CS. However, nei- ther chitin nor CS respectively exist 100% acetylated or 100% deacetylated, but both exist as a copolymer. Therefore, the difference between chitin and CS is the acetyl content of the copolymer. The degree of acetyla- tion refers to the number N-acetyl-D-glucosamine units that are present and when greater than 50%, the copoly- mer is termed chitin. When the D-glucosamine (amino groups) are predominant (>50%) the copolymer is termed CS. The number of amino groups present refers to the degree of deacetylation [6]. Chitin’s use is limited compared to CS because chitin is chemically inert and is insoluble in water and acid. CS is insoluble in neutral and basic pH environments (pH > 7), and soluble in acidic environments (pH < 6) when the amino groups become protonated to facilitate solubility [7,12,13]. Besides CS being an easily obtained derivative of a natural copolymer that is moldable with reactive func- tionalities, the investigation into CS being used as a scaffold material in bone tissue engineering is largely due to a number of beneficial biological properties in- cluding being nontoxic, biocompatible, and biodegrad- able [3,10,11,14]. CS has been shown to have intrinsic antimicrobial actions on bacteria and fungi, to be haemostatic, antitumoral, and anticholestermic. Having an affinity to proteins, promoting cell adhesion and mi- gration, and enhancing wound healing list a few more favorably reported properties of CS [4,11]. CS also has been shown to promote growth and mineral rich matrix deposition by osteoblasts in culture. Implants that are CS based have generally shown a minimal foreign body reaction with little or no fibrous encapsulation, which is a helpful property that could be useful to the osteointe- gration important in bone tissue engineering that can lend significant rigidity to bone-implant systems [4,12, 13,15]. In this study, the aim was to study the degradation behavior of hybrid MPs at physiological conditions. Since the inorganic portion of bone is composed mainly of calcium and phosphate [3], hybrid MPs were also made containing CaHPO4 or CaCO3 individually to compare and see if hybrid MPs could offer any specific advantages. Regarding this, we fabricated three different types of hybrid MPs including CS, CS-10% calcium phosphate (CaHPO4), and CS-10% calcium carbonate (CaCO3) and placed in the glass vials containing phos- phate buffered saline (PBS) medium with pH = 7.4. These vials containing MPs were incubated at 37˚C with dynamic environment (50 rpm), and refreshed medium frequently during the study. Our degradation behavior of different types of hybrid MPs was done by measuring pH and calcium release, and analyzing both surface and bulk morphology using scanning electron microscopy (SEM) over an extended time period of 30 weeks. 2. MATERIALS AND METHODS 2.1. Materials CS from crab shells, practical grade and > 85% deacety- lated, was purchased from Sigma-Aldrich. Other chemi- cals bought from Sigma-Aldrich (St. Louis, MO, USA) include: cottonseed oil, CaHPO4, CaCO3, sodium tri- polyphosphate (TPP), Span 85, hexane, and acetic acid. Acetone was supplied by Fisher Science (Hanover, IL, USA). GIBCO PBS 7.4 (1X) liquid from Invitrogen (Grand Island, NY, USA) was used throughout the ex- periment. QuanticChrom calcium Assay kit was used from BioAssay Systems (Hayward, CA, USA). All sol- vents and chemicals used, unless specified were of ana- lytical grade. 2.2. Hybrid MP Preparation CS MPs (1.5%, w/v) were prepared by emulsification technique using our scale-up method [16,17]. CS solu- tion was prepared by measuring 750 mg of CS that was diluted by adding 50 mL of 1% (v/v) acetic acid at room temperature. Using a stir plate, the mixture was allowed to reach homogeneity and then it was filtered through a nylon mesh 50 micron cloth to remove any remaining insoluble particulates. The CS solution (25 ml) was quickly mixed with an equal amount (25 ml) of acetone. This CS-acetone mixture (36 ml) was emulsified by adding the mixture drop wise using 20 ml syringes (Becton Dickinson [B-D], Franklin Lakes, NJ) and 20 gauge 1’1/2” needles (B-D) into mechanically stirred cottonseed oil (600 ml) that included surfactant Span 85 (4 ml) at 35˚C - 40˚C and 850 rpm (Corning Laboratory Stirring/Hotplate model, USA). The system continued C opyright © 2011 SciRes. JBiSE 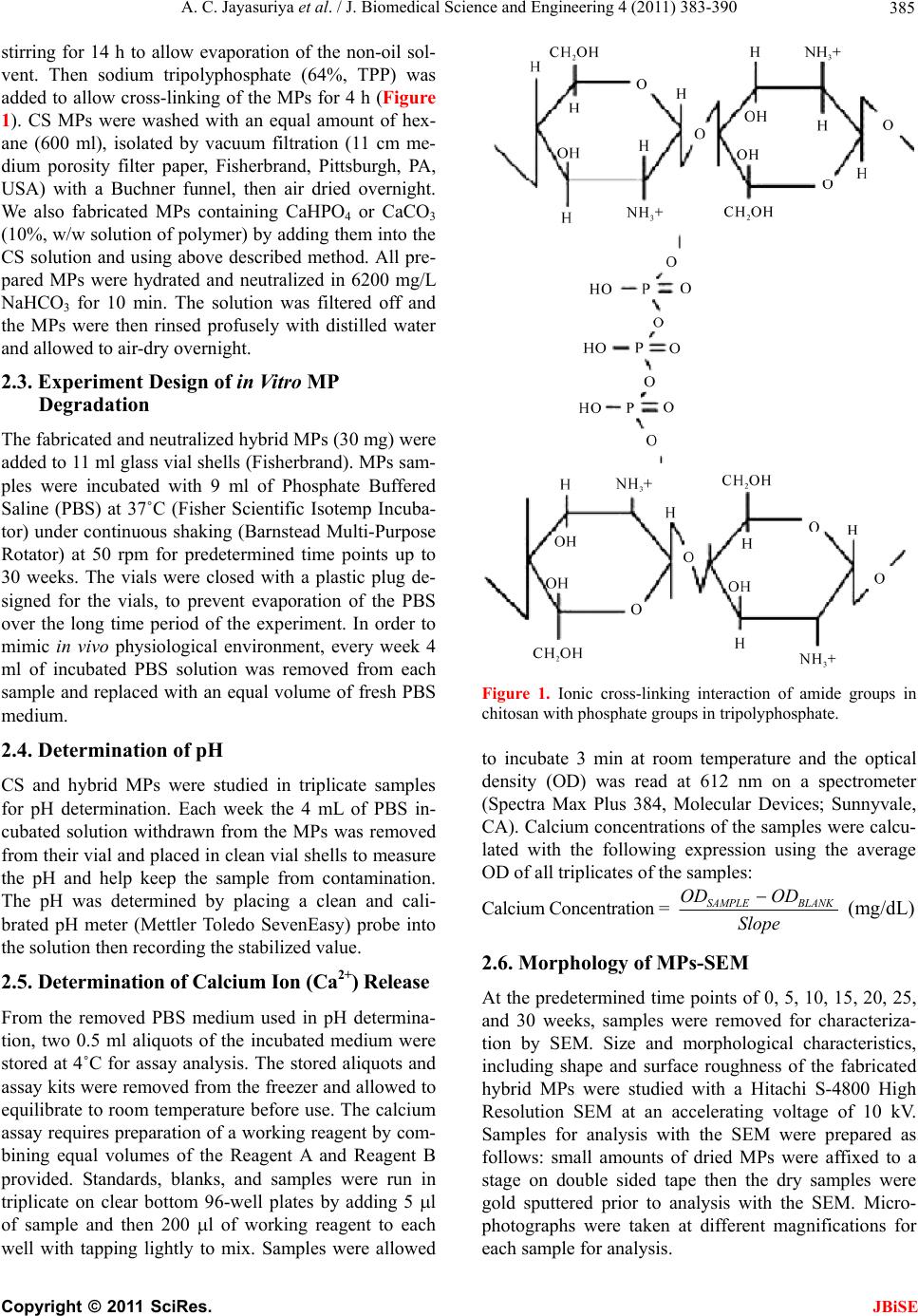 A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 385 stirring for 14 h to allow evaporation of the non-oil sol- vent. Then sodium tripolyphosphate (64%, TPP) was added to allow cross-linking of the MPs for 4 h (Figure 1). CS MPs were washed with an equal amount of hex- ane (600 ml), isolated by vacuum filtration (11 cm me- dium porosity filter paper, Fisherbrand, Pittsburgh, PA, USA) with a Buchner funnel, then air dried overnight. We also fabricated MPs containing CaHPO4 or CaCO3 (10%, w/w solution of polymer) by adding them into the CS solution and using above described method. All pre- pared MPs were hydrated and neutralized in 6200 mg/L NaHCO3 for 10 min. The solution was filtered off and the MPs were then rinsed profusely with distilled water and allowed to air-dry overnight. 2.3. Experiment Design of in Vitr o MP Degradation The fabricated and neutralized hybrid MPs (30 mg) were added to 11 ml glass vial shells (Fisherbrand). MPs sam- ples were incubated with 9 ml of Phosphate Buffered Saline (PBS) at 37˚C (Fisher Scientific Isotemp Incuba- tor) under continuous shaking (Barnstead Multi-Purpose Rotator) at 50 rpm for predetermined time points up to 30 weeks. The vials were closed with a plastic plug de- signed for the vials, to prevent evaporation of the PBS over the long time period of the experiment. In order to mimic in vivo physiological environment, every week 4 ml of incubated PBS solution was removed from each sample and replaced with an equal volume of fresh PBS medium. 2.4. Determination of pH CS and hybrid MPs were studied in triplicate samples for pH determination. Each week the 4 mL of PBS in- cubated solution withdrawn from the MPs was removed from their vial and placed in clean vial shells to measure the pH and help keep the sample from contamination. The pH was determined by placing a clean and cali- brated pH meter (Mettler Toledo SevenEasy) probe into the solution then recording the stabilized value. 2.5. De t e r m i n a tio n of C a l c i u m I o n ( C a 2+) Release From the removed PBS medium used in pH determina- tion, two 0.5 ml aliquots of the incubated medium were stored at 4˚C for assay analysis. The stored aliquots and assay kits were removed from the freezer and allowed to equilibrate to room temperature before use. The calcium assay requires preparation of a working reagent by com- bining equal volumes of the Reagent A and Reagent B provided. Standards, blanks, and samples were run in triplicate on clear bottom 96-well plates by adding 5 l of sample and then 200 l of working reagent to each well with tapping lightly to mix. Samples were allowed Figure 1. Ionic cross-linking interaction of amide groups in chitosan with phosphate groups in tripolyphosphate. to incubate 3 min at room temperature and the optical density (OD) was read at 612 nm on a spectrometer (Spectra Max Plus 384, Molecular Devices; Sunnyvale, CA). Calcium concentrations of the samples were calcu- lated with the following expression using the average OD of all triplicates of the samples: Calcium Concentration = SAMPLE BLANK OD OD Slope (mg/dL) 2.6. Morphology of MPs-SEM At the predetermined time points of 0, 5, 10, 15, 20, 25, and 30 weeks, samples were removed for characteriza- tion by SEM. Size and morphological characteristics, including shape and surface roughness of the fabricated hybrid MPs were studied with a Hitachi S-4800 High Resolution SEM at an accelerating voltage of 10 kV. Samples for analysis with the SEM were prepared as follows: small amounts of dried MPs were affixed to a stage on double sided tape then the dry samples were gold sputtered prior to analysis with the SEM. Micro- photographs were taken at different magnifications for each sample for analysis. C opyright © 2011 SciRes. JBiSE  A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 386 2.7. Statistical Analysis Statistical analysis was performed on pH and calcium release with a two-way ANOVA without replication through the Analysis ToolPak for data analysis in Excel. The differences were considered significant at the level of p < 0.05. 3. RESULTS 3.1. Study of pH We recorded the averaged pH changes (n = 3) for the different types of MPs when they immersed in the PBS medium over the 30 weeks (Figure 2). In the figure it is clear that the MPs are causing changes in the pH of the PBS medium. Each MP type has a specific pH range that varies over time, but stays within the range that is com- patible for living tissue [18]. CS MPs kept the highest pH range among all MP types, while CS-10%CaHPO4 MPs had the lowest pH range (p < 0.05) for most of the study. While CS MPs maintained the pH range in 7.4 - 7.2, CS-10%CaCO3 MPs maintained the pH in 7.2 - 7.0. CS-10%CaHPO4 MPs showed the lowest pH range, 6.8 - 7.0 among the three MP groups. 3.2. Calcium Ion (Ca2+) Release We fabricated MPs that integrated CaHPO4 or CaCO3 into their own respective structures as they formed. The calcium ion release in mg/dl is reported in Figure 3 over the 25 weeks for each type of hybrid MPs (n = 3). There was no calcium release during the 4 weeks period from CS MPs. CS-10%CaCO3 MPs have the highest variation of calcium release during the initial 6 weeks compared to all the groups (p < 0.05). Calcium release from CS-10%CaCO3 MPs was decreased at 18 week and then again started to increase (p < 0.05). Other two types of MPs was also followed the same pattern after 18 week. Calcium release in the surface of the CS-10%CaCO3 MPs is seems to be faster than compared to that of CS-10%CaHPO4 MPs. 3.3. Morphology Change—SEM The size, shape, and surface roughness of MPs can in- fluence cell attachment, proliferation, and differentiation [2]. SEM microphotographs were used to determine these characteristics of hybrid MPs. Almost prepared MPs appeared to be spherical and distinctively separate at Week 0, which means just after placing the MPs in the vials containing PBS for 10 - 15 min but not yet started to incubate them (Figures 4-6). All MPs types were measured had their smallest diameter approximately 20 m. The most CS MPs were in the size range between 20 - 75 m. However, there were a few MPs were over the upper limit of the size range. The CS-10%CaCO3 and CS-10%CaHPO4 MPs were slightly larger compare to the CS MPs and the most MPs were in the size of 20 - 90 m, and 20 - 100 m, respectively. Similar to CS MPs there were a few large 10%CaCO3 and CS-10% CaHPO4 MPs in the out of above size range. In Figure 4, microphotographs show the changes in surface morphology of CS MPs with low (50X) and high magnification (700X). Week 0 had smooth, spherical morphology with slight ridges elevations. At Week 5 we noticed a change to the outer surface morphology of most CS MPs. Larger ridges and wrinkling were ob- served. The wrinkles seem to become more predominant as the time points passed. At Week 25, we were inter- ested that the microphotographs revealed that the some MPs were peeling but the shape was not affected. At week 30, small pieces were broken from the some CS MPs. However, we did not observe the complete bulk degradation of MPs suggesting that bulk degradation initiate at this time period. Figure 2. The pH data for hybrid MPs as a function of time up to 30 week. The line with solid spheres shows the pH of the replacement PBS medium used. Figure 3. Calcium release data for each type of MPs over the 25-weeks. C opyright © 2011 SciRes. JBiSE 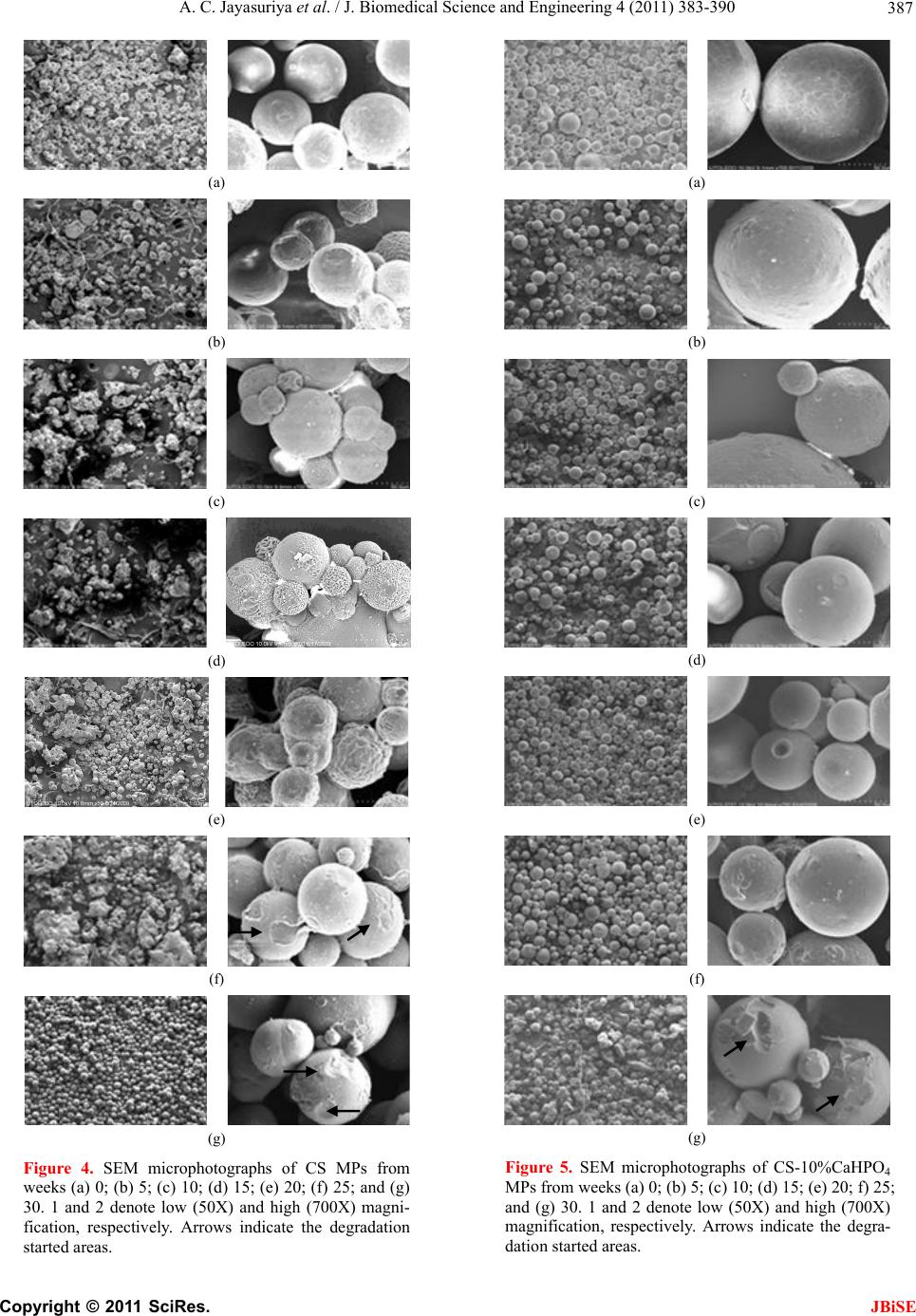 A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 387 (a) (b) (c) (d) (e) (f) (g) Figure 4. SEM microphotographs of CS MPs from weeks (a) 0; (b) 5; (c) 10; (d) 15; (e) 20; (f) 25; and (g) 30. 1 and 2 denote low (50X) and high (700X) magni- fication, respectively. Arrows indicate the degradation started areas. (a) (b) (c) (d) (e) (f) (g) Figure 5. SEM microphotographs of CS-10%CaHPO4 MPs from weeks (a) 0; (b) 5; (c) 10; (d) 15; (e) 20; f) 25; and (g) 30. 1 and 2 denote low (50X) and high (700X) magnification, respectively. Arrows indicate the degra- dation started areas. C opyright © 2011 SciRes. JBiSE 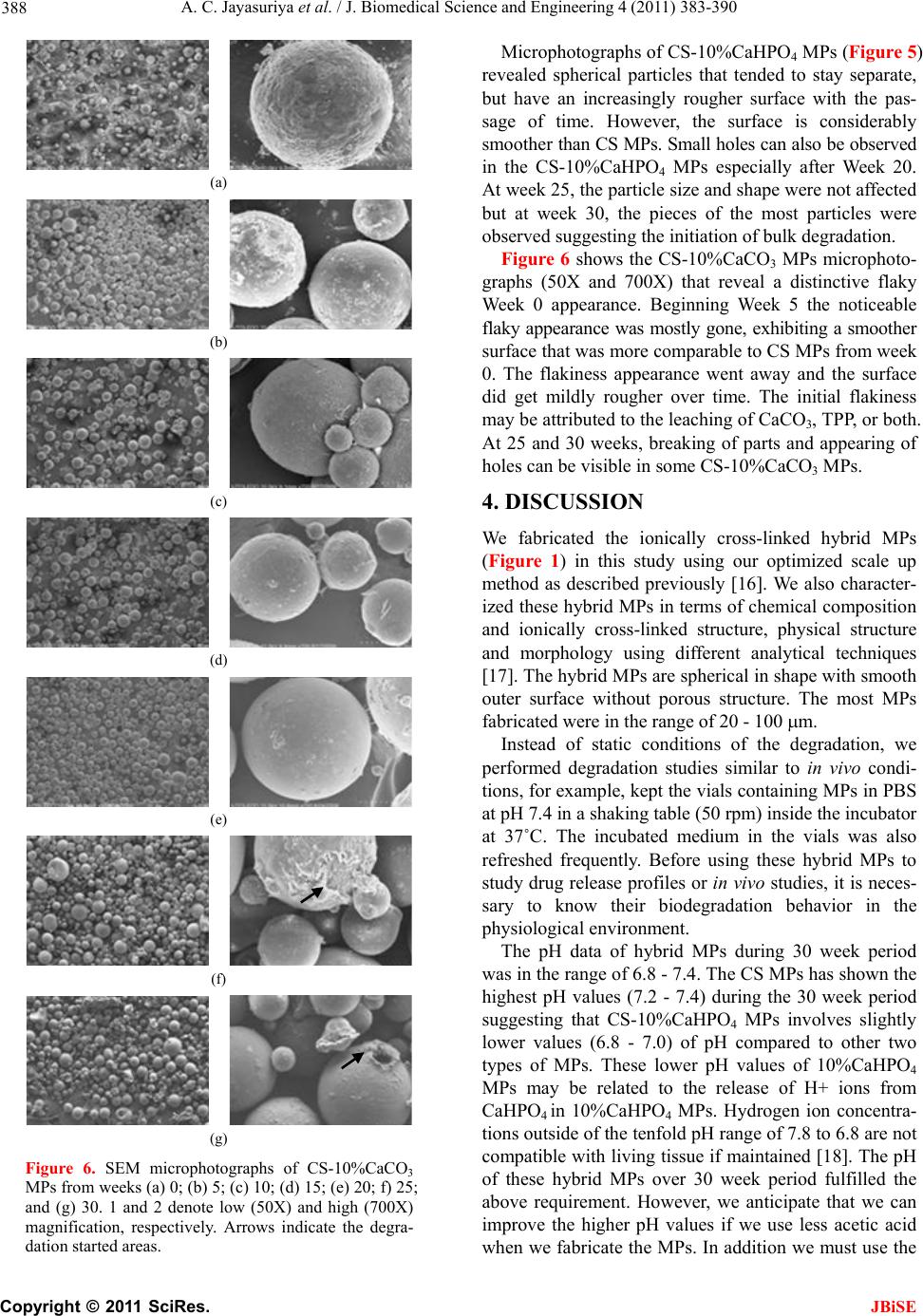 A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 388 (a) (b) (c) (d) (e) (f) (g) Figure 6. SEM microphotographs of CS-10%CaCO3 MPs from weeks (a) 0; (b) 5; (c) 10; (d) 15; (e) 20; f) 25; and (g) 30. 1 and 2 denote low (50X) and high (700X) magnification, respectively. Arrows indicate the degra- dation started areas. Microphotographs of CS-10%CaHPO4 MPs (Figure 5) revealed spherical particles that tended to stay separate, but have an increasingly rougher surface with the pas- sage of time. However, the surface is considerably smoother than CS MPs. Small holes can also be observed in the CS-10%CaHPO4 MPs especially after Week 20. At week 25, the particle size and shape were not affected but at week 30, the pieces of the most particles were observed suggesting the initiation of bulk degradation. Figure 6 shows the CS-10%CaCO3 MPs microphoto- graphs (50X and 700X) that reveal a distinctive flaky Week 0 appearance. Beginning Week 5 the noticeable flaky appearance was mostly gone, exhibiting a smoother surface that was more comparable to CS MPs from week 0. The flakiness appearance went away and the surface did get mildly rougher over time. The initial flakiness may be attributed to the leaching of CaCO3, TPP, or both. At 25 and 30 weeks, breaking of parts and appearing of holes can be visible in some CS-10%CaCO3 MPs. 4. DISCUSSION We fabricated the ionically cross-linked hybrid MPs (Figure 1) in this study using our optimized scale up method as described previously [16]. We also character- ized these hybrid MPs in terms of chemical composition and ionically cross-linked structure, physical structure and morphology using different analytical techniques [17]. The hybrid MPs are spherical in shape with smooth outer surface without porous structure. The most MPs fabricated were in the range of 20 - 100 m. Instead of static conditions of the degradation, we performed degradation studies similar to in vivo condi- tions, for example, kept the vials containing MPs in PBS at pH 7.4 in a shaking table (50 rpm) inside the incubator at 37˚C. The incubated medium in the vials was also refreshed frequently. Before using these hybrid MPs to study drug release profiles or in vivo studies, it is neces- sary to know their biodegradation behavior in the physiological environment. The pH data of hybrid MPs during 30 week period was in the range of 6.8 - 7.4. The CS MPs has shown the highest pH values (7.2 - 7.4) during the 30 week period suggesting that CS-10%CaHPO4 MPs involves slightly lower values (6.8 - 7.0) of pH compared to other two types of MPs. These lower pH values of 10%CaHPO4 MPs may be related to the release of H+ ions from CaHPO4 in 10%CaHPO4 MPs. Hydrogen ion concentra- tions outside of the tenfold pH range of 7.8 to 6.8 are not compatible with living tissue if maintained [18]. The pH of these hybrid MPs over 30 week period fulfilled the above requirement. However, we anticipate that we can improve the higher pH values if we use less acetic acid when we fabricate the MPs. In addition we must use the C opyright © 2011 SciRes. JBiSE  A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 389 lyophilizer to get rid of any remaining residual acetyl group in the MPs. We examined the calcium release for all types of MPs during the 25 week period (Figure 3). The calcium re- lease from MPs was less than 2 mg/dl at a time during the 25 week period. This released calcium amount seems to be less than the calcium content in the human serum which is 8.5 - 10.5 mg/dl [19]. Our MPs contain only 10% (w/w) of CaHPO4 or CaCO3 relative to CS amount. We can anticipate that higher release of calcium at a time if we incorporate the higher amount of calcium contain- ing compounds into the hybrid MPs. The initial calcium release seems to be attributing to the diffusion and later release after 18 week may be due to the more degrada- tion of the MPs. The CS MPs may not possess calcium since we did not incorporate any materials containing calcium. However, our data has shown the calcium re- lease over time from CS MPs. We would like to note that Chestnutt et al. [20] also reported the interesting release of calcium in pure CS scaffolds. A fact that was attrib- uted to the use of CS from crab shells containing resid- ual mineral content. SEM data (Figures 4-6) has clearly shown that these MPs did not change its shape or size until 25 week. At or after 25 week all types of MPs seem to show the initia- tion of bulk degradation. Generally, the degradation of CS is related to the molecular weight and deacetylation. These MPs were strong not only having the higher deacetylation (>85%) but these MPs were ionically cross-linked with TPP. This interaction leads to the fab- rication of physically strong particles according to our optimization and scale-up procedures [16]. It has been demonstrated that CS can be degraded en- zymatically by chitinases, chitosanases, and lysozyme. Past research has shown CS to be degraded mainly by lysozyme in human serum [21,22]. Lysozyme appears to target the acetylated units on CS, leaving CS oligosac- charides products of different lengths that can be incor- porated into glycosaminoglycans and glycoproteins pathways or excreted [13,23]. Many investigations have published that the factor in controlling the rate of degra- dation has been shown to be inversely associated with CS’s degree of deacetylation. Highly acetylated CS de- grades rapidly, and highly deacetylated (>80%) CS has low degradation rates and may remain several months in vivo [5,6,10,12,13,22]. There is an increased demand towards surgical inter- ventions being minimally invasive. Preferable scaffold constructs in bone tissue engineering for such surgeries would be moldable and/or injectable to the defect site. Some studies have shown the possibility of such osteo- genic constructs with pre-cultured ceramic MPs. How- ever, low biodegradability and brittleness can restrict the use of ceramics [2,4]. A goal of our studies is to help characterize and design CS based hybrid MPs that could become an injectable biomaterial suitable for a scaf- fold-like role in bone tissue engineering. We believe it to be important to understand the process that is happening to the MPs without enzyme for background results that can be used comparatively with what happens with en- zymatic degradation in future studies. 5. CONCLUSIONS In this study using physiological conditions we have shown that morphological changes occur on the surface followed by initiation of bulk degradation of 85% deacetylated CS based hybrid MPs without enzyme hy- drolysis over 30 week period. The size and shape of the hybrid MPs were not affected until week 30. The broken pieces were observed at Week 30 from the all types of hybrid MPs. Our results show that each MP type main- tain the physiologically relevant pH range during the 30 week period. We conclude that investigation of using CS based hybrid MPs for a bone tissue engineering purpose is encouraged by our biodegradation results, and the feasibility of using these MPs for a long-term scaf- fold-like role is possible. 6. ACKNOWLEDGEMENTS We would like to thank National Science Foundation (NSF) grant number 0652024 and National Institute of Health (NIH) grant number DE019508 for providing financial support to accomplish this work. REFERENCES [1] Kakar, S. and Einhorn, T.A. (2005) Chapter 11: Tissue engineering of bone. In: T. Einhorn & J. O. Hollinger, Eds., Bone Tissue Engineering, CRC Press, Boca Raton, 277-302. [2] Kruyt, M.C., Persson, C., Johansson, G., Dhert, W.J. and De Bruijn, J.D. (2006) Towards injectable cell-based tis- sue-engineered bone: The effect of different calcium phosphate mps and pre-culturing. Tissue Engineering, 12, 309-317. doi:10.1089/ten.2006.12.309 [3] Zhang, Y . and Zhang, M. (2001) Synthesis and charac- terization of macroporous CS/calcium phosphate com- posite scaffolds for tissue engineering. Journal of Bio- medical Materials Research, 55, 304-312. doi:10.1002/1097-4636(20010605)55:3<304::AID-JBM1 018>3.0.CO;2-J [4] Kim, I.Y., Seo, S.J., Moon, H.S., Yoo, M.K., Park, I.Y. and Kim, B.C. et al. (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnology Ad- vances, 26, 1-21. doi:10.1002/1097-4636(20010605)55:3<304::AID-JBM1 018>3.0.CO;2-J [5] Jiang, T., Kumbar, S.G., Nair, L.S. and Laurencin, C.T. (2008) Biologically active CS systems for tissue engi- neering and regenerative medicine. Current Topics in Medicinal Chemistry, 8, 354-364. C opyright © 2011 SciRes. JBiSE  A. C. Jayasuriya et al. / J. Biomedical Science and Engineering 4 (2011) 383-390 Copyright © 2011 SciRes. 390 JBiSE doi:10.2174/156802608783790974 [6] Khor, E. (2001). Chitin: Fulfilling a biomaterials promise. Elsevier, New York. [7] Shi, C., Zhu, Y., Ran, X., Wang, M., Su, Y. and Cheng, T. (2006) Therapeutic potential of CS and its derivatives in regenerative medicine. Journal of Surgical Research, 133, 185-192. doi:10.1016/j.jss.2005.12.013 [8] Kato, Y., Onishi, H. and Machida, Y. (2003) Application of chitin and CS derivatives in the pharmaceutical field. Current Pharmaceutical Biotechnology, 4, 303-309. doi:10.2174/1389201033489748 [9] Abdel-Fattah, W.I., Jiang, T., El-Bassyouni, G.E.T. and Laurencin, C.T. (2007) Synthesis, characterization of CSs and fabrication of sintered CS microsphere matrices for bone tissue engineering. Acta Biomaterialia, 3, 503-514. doi:10.1016/j.actbio.2006.12.004 [10] Freier, T., Koh, H.S., Kazazian, K. and Shoichet, M.S. (2005) Controlling cell adhesion and degradation of CS films by N-acetylation. Biomaterials, 26, 5872-5878. doi:10.1016/j.biomaterials.2005.02.033 [11] Couto, D.S., Hong, Z. and Mano, J.F. (2009) Develop- ment of bioactive and biodegradable CS-based injectable systems containing bioactive glass nanoparticles. Acta Biomaterialia, 5, 115-123. doi:10.1016/j.biomaterials.2005.02.033 [12] Di Martino, A., Sittinger, M. and Risbud, M.V. (2005) Chitosan: A versatile biopolymer for orthopaedic tis- sue-engineering. Biomaterials, 26, 5983-5990. doi:10.1016/j.biomaterials.2005.03.016 [13] Francis Suh, J.K. and Matthhew, H.W. (2000) Applica- tion of CS-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials, 21, 2589- 2598. doi:10.1016/S0142-9612(00)00126-5 [14] Anal, A.K., Stevens, W.F. and Remunan-Lopez, C. (2006) Ionotropic cross-linked CS microspheres for controlled release of ampicillin. International Journal of Pharma- ceutics, 312, 166-173. doi:10.1016/j.ijpharm.2006.01.043 [15] Costantino, P.D., Hiltzik, D., Govindaraj, S. and Moche, J. (2002) Bone healing and bone substitutes. Facial Plas- tic Surgery, 18, 13-26. doi:10.1055/s-2002-19823 [16] Jayasuriya, A.C. and Bhat, A. (2009) Optimization of scaled-up chitosan microparticles for bone regeneration, Biomedical Materials, 4, 55006. doi:10.1088/1748-6041/4/5/055006 [17] Jayasuriya, A.C. and Bhat, A. (2010) Fabrication and characterization of novel hybrid organic/inorganic mi- croparticles to apply in bone regeneration. Journal of Biomedical Materials Research A, 93A, 1280-1288. [18] Widmaier, E.P., Raff, H. and Strang, K.T. (2006). Chapter 2: Chemical composition of the body. In: Vander, Ed., Human Physiology: The Mechanisms of Body Function, McGraw-Hill, Boston, pp. 21-46. [19] Seltzer, M.H., Rosato, F.E. and Fletcher, M.J. (1970) Serum and tissue in human breast carcinoma. Cancer Research, 30, 615-616. [20] Chesnutt, B.M., Viano, A.M., Yuan, Y., Yang, Y., Guda, T., Appleford, M.R. et al. (2009) Design and characteri- zation of a novel CS/nanocrystalline calcium phosphate composite scaffold for bone regeneration. Journal of Biomedical Materials Research Part A, 88A, 491-502. doi:10.1002/jbm.a.31878 [21] Vårum, K.M., Myhr, M.M., Hjerde, R.J., Smidsrød, O. (1997) In vitro degradation rates of partially N-acetylated CSs in human serum. Carbohydrate Research, 299, 99-101. doi:10.1016/S0008-6215(96)00332-1 [22] Ren, D., Yi, H., Wang, W. and Ma, X. (2005) The enzy- matic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbo- hydrate Research, 340, 2403-2410. doi:10.1016/S0008-6215(96)00332-1 [23] Tığlı, R.S., Karakeçili, A. and Gümüşderelioğlu, M. (2007) In vitro characterization of CS scaffolds: Influ- ence of composition and deacetylation degree. Journal of Materials Science: Materials in Medicine, 18, 1665-1674. doi:10.1007/s10856-007-3066-x [24] Young, M.A., Ravishanker, G., Beveridge, D.L. and Berman, H.M. (1995) Analysis of local helix bending in crystal structures of DNA oligonucleotides and DNA- rotein complexes. Biophysical Journal, 68, 2454-2468. doi:10.1016/S0006-3495(95)80427-3
|