Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 301-310 doi:10.4236/jbnb.2011.23037 Published Online July 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB 301 Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Michael M. Porter, Jian Yu Hawai’i Natural Energy Institute, University of Hawai’i at Mānoa, Honolulu, Hawai’i. Email: jianyu@hawaii.edu Received February 4th, 2011; revised May 4th 2011; accepted June 1st, 2011. ABSTRACT Poly(3-hydroxybutyrate) (PHB) is a natural biopolyester accumulated in microbial cells as tiny amorphous granules. The nano- micro-particles have a variety of potential applications and behave differently in different environments. In this work, the in situ crystallization of native PHB granules was investigated under different environmental conditions. The isothermal crystallization kinetics of the granules was shown to follow Avrami’s equation. The model parameter describing crystal growth is a function of temperature or pH and estimated from in situ crystallization measurements with attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy. Empirical equations describing crystal growth are derived for the parameter values. PHB granules heated at 80˚C - 140˚C in acidic solution (pH 2) up to 4 hr showed an increase in crystallinity from about 5% to 35% and moderate particle aggregation. PHB granules suspended in alkaline solutions (pH 9-12) at room temperature up to 4 hr showed an increase in crystallinity up to 45% and very little particle aggregation. It was found that the amorphousness of PHB granules in vivo is stabilized by water, lipids and proteins. Upon removal of these impurities, partial crystallization is induced which may inhibit extensive particle aggregation. Keywords: Avrami Model, Crystallization Kinetics, PHA, PHB Granules, Biopolyester Particles 1. Introduction Poly(3-hydroxybutyrate) (PHB) is a representative, natu- rally occurring bacterial polyhydroxyalkanoate (PHA) that can be produced from renewable feedstocks as an eco-friendly bioplastic [1,2]. In nature, PHB biopoly- esters are accumulated in microbial cells as tiny intracel- lular amorphous granules (0.2 - 0.5 μm in diameter) (see Figure 1(a)) [1-5]. PHB granules can be extracted from microbial cells and purified by a number of different re- covery processes [6-9]. During recovery, the environ- ment of the amorphous granules changes and may cause the nano- micro-particles to crystallize [10-12]. Interest- ingly, in different environmental conditions the rate and extent of PHB crystallization varies, which may affect the degree of particle aggregation, resulting in different size and morphology of the biopolyester granules. Puri- fied PHB particles may be useful in a variety of applica- tions because PHB is completely biodegradable and bio- compatible [2,4,5]. The crystallization of PHB is an energetically favor- able process that may be induced by its environment. Purified PHB granules become a semi-crystalline mate- rial (~60% crystallinity) [13-15]. When pure PHB is heated above its melting point (~180˚C), it becomes a fully amorphous melt [14,16-20]. Upon cooling, the PHB molecules crystallize, forming antiparallel helical chains linked by C – H ··· O hydrogen bonds between the car- bonyl (C=O) and methyl (CH3) groups of the polyester backbone [18,21,22]. Crystallization begins as groups of amorphous molecules aggregate into tiny clusters form- ing nuclei [19,23]. Then, sheet-like structures known as lamellae grow radially outward from the nuclei in the form of spherulites [19,23]. The kinetics of PHB crystal- lization depend on the thermodynamics of phase change (i.e., amorphous to crystalline), the nature of nucleation and spherulitic crystal growth, and environmental factors such as temperature and chemical potential [19,23,24]. In vivo, PHB granules are fully amorphous and sur- rounded by a monolayer membrane composed of phos- pholipids and proteins [3,10,11,25]. The amorphousness 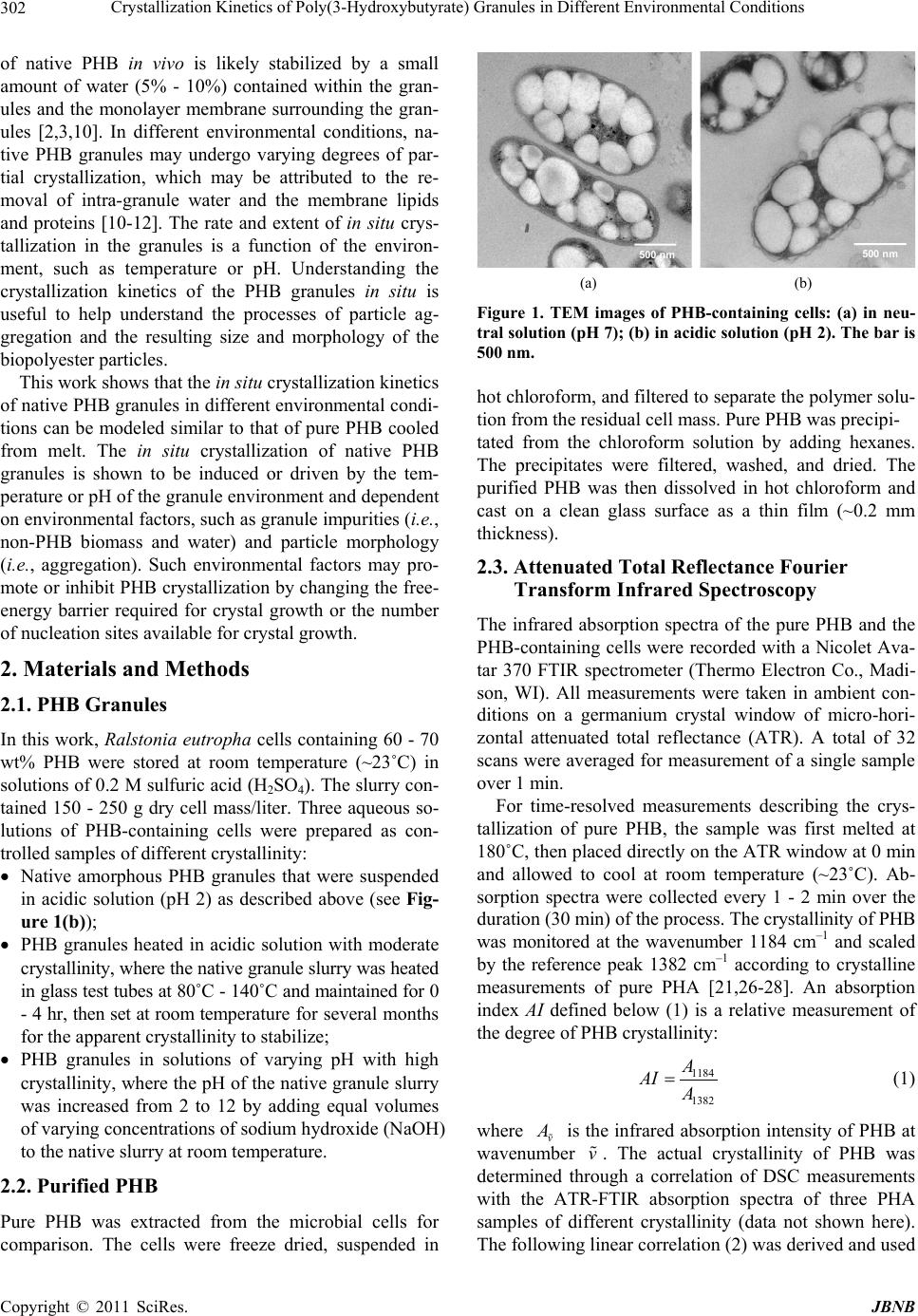 Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 302 of native PHB in vivo is likely stabilized by a small amount of water (5% - 10%) contained within the gran- ules and the monolayer membrane surrounding the gran- ules [2,3,10]. In different environmental conditions, na- tive PHB granules may undergo varying degrees of par- tial crystallization, which may be attributed to the re- moval of intra-granule water and the membrane lipids and proteins [10-12]. The rate and extent of in situ crys- tallization in the granules is a function of the environ- ment, such as temperature or pH. Understanding the crystallization kinetics of the PHB granules in situ is useful to help understand the processes of particle ag- gregation and the resulting size and morphology of the biopolyester particles. This work shows that the in situ crystallization kinetics of native PHB granules in different environmental condi- tions can be modeled similar to that of pure PHB cooled from melt. The in situ crystallization of native PHB granules is shown to be induced or driven by the tem- perature or pH of the granule environment and dependent on environmental factors, such as granule impurities (i.e., non-PHB biomass and water) and particle morphology (i.e., aggregation). Such environmental factors may pro- mote or inhibit PHB crystallization by changing the free- energy barrier required for crystal growth or the number of nucleation sites available for crystal growth. 2. Materials and Methods 2.1. PHB Granules In this work, Ralstonia eutropha cells containing 60 - 70 wt% PHB were stored at room temperature (~23˚C) in solutions of 0.2 M sulfuric acid (H2SO4). The slurry con- tained 150 - 250 g dry cell mass/liter. Three aqueous so- lutions of PHB-containing cells were prepared as con- trolled samples of different crystallinity: Native amorphous PHB granules that were suspended in acidic solution (pH 2) as described above (see Fig- ure 1(b)); PHB granules heated in acidic solution with moderate crystallinity, where the native granule slurry was heated in glass test tubes at 80˚C - 140˚C and maintained for 0 - 4 hr, then set at room temperature for several months for the apparent crystallinity to stabilize; PHB granules in solutions of varying pH with high crystallinity, where the pH of the native granule slurry was increased from 2 to 12 by adding equal volumes of varying concentrations of sodium hydroxide (NaOH) to the native slurry at room temperature. 2.2. Purified PHB Pure PHB was extracted from the microbial cells for comparison. The cells were freeze dried, suspended in 500 nm500 nm (a) (b) Figure 1. TEM images of PHB-containing cells: (a) in neu- tral solution (pH 7); (b) in acidic solution (pH 2). The bar is 500 nm. hot chloroform, and filtered to separate the polymer solu- tion from the residual cell mass. Pure PHB was precipi- tated from the chloroform solution by adding hexanes. The precipitates were filtered, washed, and dried. The purified PHB was then dissolved in hot chloroform and cast on a clean glass surface as a thin film (~0.2 mm thickness). 2.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy The infrared absorption spectra of the pure PHB and the PHB-containing cells were recorded with a Nicolet Ava- tar 370 FTIR spectrometer (Thermo Electron Co., Madi- son, WI). All measurements were taken in ambient con- ditions on a germanium crystal window of micro-hori- zontal attenuated total reflectance (ATR). A total of 32 scans were averaged for measurement of a single sample over 1 min. For time-resolved measurements describing the crys- tallization of pure PHB, the sample was first melted at 180˚C, then placed directly on the ATR window at 0 min and allowed to cool at room temperature (~23˚C). Ab- sorption spectra were collected every 1 - 2 min over the duration (30 min) of the process. The crystallinity of PHB was monitored at the wavenumber 1184 cm–1 and scaled by the reference peak 1382 cm–1 according to crystalline measurements of pure PHA [21,26-28]. An absorption index AI defined below (1) is a relative measurement of the degree of PHB crystallinity: 1184 1382 A AI A (1) where v A is the infrared absorption intensity of PHB at wavenumber v . The actual crystallinity of PHB was determined through a correlation of DSC measurements with the ATR-FTIR absorption spectra of three PHA samples of different crystallinity (data not shown here). The following linear correlation (2) was derived and used  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 303 to calculate the crystallinity from the absorption index: 2.91 3.04XAI (2) where X is the apparent crystallinity of PHB. For in situ measurements of the PHB-containing cells, a small drop (~2 μL) of aqueous solution was placed di- rectly on the ATR window and allowed to evaporate at room temperature. During water evaporation, the PHB- containing cells deposited onto the window via sedimen- tation and absorption spectra were collected every 1 - 2 min for the duration (30 min) of the process. The absorp- tion spectra were divided into three distinct stages: an initial scattering stage predominated by water absorption, a stable secondary stage representing the true, in situ crystallinity, and a final “artificial” crystallization stage caused by excess dehydration. The apparent, in situ crys- tallinity was measured during the stable, secondary stage of the measurement at the wavenumber 1184 cm–1, scaled by the reference peak 1382 cm–1 [21,26-28]. Spec- tral interference from background absorptions caused by water and other non-PHB cellular components were de- termined to be constant (data not shown here) and sub- tracted from the total absorption spectra. The in situ PHB crystallinity measurements were quantified using the same DSC/ATR-FTIR correlation mentioned above in (1) and (2). 2.4. Transmission Electron Microscopy The transmission electron microscopy (TEM) images were viewed on a LEO 912 EFTEM (Zeiss, Germany) at 100 kV and photographed with a frame-transfer CCD camera (Proscan, Germany). The cells were fixed with glutaraldehyde and calcium chloride in a sodium caco- dylate buffer, then post-fixated with osmium tetroxide, stained with uranyl acetate, dehydrated with ethanol and embedded in epoxy. Ultrathin (60 - 80 nm) sections were obtained on an Ultracut E ultramicrotome (Reichert, Austria), double stained with uranyl acetate and lead cit- rate. 2.5. Environmental Factors and Kinetics Modeling To determine the factors that may stabilize amorphous PHB granules, aqueous solutions of PHB-containing cells were dehydrated by freeze-drying and acetone ex- traction. The in situ crystallization behavior of the native granules in different environmental conditions was in- vestigated by suspending PHB-containing cells in an acidic solution (pH 2) at high temperatures (80˚C - 140˚C) or in aqueous solutions of increasing pH (2 - 12) in am- bient conditions. The crystallization of pure PHB from melt was ana- lyzed and compared to the in situ crystallization of the PHB granules. Crystallinity measurements were obtained via ATR-FTIR and fit to Avrami’s Equation (3) [21,28, 29]: 1exp n X kt (3) where X is the crystallinity, t is the crystallization time, k is a rate constant dependent on the formation of nuclei and spherulitic crystal growth, and n is Avrami’s expo- nent dependent on the nature of nucleation and geometry of crystal growth. Empirical equations to describe the growth rate parameter (k) as a function of temperature or pH were derived. Based on the crystallization kinetics of polymers similar to PHB, the growth rate parameter may be described by the Arrhenius Equation (4) as a function of temperature [29,30]: 1exp na o E kk RT (4) where a E is the crystallization activation energy, R is the gas constant (8.314 J/K·mol), ko is a pre-exponential factor independent of temperature, and T is the isother- mal crystallization temperature in Kelvin. The growth rate parameters and Avrami’s exponents were estimated by fitting the measured values to the empirical equations and discussed in terms of thermodynamic energy, gran- ule geometry, spherulitic growth rate, and the nature of nucleation. Transmission electron microscopy (TEM) images of the granules in different environments were taken to show the extent of granule aggregation. 3. Results 3.1. Crystallization of Pure PHB from Melt Figure 2(a) shows a time development of the infrared absorption spectra illustrating the crystallization of pure PHB cooled from an amorphous melt at 180˚C (solid line) to a semi-crystalline solid at room temperature (dashed line) over 30 min in ambient conditions. As seen in the figure, the absorption intensity at 1184 cm–1 decreases with time, while the absorption intensity at 1382 cm–1 does not change with time and is taken as the reference peak [31]. Using the data from Figure 2(a) with (1) and (2), the corresponding crystallinity of PHB versus cool- ing time is plotted in Figure 2(b). Primary crystallization, which includes nucleation and spherulitic crystal growth, is represented by the solid data points and occurs be- tween 2 - 15 min. Secondary crystallization, or the rear- rangement of PHB molecules into more energetically favorable structures, is represented by the hollow data points and occurs after 15 min. The initial data points (0 - 2 min) are neglected in this analysis because the thermal history of the sample may be unstable and is not known. 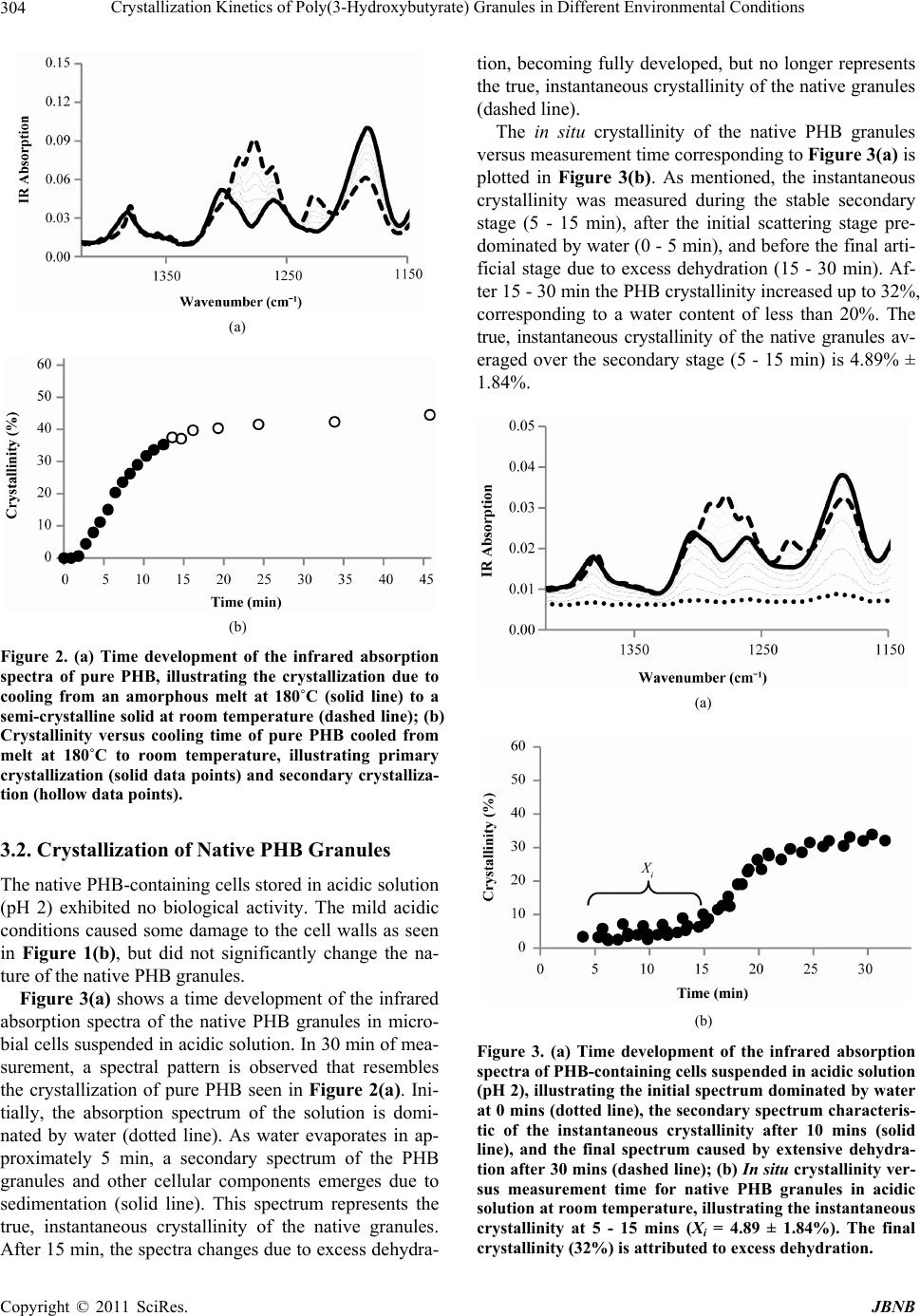 Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 304 (a) (b) Figure 2. (a) Time development of the infrared absorption spectra of pure PHB, illustrating the crystallization due to cooling from an amorphous melt at 180˚C (solid line) to a semi-crystalline solid at room temperature (dashed line); (b) Crystallinity versus cooling time of pure PHB cooled from melt at 180˚C to room temperature, illustrating primary crystallization (solid data points) and secondary crystalliza- tion (hollow data points). 3.2. Crystallization of Native PHB Granules The native PHB-containing cells stored in acidic solution (pH 2) exhibited no biological activity. The mild acidic conditions caused some damage to the cell walls as seen in Figure 1(b), but did not significantly change the na- ture of the native PHB granules. Figure 3(a) shows a time development of the infrared absorption spectra of the native PHB granules in micro- bial cells suspended in acidic solution. In 30 min of mea- surement, a spectral pattern is observed that resembles the crystallization of pure PHB seen in Figure 2(a). Ini- tially, the absorption spectrum of the solution is domi- nated by water (dotted line). As water evaporates in ap- proximately 5 min, a secondary spectrum of the PHB granules and other cellular components emerges due to sedimentation (solid line). This spectrum represents the true, instantaneous crystallinity of the native granules. After 15 min, the spectra changes due to excess dehydra- tion, becoming fully developed, but no longer represents the true, instantaneous crystallinity of the native granules (dashed line). The in situ crystallinity of the native PHB granules versus measurement time corresponding to Figure 3(a) is plotted in Figure 3(b). As mentioned, the instantaneous crystallinity was measured during the stable secondary stage (5 - 15 min), after the initial scattering stage pre- dominated by water (0 - 5 min), and before the final arti- ficial stage due to excess dehydration (15 - 30 min). Af- ter 15 - 30 min the PHB crystallinity increased up to 32%, corresponding to a water content of less than 20%. The true, instantaneous crystallinity of the native granules av- eraged over the secondary stage (5 - 15 min) is 4.89% ± 1.84%. (a) (b) Figure 3. (a) Time development of the infrared absorption spectra of PHB-containing cells suspe nded in acidic solution (pH 2), illustrating the initial spectrum dominated by water at 0 mins (dotted line), the secondary spectrum characteris- tic of the instantaneous crystallinity after 10 mins (solid line), and the final spectrum caused by extensive dehydra- tion after 30 mins (dashed line); (b) In situ crystallinity ver- sus measurement time for native PHB granules in acidic solution at room temperature, illustrating the instantaneous crystallinity at 5 - 15 mins (Xi = 4.89 ± 1.84%). The final crystallinity (32%) is attributed to excess dehydration.  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 305 In further analysis, dehydration of the PHB-containing cells was observed to induce partial crystallization in the native granules. Cells freeze-dried for 3 hr exhibited a slow crystallization of PHB, increasing up to 33% over the first 24 hr after drying. In contrast, cells dehydrated with acetone extraction showed a much faster crystalliza- tion of PHB up to 52% after 24 hr. The increased crystal- linity observed from acetone extraction is attributed to the simultaneous removal of water, as well as, lipids from the granules. 3.3. Crystallization of PHB Granules Subjected to High Temperatures PHB-containing cells heated to and maintained at 80˚C - 140˚C for 0 - 4 hr in acidic solution (pH 2) showed an increase in PHB crystallization and granule aggregation with increasing temperature and exposure time. A sig- nificant increase in crystallinity observed (20% - 35%) in the granules heated above 80˚C suggests that heating the cell slurry may have induced partial crystallization where intra-granule water, proteins and lipids were displaced from the PHB particles. Figure 4(a) contains a represen- tative TEM image of the granules heated at 120˚C for 1 hr in acidic solution (pH 2). As seen in the figure, heating the PHB-containing cells induced moderate granule ag- gregation. The instantaneous crystallinity of the PHB granules at 120˚C for 1 hr, for instance, reached 22.52%. 3.4. Crystallization of PHB Granules Subjected to Varying pH Raising the pH of the PHB-containing cells from 2 to 12 in ambient conditions showed an increase in granule crystallinity with increasing pH. Increasing the pH, how- ever, did not seem to induce granule aggregation and the in situ crystallization of the PHB granules seemed to oc- cur instantaneously. Most likely, increasing the pH may have dissolved lipids and proteins from the granule membranes, inducing a quick partial crystallization at the particle surfaces where the granule membranes were re- moved. Figure 4(b) contains a representative TEM im- age of the granules at pH 12 for 1 hr in room temperature. As seen in the figure, increasing the pH of the PHB-con- taining cells induced very little granule aggregation. The instantaneous crystallinity of the PHB granules at pH 12 for 1 hr, for instance, reached 40.59%. 3.5. Modeling the Crystallization Kinetics of PHB The crystallization of PHB is well described by Avrami’s Equation (3) for isothermal crystallization, and is con- veniently rewritten in linear form (5) [21,28,29]: logln 1loglog X kn t (5) 500 nm 500 nm (a) (b) Figure 4. TEM images of PHB granules. (a) heated in acidic solution (pH 2) at 120˚C for 1 hr; (b) suspended in alkaline solution (pH 12) at room temperature for 1 hr. The bar is 500 nm. Referring to Figure 2(b), the primary crystallization stage (2 - 15 min) of pure PHB can be fit to Avrami’s equation by plotting logln 1 X versus log t. Figure 5 contains a log-log plot for the crystallization of pure PHB from melt. Numerical values of Avrami’s ex- ponent and the growth rate parameter were obtained from the slope and intercept to be n = 1.5010 and k = 0.0118, respectively. When compared to published results, these values are close to the documented range (n = 1.58 – 1.92; k = 0.04 – 0.13) for the isothermal crystallization of neat PHB [31]. Similar to the crystallization of pure PHB, the in situ crystallization of PHB granules subjected to heating or varying pH can be modeled by Avrami’s Equation (3), where X is the in situ crystallinity of the PHB granules and t is the period of time the granules are exposed to the different environmental conditions. Table 1 gives the values of n and k obtained from lin- ear correlations of the crystallinity data from the Avrami analysis of the PHB granules heated at 80˚C - 140˚C for 0 - 4 hr in acidic solution (pH 2). Table 1 also shows the measured in situ crystallinity of the granules after 4 hr of heating at each temperature. From observation it is ap- parent that Avrami’s exponent remains constant with temperature, so that n = 0.2140. Further analysis shows that the crystallization growth rate parameter increases with temperature and may follow the Arrhenius equation previously mentioned (4). Rewriting the Arrhenius equa- tion in linear form (6) [29,30]: 11 ln lna o E kk nRT (6) and plotting 1lnnk versus 1T, as seen in Figure 6, the pre-exponential factor and activation energy were found from the linear correlation to be o k = 78.73 min–1 and a E = 48.82 kJ/mol, respectively. When compared to the activation energy of polypropylene (163 kJ/mol),  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 306 Table 1. Crystallization of PHB granules heated in acidic solution (pH 2). T (˚C) X (%) after 4 hr n k (min–1) R2 25 4.89 --- --- --- 80 21.00 0.2159 0.0711 0.9915 100 23.26 0.1922 0.0904 0.9806 120 27.19 0.2018 0.1042 0.9274 140 35.54 0.2459 0.1200 0.9754 AVG --- 0.2140 --- --- STD --- 0.0234 --- --- Figure 5. Determination of the crystallization rate constant k and the Avrami exponent n for the crystallization of pure PHB at room temperature during primary crystallization (2 - 15 min). Figure 6. Crystallization growth rate parameter k as a function of temperature for heated PHB granules. the activation energy determined for the heated PHB granules (48.82 kJ/mol) is much lower, but of similar magnitude [29]. Table 2 contains the values of n and k obtained from linear correlations of the crystallinity data from the Avrami analysis of the PHB granules suspended in solu- tions of varying pH (2-12) for 0 - 4 hr at room tempera- ture. Table 2 also shows the in situ crystallinity of the PHB granules averaged over the 4 hr of exposure time. The value of the Avrami exponent (n) was found to be around zero. As previously mentioned, raising the pH of the cell slurry caused the PHB granules to crystallize nearly instantaneously. Therefore, the in situ crystallinity was assumed to be independent of the exposure time. Figure 7 contains a plot of the term logln 1 X from Avrami’s equation versus pOH (= 14 – pH). From the log plot in Figure 7, it is apparent that the PHB crys- tallinity is linearly correlated to the pOH of the cell slurry, such that: logln 1pOHX (7) where the constants = –0.0891 and = –0.1219. Then combining (5) and (7) with the observation that N = 0, the growth rate parameter is expressed as: 10 OHk (8) where OH is the concentration of hydroxide ions in the solution. No temperature dependence appears in this empirical relation because the pH of the granules was adjusted at a constant room temperature (~23˚C). Table 3 outlines the kinetics models describing the crystallization of pure PHB cooled from melt, PHB granules heated in acidic solution, and PHB granules of Table 2. Crystallization of PHB granules in solutions of varying pH at room temperature (~23˚C). pH X ± STD (%) n k (min–1) 2.00 6.99 ± 1.41 0.00 0.0645 3.55 7.61 ± 1.36 0.0886 9.30 26.70 ± 0.52 0.2881 11.06 28.43 ± 2.65 0.4133 11.80 41.14 ± 1.96 0.4811 12.12 41.25 ± 4.63 0.5137 Figure 7. Log plot illustrating the linearity of in situ crystal- lization versus pOH of PHB granules suspended in solutions of varying pH.  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 307 Table 3. Crystallization models of pure PHB and PHB granules in different environmental conditions by Avrami analysis. Pure PHB PHB Granules (Heat) PHB Granules (pH) Crystallinity 1exp n Xkt 1expn Xkt 1expn Xkt 1exp na o E kk R T 10 OHk Growth Rate Parameter 1exp na o E kk R T ko = 78.73 min–1; a E = 48.82 kJ/mol = –0.0891; = –0.1219 n 1.5010 0.2140 0 k (min–1) 0.0118 0.0725 - 0.1215a 0.0428 - 0.7553b R2 0.9761 0.9903 0.9727 aRange of k for temperatures 80˚C - 140˚C; bRange of k for pH 0 - 14. varying pH. Table 3 also includes numerical values of the empirical constants, Avrami exponents (n), and growth rate parameters (k) determined for each of the three cases. 4. Discussion 4.1. Stabilizing Factors of Amorphous PHB Granules According to the observation on dehydration of the na- tive PHB granules, removal of water from the granules, as well as, removal of lipids from the granule membranes induced some partial crystallization. It is widely accepted that PHB granules are fully amorphous in vivo having a crystallinity of 0% in a neutral culture medium (pH 7) [3, 10]. When partially isolated and/or subjected to varying environmental conditions, however, the PHB granules undergo varying degrees of in situ crystallization [10-12]. As expected, the mild acidic solution (pH 2) used to store the PHB-containing cells triggered very minuscule changes in crystallinity (~5%). It seems that the intra- granule water and granule membranes remained largely intact, stabilizing the native PHB granules in a mostly amorphous state. Furthermore, the crystallization that occurred due to excess dehydration during ATR- FTIR measurement and dehydration by freeze-drying the cells did not exceed 35%. Hence, the remaining granule mem- branes obviously kept the PHB granules from reaching a fully crystalline state (~60%). When dehydrated with acetone, on the other hand, the native granules became highly crystalline (~ 52%) in 24 hr, further suggesting that the lipids dissolved in acetone stabilized the native granules in a prior amorphous state. The crystallization of PHB is primarily driven by C – H ··· O hydrogen bonding between the C = O and CH3 groups in PHB [18,21,22]. Weakening the hydrogen bonds by melting or disrupting the hydrogen bonds with environmental impurities may inhibit the crystallization of PHB [10,13-15]. As noted, the amorphousness of na- tive PHB granules in vivo must be stabilized by the monolayer membrane surrounding the particles and in- tra-granule water [2,3,10]. The 5% - 10% of water pre- sent in the granules is thought to act as a plasticizer, forming hydrogen bonds with the C=O and CH3 groups of the biopolyester backbones [10]. These hydrogen bonds prevent strong intra- and intermolecular interac- tions from occurring between the C=O and CH3 groups in PHB [3,10,12]. Similarly, the granule membranes pre- vent strong interactions from occurring between PHB molecules in adjacent granules. Removal of the in- tra-granule water or the granule membrane promotes in situ crystallization due to increased C–H ··· O hydrogen bonding, which is analogous to the cooling crystalliza- tion process in pure PHB [10]. If there is very little or no granule crystallization, regions of particles without gran- ule membranes tend to aggregate. 4.2. Interpretation of Avrami Exponents and Growth Rate Parameters Table 3 contains the equations and empirical constants determined from the Avrami analyses for the crystalliza- tion of pure PHB, PHB granules heated in acidic solution (pH 2), and PHB granules in solutions of varying pH at room temperature. As previously shown, Avrami’s equa- tion can be used to describe the crystallization of pure PHB, as well as, the in situ crystallization of native PHB granules in different environmental conditions. Differ- ences in the Avrami exponents (n) and the growth rate parameters (k) for each condition provide the insight necessary to understand the different crystallization proc- esses. To help visualize the geometric nature of crystal- lization, it is useful to introduce a theoretical size pa- rameter (w) describing crystal growth: n s r wr (9)  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 308 and the critical nuclei radii (cr r) required to overcome the free energy barrier for nucleation [23,24]: 2 cr v rG (10) where the average radii of the nuclei and corresponding spherulites are n r and s r, and the surface and volumet- ric energies associated with nucleation are and v G , respectively. The Avrami exponent (n) is a measure of the geomet- ric constraints associated with isothermal crystallization, describing the type of crystal growth (i.e., geometry of spherulites) and the nature of nucleation (i.e., number and size of nucleation points). A large Avrami exponent (1n) signifies relatively large spherulite growth from infinitesimal, point-source nuclei (0w), as seen in the observation of pure PHB where n = 1.5010. Because pure PHB is a neat homopolymer, it undergoes favorable, homogeneous nucleation, in which volumetric energy dominates, so that ncr rr [23,24]. A small Avrami exponent (1n), represents small spherulite growth from tiny, surface-source nuclei (01w), as seen in the heated granules where n = 0.2140 and the granules at high pH where n = 0. This heterogeneous nucleation phenomenon most likely occurs at interfacial surfaces created by the removal of non-PHB biomass and water, lowering the free energy barrier and allowing a greater number of smaller nuclei to crystallize, so that ncr rr. As the Avrami exponent approaches zero (0n), the size of spherulites may be restricted by material avail- ability (i.e., amount of PHB per granule) or the im- pingement of adjacent crystallites, and unable grow be- yond the size of the initial nuclei (1w). This behavior is seen in the granules at high pH where n = 0, which is indicative of nearly instantaneous, time independent crystal growth where crystalline regions are composed of several tiny crystallites roughly the size of individual nuclei. The growth rate parameter (k) is a measure associated with the frequency of nucleation (i.e., number of nuclei) and subsequent spherulitic crystal growth. That is, a ma- terial exhibiting a small growth rate parameter may crys- tallize from very few nucleation points into relatively large spherulites. Conversely, a material exhibiting a larger growth rate parameter may crystallize from several nucleation points into many tiny spherulites. As seen in Tables 1-3, the growth rate parameter is smallest for pure PHB where k = 0.0118 and increases with increas- ing temperature (0.1215k) and increasing pH (0.7553k). This suggests that, although the average growth rate of an individual spherulite of PHB may not differ in varying environments, the overall crystallization growth rate of PHB is affected by the frequency of nu- cleation; which, in pure PHB is significantly less than that of the heated granules and the granules at high pH. The growth rate parameter describing the heated granules increases with temperature most likely because hetero- geneous nucleation is more favorable at higher tempera- tures. The granules at high pH, on the other hand, show increasing growth rate parameters with increasing pH because the removal of lipids and proteins from the granules create surfaces where heterogeneous nucleation is more favorable. Referring to Figure 4, the heated granules were generally less than 2 μm in diameter (Fig- ure 4(a)), while the granules at high pH were generally less than 0.5 μm in diameter (Figure 4(b)). The relative size of the PHB granules is dependent on the extent of particle aggregation, which occurs only at regions where PHB is amorphous and intermolecular entanglement can easily take place. With more surface area favorable for heterogeneous nucleation (ncr rr) than a sample of pure PHB, the frequency of nucleation in smaller PHB gran- ules is much greater, resulting in larger values of the growth rate parameter. 5. Conclusions The in situ crystallization of PHB in its native form is similar to that of pure PHB and can be modeled by Avrami’s equation under isothermal conditions. The growth rate parameter (k) is dependent on the crystalliza- tion environment (i.e., temperature and pH) and de- scribes the frequency of nucleation and subsequent spherulitic crystal growth. The Avrami exponent (n) is dependent on the geometric nature of crystallization and is related to the number and size of nucleation points and spherulites. In summary, the empirical values of n and k increase and decrease respectively, with the amount of available material (i.e., amount of PHB per crystal/granule) for nucleation and crystal growth. Pure PHB crystallized into large spherulitic structures from homogeneous nuclei (n = 1.5010) at a low growth rate (k = 0.0118). PHB gran- ules heated (80 - 140˚C) in acidic solution (pH 2) crystal- lized into partial spherulitic structures from heterogene- ous nuclei (n = 0.2140) at moderate growth rates (k = 0.0725 - 0.1215). PHB granules suspended in solutions of increasing pH (2-12) at room temperature crystallized instantaneously from heterogeneous nuclei at granule surfaces (n = 0) and high growth rates (k = 0.0428- 0.7553). 6. Acknowledgements This work is partially supported by the Consortium for Plant Biotechnology Research (CPBR) and the U.S. De- partment of Energy.  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 309 REFERENCES [1] W. D. Luzier, “Materials Derived from Biomass/Biode- Gradable Materials,” Proceedings of the National Acad- emy of Sciences of the United States of America, Vol. 89, No. 3, 1992, pp. 839-842. [2] A. J. Anderson and E. A. Dawes, “Occurrence, Metabo- lism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates,” Microbiological Reviews, Vol. 54, No. 4, 1990, pp. 450-472. [3] G. N. Barnard and J. K. M. Sanders, “The Poly-β-Hy- droxybutyrate Granule in Vivo: A New Insight Based on Nmr Spectroscopy of Whole Cells,” Journal of Biological Chemistry, Vol. 264, No. 6, 1989, pp. 3286-3291. [4] S. Y. Lee, “Bacterial Polyhydroxyalkanoates,” Biotech- nology and Bioengineering, Vol. 49, No. 1, 1996, pp. 1- 14. [5] T. V. Ojumu, J. Yu and B. O. Solomon, “Production of Polyhydroxyalkanoates, a Bacterial Biodegradable Poly- mer,” African Journal of Biotechnology, Vol. 3, No. 1, 2004, pp. 18-24. [6] Y. Chen, J. Chen, C. Yu, G. Du and S. Lun, “Recovery of Poly-3-Hydroxybutyrate from Alcaligenes Eutrophus by Surfactant-Chelate Aqueous System,” Process Biochem- istry, Vol. 34, No. 2, 1999, pp. 153-157. [7] J.-I. Choi and S. Y. Lee, “Efficient and Economical Re- covery of Poly(3-Hydroxybutyrate) from Recombinant Escherichia Coli by Simple Digestion with Chemicals,” Biotechnology and Bioengineering, Vol. 62, No. 5, 1999, pp. 546-553. [8] J. S. Herron, J. D. King and D. C. White, “Recovery of Poly-β-Hydroxybutyrate from Estuarine Microflora,” Ap- plied and Environmental Microbiology, Vol. 35, No. 2, 1978, pp. 251-257. [9] S. K. Hahn, Y. K. Chang and S. Y. Lee, “Recovery and Characterization of Poly(3-Hydroxybutyric Acid) Synthe- sized in Alcaligenes Eutrophus and Recombinant Es- cherichia coli,” Applied and Environmental Microbiology, Vol. 61, No. 1, 1995, pp. 34-39. [10] K. Sudesh, H. Abe and Y. Doi, “Synthesis, Structure And Properties of Polyhydroxyalkanoates: Biological Polyes- ters,” Progress in Polymer Science, Vol. 25, No. 10, 2000, pp. 1503-1555. [11] E. S. Stuart, A. Tehrani, H. E. Valentin, D. Dennis, R. W. Lenz and R. C. Fuller, “Protein Organization on the PHA Inclusion Cytoplasmic Boundary,” Journal of Biotechno- logy, Vol. 64, No. 2-3, 1998, pp. 137-144. [12] D. M. Horowitz and J. K. M. Sanders, “Amorphous, Biomimetic Granules of Polyhydroxybutyrate: Preparation, Characterization, and Biological Implications,” Journal of the American Chemical Society, Vol. 116, No. 7, 1994, pp. 2695-2702. [13] P. J. Barham, A. Keller, E. L. Otun and P. A. Holmes, “Crystallization and Morphology of a Bacterial Thermo- plastic: Poly-3-Hydroxybutyrate,” Journal of Materials Science, Vol. 19, No. 9, 1984, pp. 2781-2794. [14] L. M. W. K. Gunaratne, R. A. Shanks and G. Amaras- inghe, “Thermal History Effects on Crystallisation and Melting of Poly(3-Hydroxybutyrate),” Thermochimica Acta, Vol. 423, No. 1-2, 2004, pp. 127-135. [15] D. S. Conti, M. I. Yoshida, S. H. Pezzin and L. A. F. Coelho, “Miscibility and Crystallinity of Poly(3-Hy- Dro- xybtyrate)/Poly(3-Hydroxybutyrate-co-3-Hydroxyl-Valer ate) Blends,” Thermochimica Acta, Vol. 450, 2006, pp. 61-66. [16] J. Cornibert and R. H. Marchessault, “Conformational Isomorphism. A General 21 Helical Conformation for Poly(Β-Alkanoates),” Macromolecules, Vol. 8, No. 3, 1975, pp. 296-305. [17] A. Padermshoke, Y. Katsumoto, H. Sato, S. Ekgasit, I. Noda and Y. Ozaki, “Melting Behavior of Poly(3-Hy- droxybutyrate) Investigated by Two-Dimensional Infrared Correlation Spectroscopy,” Spectrochimica Acta Part A, Vol. 61, No. 4, 2005, pp. 541-550. [18] H. Sato, R. Murakami, A. Padermshoke, F. Hirose, K. Senda, I. Noda and Y. Ozaki, “Infrared Spectroscopy Studies of CH···O Hydrogen Bondings and Thermal Be- havior of Biodegradable Poly(Hydroxyalkanoate),” Mac- romolecules, Vol. 37, No. 19, 2004, pp. 7203-7213. [19] W. D. J. Callister, “Materials Science and Engineering: An Introduction,” John Wiley & Sons, Inc., Hoboken, 2003. [20] P. J. Barham, “Nucleation Behaviour of Poly-3-Hydroxy- Butyrate ,” Journal of Materials Science, Vol. 19, No. 12, 1984, pp. 3826-3834. [21] J. Zhang, H. Sato, I. Noda and Y. Ozaki, “Conformation Rearrangement and Molecular Dynamics of Poly(3-Hy- droxybutyrate) During the Melt-Crystallization Process Investigated by Infrared and Two-Dimensional Infrared Correlation Spectroscopy,” Macromolecules, Vol. 38, No. 10, 2005, pp. 4274-4281. [22] T. Furukawa, H. Sato, R. Murakami, J. Zhang, Y.-X. Duan, I. Noda, S. Ochiai and Y. Ozaki, “Structure, Dis- persibility, and Crystallinity of Poly(Hydroxybutyrate)/ Poly(L-Lactic Acid) Blends Studied by FT-IR Micros- pectroscopy and Differential Scanning Calorimetry,” in Macromolecules, Vol. 38, No. 15, 2005, pp. 6445- 6454. [23] A. S. Myerson, “Molecular Modeling Applications In Crystallization,” Cambridge University Press, Cambridge, 1999. doi:10.1017/CBO9780511529610 [24] M. D. Ward, “Bulk Crystals To Surfaces: Combining X- Ray Diffraction and Atomic Force Microscopy to Probe the Structure and Formation of Crystal Interfaces,” Chemical Reviews, Vol. 101, No. 6, 2001, pp. 1697-1726. [25] K. Grage, A. C. Jahns, N. Parlane, R. Palanisamy, I. A. Rasiah, J. A. Atwood and B. H. A. Rehm, “Bacterial Poly- hydroxyalkanoate Granules: Biogenesis, Structure, and Po- tential Use as Nano-/Micro-Beads in Biotechnological and Biomedical Applications,” Biomacromolecules, Vol. 10, No. 4, 2009, pp. 660-669. [26] S. Bloembergen, D. A. Holden, G. K. Hamer, T. L. Bluhm and R. H. Marchessault, “Studies of Composition and Crystallinity of Bacterial Poly(β-Hydroxybutyrate- co-β-Hydroxyvalerate),” Macromolecules, Vol. 19, No.  Crystallization Kinetics of Poly(3-Hydroxybutyrate) Granules in Different Environmental Conditions Copyright © 2011 SciRes. JBNB 310 11, 1986, pp. 2865-2871. [27] A. Paermshoke, Y. Katsumoto, H. Sato, S. Ekgasit, I. Noda and Y. Ozaki, “Melting Behavior of Poly(3-Hy- droxybutyrate) Investigated by Two-Dimensional Infrared Correlation Spectroscopy,” Spectrochimica Acta Part A, Vol. 61, No. 4, 2005, pp. 541-550. [28] J. Xu, B.-H. Guo, R. Yang, Q. Wu, G.-Q. Chen and Z.-M. Zhang, “In situ FTIR Study on Melting and Crystalliza- tion of Polyhydroxyalkanoates,” Polymer, Vol. 43, No. 25, 2002, pp. 6893-6899. [29] J. Li, C. Zhou, G. Wang, Y. Tao, Q. Liu and Y. Li, “Iso- thermal and Nonisothermal Crystallization Kinetics of Elastomeric Polypropylene,” Polymer Testing, Vol. 21, No. 5, 2002, pp. 583-589. [30] M. Liu, Q. Zhao, Y. Wang, C. Zhang, Z. Mo and S. Cao, “Melting Behaviors, Isothermal and Non-Isothermal Crys- tallization Kinetics of Nylon 1212,” Polymer, Vol. 44, No. 8, 2003, pp. 2537-2545. [31] Y. An, L. Dong, L. Li, Z. Mo and Z. Feng, “Isothermal Crystallization Kinetics and Melting Behavior of Poly(Β- Hydroxybutyrate)/Poly(Vinyl Acetate) Blends,” Euro- pean Polymer Journal, Vol. 35, No. 3, 1999, pp. 365-369. |

