Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 346-354 doi:10.4236/msa.2011.25045 Published Online May 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Jari Vartiainen, Ali Harlin VTT Technical Research Centre of Finland, Espoo, Finland. Email: jari.vartiainen@vtt.fi Received March 17th, 2011; revised March 25th, 2011; accepted March 29th, 2011. ABSTRACT Chitosan-nanoclay bio-hybrid films were successfully crosslinked with glutaraldehyde, genipin and glyoxal. Moisture sensitivity of films decreased as a result of crosslinking which led to improved barrier properties against water vapor and oxygen. Films containing chitosan (6.6 g/m2) with genipin (3.3 g/m2) and nanoclay (6.6 g/m2) had water vapor transmission rate of 72 g × 100 µm/(m2 × 24 h) which was 34% lower as compared to pure chitosan and 30% lower as compared to chitosan/nanoclay without crosslinkers. Glyoxal induced crosslinking resulted in 92% reduction in oxygen transmission rate at 80% relative humidity as compared to pure chitosan films. Oxygen transmission through glyoxal (3.3 g/m2) treated chitosan/nanoclay film was 2.8 cm3 × 100 µm/( m2 × 24 h) which was 53% lower as compared to chi- tosan/nanoclay without crosslinkers. In addition, nanoclay and especially glyoxal crosslinking prevented the water va- por sorption of chitosan considerably. Crosslinking may be used as an efficient tool for enhancing the exploitability of naturally hydrophilic biopolymers towards new high-value applications, such as food packaging. Keywords: Chitosan, Nanoclay, Crosslinking, Barrier, Packaging, Glutaraldehyde, Genipin, Glyoxal 1. Introduction Green economy, also referred to as biobased economy, utilizes biomass derived raw materials for high-volume applications, such as packaging. Barrier properties are extremely important for biobased food packaging mate- rials as both gas and water vapor transmission through packaging reduce the quality of food resulting shorter shelf-lives, increased costs and eventually more waste. Nanoclays (or nanolayered silicates) such as hectorite, saponite and montmorillonite are promising additives with high aspect ratio and surface area [1-3]. Due to their unique platelet-like structure nanoclays have been widely studied as regards the barrier properties. Chitosan is a polysaccharide prepared by the N-deacetylation of chitin, the second most abundant natural biopolymer after cel- lulose. As chitosan is both hydrophilic and cationic in acidic conditions, it has usually good miscibility with negatively charged nanoclays. Chitosan chains may eas- ily intercalate into the clay interlayer by means of catio- nic exchange [4]. Chitosan/layered silicate nanocompo- sites have been used to improve the barrier properties against oxygen, water vapor, grease and UV-light trans- mission as well as the mechanical, thermal and antimi- crobial properties [5-9]. Studies on the barrier properties of polymer/layered silicate nanocomposites have tradi- tionally focused on systems with low organoclay con- tents. Recently, it has been reported that nanoclays can produce substantial improvements in barrier properties over the full range of compositions even up to organoc- lay content of 80 vol% withou t loosing th e flexibility an d transparency [10]. Correspondingly, we have recently demonstrated an 88% improvement in the oxygen barrier properties of chitosan films in high humidity conditions with 67 wt% of nanoclay. The main goal of this work was to verify the effects of crosslinking on the barrier properties of chitosan in high humidity. Glutaraldehyde, genipin and glyoxal were selected as crosslinking agents due to their well-recognised efficiency in cross- linking chitosan [11-15]. 2. Experimental 2.1. Materials Chitosan was obtained from Fluka BioChemika (low-vis-  Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 347 cous with a molecular weight of 150 kDa) and hydro- philic bentonite nanoclay (Nanomer PGV) from Aldrich. According to manufacturer, nanoclay was untreated (no organic modification) hydrophilic clay (> 98% montmo- rillonite) with aspect ratio of 150 - 200. Glutaraldehyde, 25% solution, was obtained from Merck-Schuchardt, ge- nipin powder from Challenge Bioproducts and glyoxal, 40% solution, from Sigma-Aldrich. 2.2. Preparation of Nanocomposite Films 1% nanoclay was swelled in 50 mL of distilled water and dispersed using ultrasonification tip (Branson Digital Sonifier) for 10 min. The dispersion was added into 50 mL of 1% chitosan in 1% acetic acid, followed by soni- cation for 10 min. Finally, 0.25% or 0.025% crosslinker (glutaraldeh yde, g enip in or glyox al) w as d isso lv ed in 100 mL of ethanol and added under rigorous mixing. 15 mL of each solution was cast onto polystyrene Petri dish (Ø 8.5 cm) and dried at room temperature. The obtained films were peeled off from the Petri dishes and stored at room temperature and 50% relative hu midity before tests. The composition of the films can be found from Table 1. 2.3. Viscosity Viscosity increment of chitosan solutions after adding crosslinkers was measured using Brookfield Model DVIII Rheometer at 23˚C with spindel DIN87, model LV and rotation speed of 25 rpm. Table 1. Composition of films expressed as grammages of each component in film. Crosslinker Crosslinker/nanoclay/chitosan [g/m2] - 0/0/6.6 - 0/6.6/6.6 Glutaraldehyde 3.3/6.6/6.6 Glutaraldehyde 0.3/6.6/6.6 Glutaraldehyde 3.3/0/6.6 Glutaraldehyde 0.3/0/6.6 Genipin 3.3/6.6/6.6 Genipin 0.3/6.6/6.6 Genipin 3.3/0/6.6 Genipin 0.3/0/6.6 Glyoxal 3.3/6.6/6.6 Glyoxal 0.3/6.6/6.6 Glyoxal 3.3/0/6.6 Glyoxal 0.3/0/6.6 2.4. Scanning Electron Microscopy (SEM) Structures of pure nanoclay in sonicated dispersions were analyzed using scanning electron microscopy (SEM, LEO DSM 982 Gemini FEG-SEM). SEM samples of aqueous dispersions of pure nanoclay were prepared by spreading dispersions on a polyvinyl amine premodified silica surface using fast spinning (2800 rpm for 1 min). Typically, no conductive coating was applied on the spe- cimen prior SEM imaging. However, in some cases a thin layer (~10 nm) of platinum was sputter coated on the surface to improve conductivity and stability of the spe- cimen. The SEM analyses of the aqueous dispersions were conducted using electron energies of 1.0 kV and 2.0 kV [16]. 2.5. Color Hunter a- and b-values were measured using a colorime- ter (CR200 Minolta Chroma Meter, Minolta Camera Co., Osaka, Japan). The values indicate the color directions: +a (magenta), –a (green), +b (yellow) and –b (blue). Color values were determined randomly at three different positions on each film. 2.6. X-Ray Diffraction (XRD) X-ray diffraction was used to determine the interlayer distance of layered nanoclays and crosslinked chitosan nanocomposites. Interlayer distances were calculated by the Bragg’s equation: 2dsinθ = λ, where d is the interlay- er distance, 2θ is the diffraction angle and λ is the wave- length of the X-ray (λ = 1.542 Å). X-ray diffractograms were run from the samples using Philips X’Pert MPD diffractometer, powder method and Cu X-ray tube. 2.7. Water Contact Angle Water contact angle of the film surface was measured using CAM200 equipment (KSV Instruments, Finland) in test conditions of 23˚C and 50% relative humidity af- ter incubation for 2 s. 2.8. Water Vapor Transmission Water vapor transmission rates of the films were deter- mined gravimetrically using a modified ASTME-96 pro- cedure. Samples with a test area of 25 cm2 were mounted on a circular aluminium dish (H.A. Buchel V/H, A.v.d. Korput, Baarn-Holland 45 M-141), which contained wa- ter. Dishes were stored in test conditions of 23˚C and 50% relative humidity and weighed periodically until a constant rate of weight reduction was attained . 2.9. Oxygen Transmission Oxygen transmission measurements were performed with Oxygen Permeation Analyser Model 8001 (Systech In-  Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 348 struments Ltd. UK). The tests were carried out at 23˚C and 80% relative humidity. 2.10. Water Vapor Sorption Water vapor sorption isotherms were measured at 20˚C with a dynamic water vapor sorption device (DVS-1, Surface Measurement Systems, UK). The device con- tained a microbalance and a humidity-regulated sample module within a temperature controlled chamber. Hu- midity was controlled by mixing dry and saturated (with water vapor) nitrogen gases, which flowed through sam- ple and reference (with an empty pan) cells. The weight of the sample was recorded once a minute until the equi- librium was reached. 3. Results and Discussion Typically, the viscosity of the solutions increases as a function of crosslinking and molecular weight. The vis- cosity measurements indicated that after adding the cros- slinkers the chitosan solutions finally converted into a crosslinked gel [17]. Reaction between glutaraldehyde and amino groups of chitosan took place immediately whereas the crosslinking induced by glyoxal and espe- cially genipin proceeded considerably slower. In each case the final product formed gels with viscosity over the range of measurement (Table 2). Nanoclay was delivered as dry powder with particle size < 25 µm. According to manufacturer, the nanoclay was composed of high purity aluminosilicate minerals, intended for use as additive to hydrophilic po lymers such as polyvinylalcohols, polysaccharides and polyacrylic acids. When fully dispersed, the nanoclay was supposed to form nanocomposites with the host polymers. Hydro- philic nanoclay was dispersed using ultrasonic energy in aqueous suspension. Chitosan dissolved in 1% acetic acid was then added to the mixture for adsorption to the separated nanoclay sheets. After adding crosslinkers, the nanocomposite films were obtained upon drying. Thick- ness of the films varied between 8 and 15 µm. Acidic pH was necessary for the protonation of amino groups of Table 2. Viscosity (mPas) of 2% chitosan in 1% acetic acid with 1% crosslinker measured 1, 150 and 500 min after adding the crosslinker. Crosslinker 1 min 150 min 500 min 180 194 184 glutaraldehyde >10 000 (gelling) >10 000 (gelling) >10 000 (gelling) genipin 160 257 >10 000 (gelling) glyoxal 165 >10 000 (gelling) >10 000 (gelling) chitosan. Adsorption process was mainly controlled by a cationic exchange mechanism due to the coulombic inte- ractions between the positive amino groups of chitosan and the negative sites in the clay structure. Since chitosan contains amino and hydroxyl groups, it can form strong intermolecular hydrogen bonds with the silanol edges of the nanoclays, which leads to the strong affinity b etween the matrix and silicate layers [4]. As can be seen in Figure 1(a), dry nanoclay powder consisted of round particles with coarse and platelety surface. Diameters of nanoclay particles varied between 3 and 25 µm. By ultrasonic dispersing the nanoclay platelets were effectively ripped off and uniformly dis- tributed on the surface. Previous studies have demon- strated that nanoclay platelets could easily orient parallel to the surface of especially solution cast coatings [18,19]. The diameter of the intercalated nanoplatelets was < 400 nm (Figure 1( b)). Chitosan dissolved in acetic acid formed completely (a) (b) Figure 1. SEM images of (a) Typical nanoclay aggregates prior dispergation and (b) Spincoated nanoclay platelets after ultrasonic dispergation. 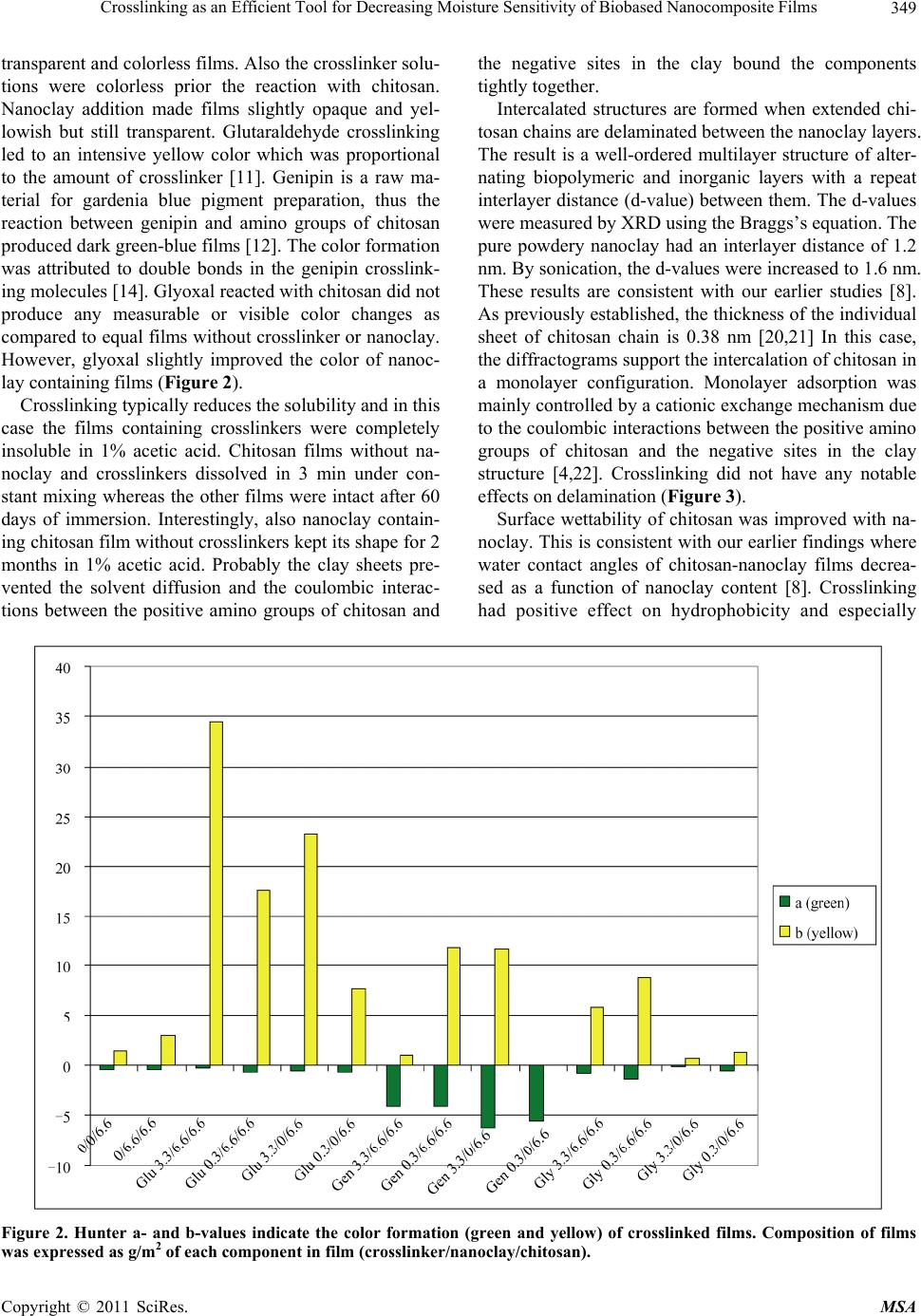 Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 349 transparent and colorless films. Also the crosslinker so lu- tions were colorless prior the reaction with chitosan. Nanoclay addition made films slightly opaque and yel- lowish but still transparent. Glutaraldehyde crosslinking led to an intensive yellow color which was proportional to the amount of crosslinker [11]. Genipin is a raw ma- terial for gardenia blue pigment preparation, thus the reaction between genipin and amino groups of chitosan produced dark green-blue films [12]. The color formation was attributed to double bonds in the genipin crosslink- ing molecules [14]. Glyoxal reacted with chitosan did not produce any measurable or visible color changes as compared to equal films without crosslinker or nanoclay. However, glyoxal slightly improved the color of nanoc- lay containing films (Figure 2). Crosslinking typically reduces the so lub ility and in this case the films containing crosslinkers were completely insoluble in 1% acetic acid. Chitosan films without na- noclay and crosslinkers dissolved in 3 min under con- stant mixing whereas the other films were intact after 60 days of immersion. Interestingly, also nanoclay contain- ing chitosan film without crosslinkers kept its shape for 2 months in 1% acetic acid. Probably the clay sheets pre- vented the solvent diffusion and the coulombic interac- tions between the positive amino groups of chitosan and the negative sites in the clay bound the components tightly together. Intercalated structures are formed when extended chi- tosan chains are delaminated between the nanoclay layers. The result is a well-ordered multilayer structure of alter- nating biopolymeric and inorganic layers with a repeat interlayer distance (d-value) between them. The d-values were measured by XRD using the Braggs’s equation. The pure powdery nanoclay had an interlayer distance of 1.2 nm. By sonication, the d-values were increased to 1.6 nm. These results are consistent with our earlier studies [8]. As previously established, the thick ness of the individual sheet of chitosan chain is 0.38 nm [20,21] In this case, the diffractograms support the intercalation of chitosan in a monolayer configuration. Monolayer adsorption was mainly controlled by a cationic exchange mechanism due to the coulombic interactions between the positive amino groups of chitosan and the negative sites in the clay structure [4,22]. Crosslinking did not have any notable effects on delam i nat i on (Figure 3). Surface wettability of chitosan was improved with na- noclay. This is consisten t with our earlier findings where water contact angles of chitosan-nanoclay films decrea- sed as a function of nanoclay content [8]. Crosslinking had positive effect on hydrophobicity and especially Figure 2. Hunter a- and b-values indicate the color formation (green and yellow) of crosslinked films. Composition of films was expressed as g/m 2 of each component in film (crosslinker/nanoclay/chitosan). 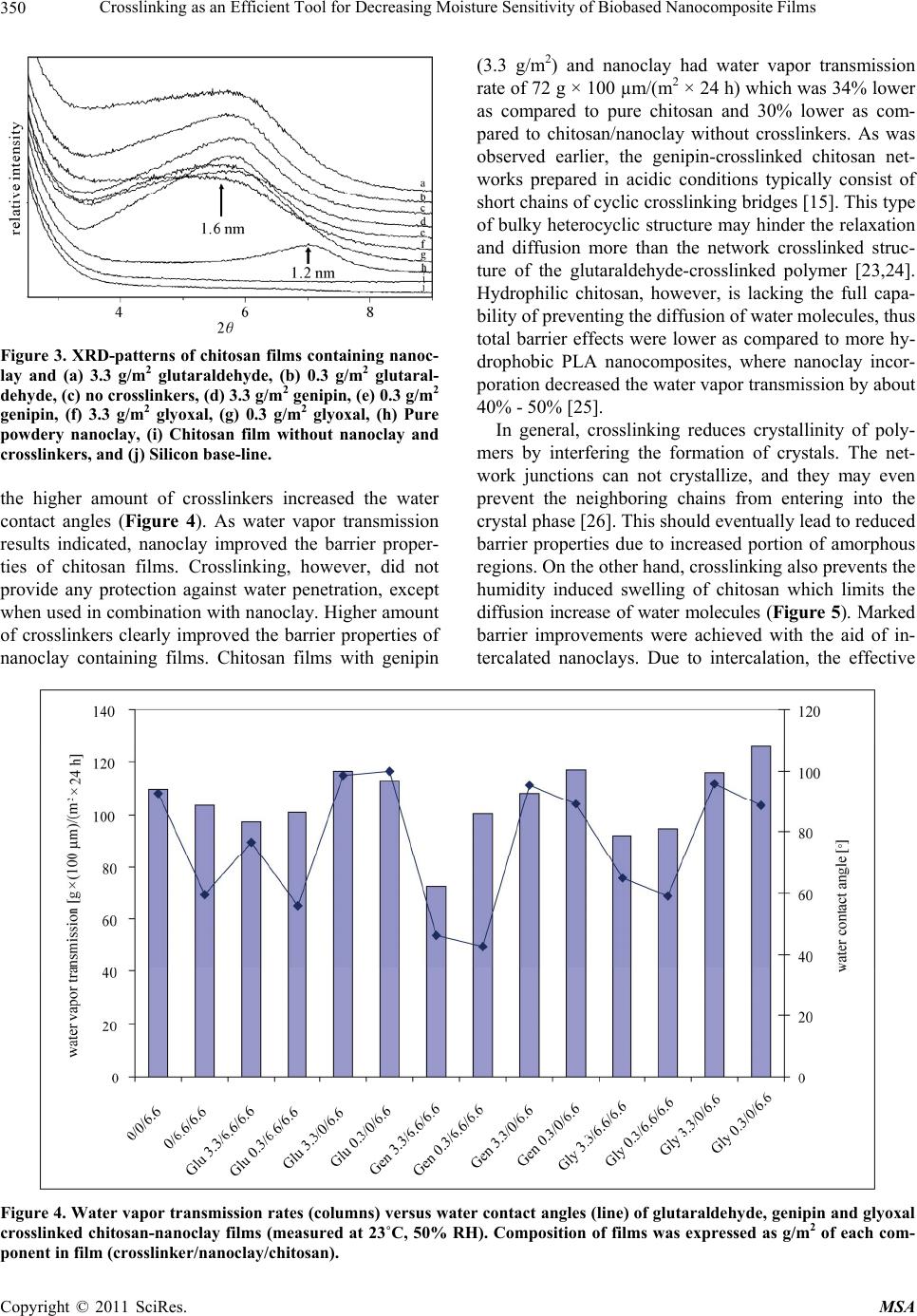 Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 350 Figure 3. XRD-patterns of chitosan films containing nanoc- lay and (a) 3.3 g/m2 glutaraldehyde, (b) 0.3 g/m2 glutaral- dehyde, (c) no crosslinker s, (d) 3.3 g/m2 genipin, (e ) 0.3 g/m2 genipin, (f) 3.3 g/m2 glyoxal, (g) 0.3 g/m2 glyoxal, (h) Pure powdery nanoclay, (i) Chitosan film without nanoclay and crosslinkers, and (j) Silicon base-line. the higher amount of crosslinkers increased the water contact angles (Figure 4). As water vapor transmission results indicated, nanoclay improved the barrier proper- ties of chitosan films. Crosslinking, however, did not provide any protection against water penetration, except when used in combination with nanoclay. Higher amount of crosslinkers clearly improved the barrier properties of nanoclay containing films. Chitosan films with genipin (3.3 g/m2) and nanoclay had water vapor transmission rate of 72 g × 100 µm/(m2 × 24 h) which was 34% lower as compared to pure chitosan and 30% lower as com- pared to chitosan/nanoclay without crosslinkers. As was observed earlier, the genipin-crosslinked chitosan net- works prepared in acidic conditions typically consist of short chains of cyclic crosslinking bridges [15]. This type of bulky heterocyclic structure may hinder the relaxation and diffusion more than the network crosslinked struc- ture of the glutaraldehyde-crosslinked polymer [23,24]. Hydrophilic chitosan, however, is lacking the full capa- bility of preventing the diffusion of water molecules, thus total barrier effects were lower as compared to more hy- drophobic PLA nanocomposites, where nanoclay incor- poration decreased the water vapor transmission by about 40% - 50% [25]. In general, crosslinking reduces crystallinity of poly- mers by interfering the formation of crystals. The net- work junctions can not crystallize, and they may even prevent the neighboring chains from entering into the crystal phase [26]. This should eventually lead to reduced barrier properties due to increased portion of amorphous regions. On the other hand, crosslinking also prevents the humidity induced swelling of chitosan which limits the diffusion increase of water molecules (Figure 5). Marked barrier improvements were achieved with the aid of in- tercalated nanoclays. Due to intercalation, the effective Figure 4. Water vapor transmission rates (columns) versus water contact angles (line) of glutaraldehyde, genipin and glyoxal crosslinked chitosan-nanoclay films (measured at 23˚C, 50% RH). Composition of films was expressed as g/m2 of each com- ponent in film (crosslinker/nanoclay/chitosan). 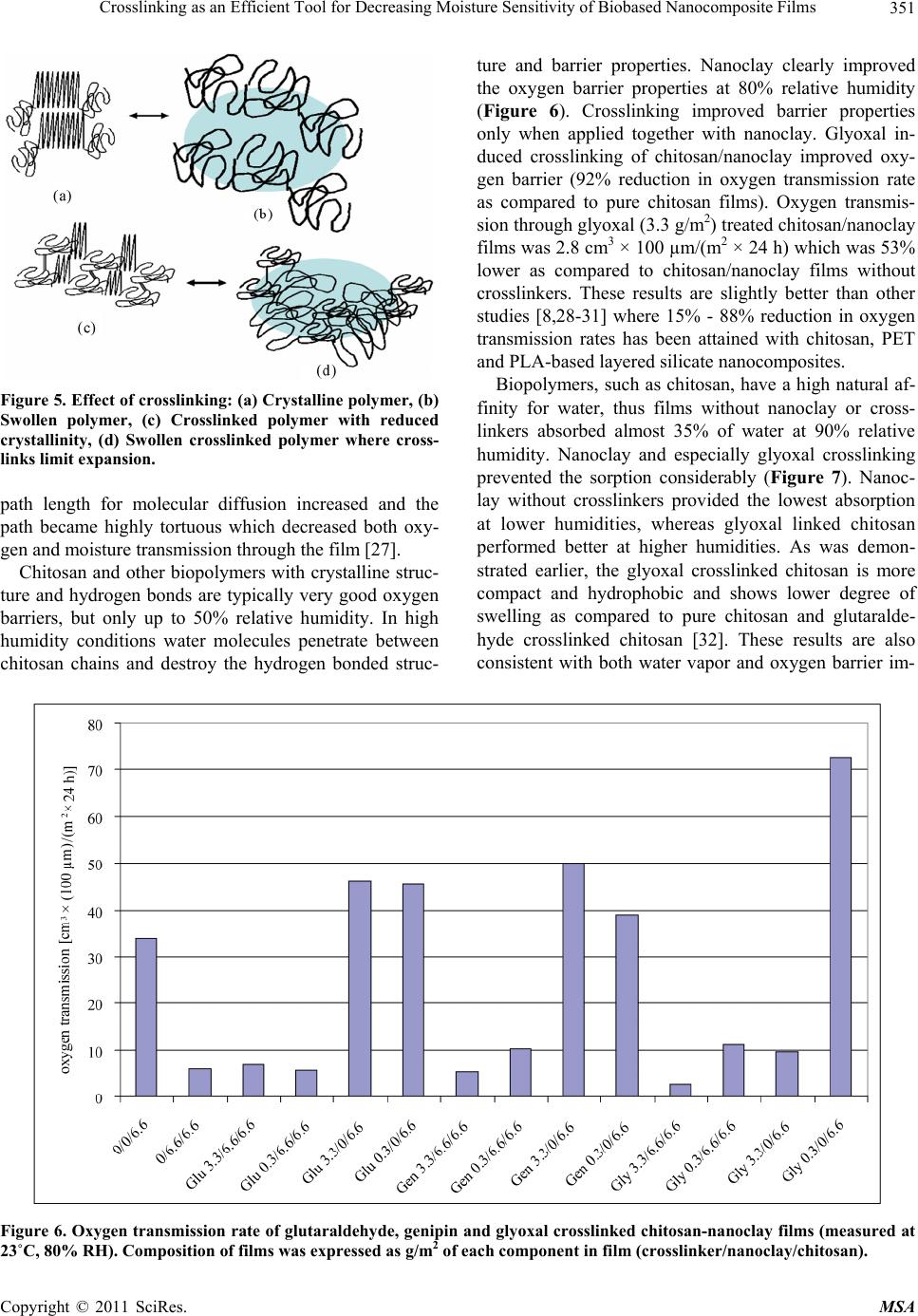 Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 351 Figure 5. Effect of crosslinking: (a) Crystalline polymer, (b) Swollen polymer, (c) Crosslinked polymer with reduced crystallinity, (d) Swollen crosslinked polymer where cross- links limit expansion. path length for molecular diffusion increased and the path became highly tortuous which decreased both oxy- gen and moisture transmission through the film [27]. Chitosan and other biopolymers with crystalline struc- ture and hydrogen bonds are typically very good oxygen barriers, but only up to 50% relative humidity. In high humidity conditions water molecules penetrate between chitosan chains and destroy the hydrogen bonded struc- ture and barrier properties. Nanoclay clearly improved the oxygen barrier properties at 80% relative humidity (Figure 6). Crosslinking improved barrier properties only when applied together with nanoclay. Glyoxal in- duced crosslinking of chitosan/nanoclay improved oxy- gen barrier (92% reduction in oxygen transmission rate as compared to pure chitosan films). Oxygen transmis- sion through glyoxal (3.3 g/m2) treated chitosan/nanoclay films was 2.8 cm3 × 100 µm/(m2 × 24 h) which was 53% lower as compared to chitosan/nanoclay films without crosslinkers. These results are slightly better than other studies [8,28-31] where 15% - 88% reduction in oxygen transmission rates has been attained with chitosan, PET and PLA-based layered silicate nanocomposites. Biopolymers, such as chitosan, have a high natural af- finity for water, thus films without nanoclay or cross- linkers absorbed almost 35% of water at 90% relative humidity. Nanoclay and especially glyoxal crosslinking prevented the sorption considerably (Figure 7). Nanoc- lay without crosslinkers provided the lowest absorption at lower humidities, whereas glyoxal linked chitosan performed better at higher humidities. As was demon- strated earlier, the glyoxal crosslinked chitosan is more compact and hydrophobic and shows lower degree of swelling as compared to pure chitosan and glutaralde- hyde crosslinked chitosan [32]. These results are also consistent with both water vapor and oxygen barrier im- Figure 6. Oxygen transmission rate of glutaraldehyde, genipin and glyoxal crosslinked chitosan-nanoclay films (measured at 23˚C, 80% RH). Composition of films was expressed as g/m2 of each component in film (crosslinker/nanoclay/chitosan). 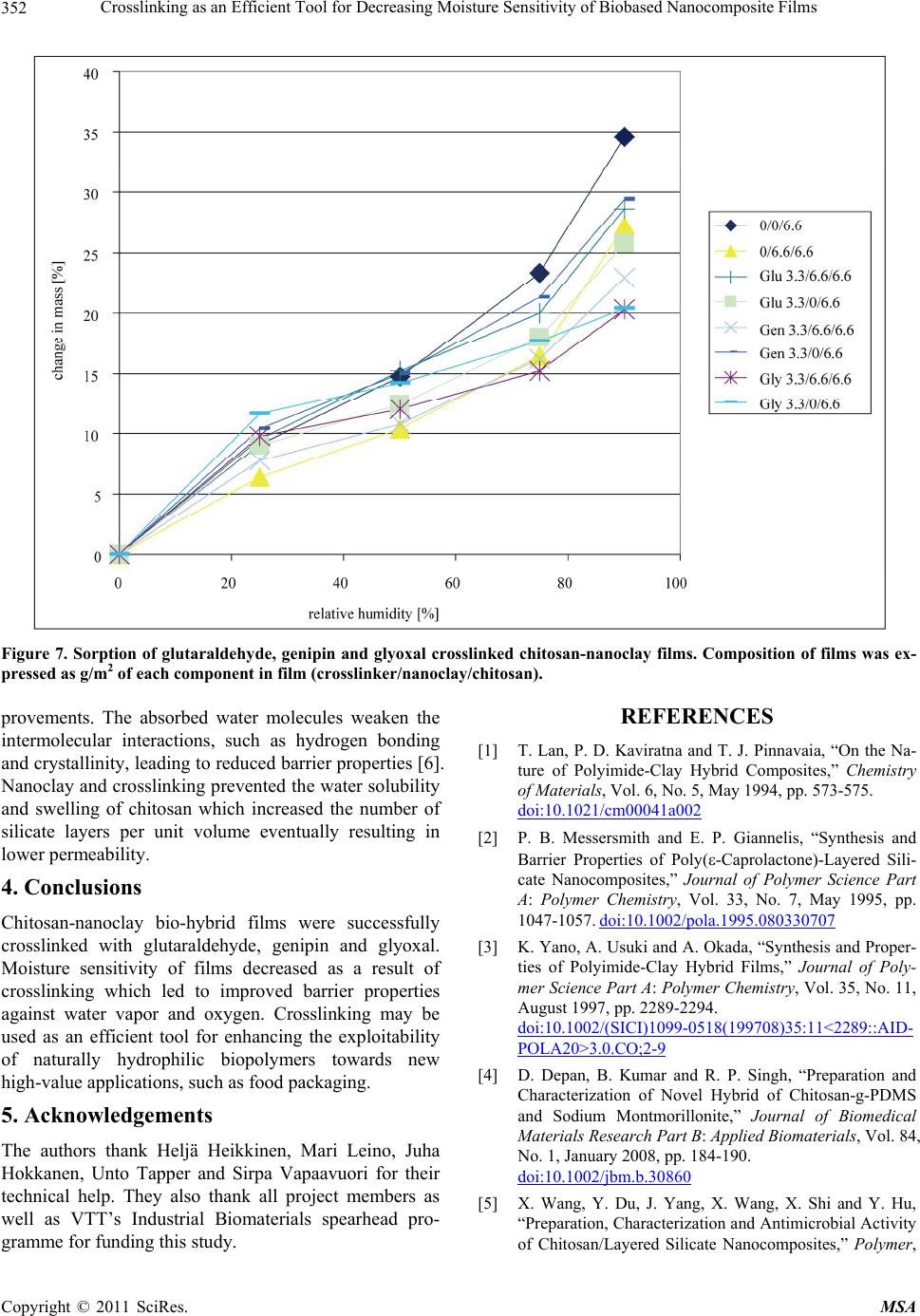 Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 352 Figure 7. Sorption of glutaraldehyde, genipin and glyoxal crosslinked chitosan-nanoclay films. Composition of films was ex- pressed as g/m2 of each component in film (crosslinker/nanoclay/chitosan). provements. The absorbed water molecules weaken the intermolecular interactions, such as hydrogen bonding and crystallinity, leading to reduced barrier properties [6 ]. Nanoclay and crosslinking p revented the water solubility and swelling of chitosan which increased the number of silicate layers per unit volume eventually resulting in lower permeability. 4. Conclusions Chitosan-nanoclay bio-hybrid films were successfully crosslinked with glutaraldehyde, genipin and glyoxal. Moisture sensitivity of films decreased as a result of crosslinking which led to improved barrier properties against water vapor and oxygen. Crosslinking may be used as an efficient tool for enhancing the exploitability of naturally hydrophilic biopolymers towards new high-value applications, such as food packaging. 5. Acknowledgements The authors thank Heljä Heikkinen, Mari Leino, Juha Hokkanen, Unto Tapper and Sirpa Vapaavuori for their technical help. They also thank all project members as well as VTT’s Industrial Biomaterials spearhead pro- gramme for funding this study. REFERENCES [1] T. Lan, P. D. Kaviratna and T. J. Pinnavaia, “On the Na- ture of Polyimide-Clay Hybrid Composites,” Chemistry of Materials, Vol. 6, No. 5, May 1994, pp. 573-575. doi:10.1021/cm00041a002 [2] P. B. Messersmith and E. P. Giannelis, “Synthesis and Barrier Properties of Poly(-Caprolactone)-Layered Sili- cate Nanocomposites,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 33, No. 7, May 1995, pp. 1047-1057. doi:10.1002/pola.1995.080330707 [3] K. Yano, A. Usuki and A. Okada, “Synthesis and Proper- ties of Polyimide-Clay Hybrid Films,” Journal of Poly- mer Science Part A: Polymer Chemistry, Vol. 35, No. 11, August 1997, pp. 2289-2294. doi:10.1002/(SICI)1099-0518(199708)35:11<2289::AID- POLA20>3.0.CO;2-9 [4] D. Depan, B. Kumar and R. P. Singh, “Preparation and Characterization of Novel Hybrid of Chitosan-g-PDMS and Sodium Montmorillonite,” Journal of Biomedical Materials Research Part B: Applied Biomaterials, Vol. 84, No. 1, January 2008, pp. 184-190. doi:10.1002/jbm.b.30860 [5] X. Wang, Y. Du, J. Yang, X. Wang, X. Shi and Y. Hu, “Preparation, Characterization and Antimicrobial Activity of Chitosan/Layered Silicate Nanocomposites,” Polymer,  Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 353 Vol. 47, No. 19, September 2006, pp. 6738-6744. doi:10.1016/j.polymer.2006.07.026 [6] C. Tang, N. Chen, Q. Zhang, K. Wang, Q. Fu and X. Zhang, “Preparation and Properties of Chitosan Nano- composites with Nanofillers of Different Dimensions,” Polymer Degradation and Stability, Vol. 94, No. 1, Janu- ary 2009, pp. 124-131. [7] X. Wang, Y. Du, J. Luo, B. Lin and J. F. Kennedy, “Chi- tosan/Organic Rectorite Nanocomposite Films: Structure, Characteristic and Drug Delivery Behaviour,” Carbohy- drate Polymers, Vol. 69, No. 1, May 2007, pp. 41-49. doi:10.1016/j.carbpol.2006.08.025 [8] J. Vartiainen, M. Tuominen and K. Nättinen, “Bio-Hybrid Nanocomposite Coatings from Sonicated Chitosan and Nanoclay, Journal of Applied Polymer Science, Vol. 116, No. 6, June 2010, pp. 3638-3647. [9] J. M. Lagarón and A. Fendler, “High Water Barrier Nanobiocomposites of Methyl Cellulose and Chitosan for Film and Coating Applications,” Journal of Plastic Film and Sheeting, Vol. 25, No. 1, January 2009, pp. 47-59. [10] E. Dunkerley and D. Schmidt, “Effects of Composition, Orientation and Temperature on the O2 Permeability of Model Polymer/Clay Nanocomposites,” Macromolecules, Vol. 43, No. 24, December 2010, pp. 10536-10544. doi:10.1021/ma1018846 [11] G. A. F. Roberts and K. E. Taylor, “Chitosan Gels, 3: The Formation of Gels by Reaction of Chitosan with Gluta- raldehyde,” Die Makromolekulare Chemie, Vol. 190, No. 5, May 1989, pp. 951-960. doi:10.1002/macp.1989.021900504 [12] R. A. A. Muzzarelli, “Genipin-Crosslinked Chitosan Hy- drogels as Biomedical and Pharmaceutical Aids,” Carbo- hydrate Polymers, Vol. 77, No. 22, pp. 1-9. [13] J. Z. Knaul, S. M. Hudson and K. A. M. Creber, “Cross- linking of Chitosan Fibers with Dialdehydes: Proposal of a New Reaction Mechanism,”Journal of Polymer Science Part B: Polymer Physics, Vol. 37, No. 1, June 1999, pp. 1079-1094. doi:10.1002/(SICI)1099-0488(19990601)37:11<1079::AI D-POLB4>3.0.CO;2-O [14] Y. Yuan, B. M. Chesnutt, G. Utturkar, W. O. Haggard, Y. Yang, J. L. Ong and J. D. Bumgardner, “The Effect of Cross-Linking of Chitosan Microspheres with Genipin on Protein Release,” Carbohydrate Polymers, Vol. 68, No. 3, April 2007, pp. 561-567. doi:10.1016/j.carbpol.2006.10.023 [15] F. L. Mi, S. S. Shy u and C. K. Peng, “Characteriz ation of Ring-Opening Polymerization of Genipin and pH-Depen- dent Cross-Linking Reactions Between Chitosan and Ge- nipin,” Journal of Polymer Science Part A: Polymer Che- mistry, Vol. 43, No. 10, May 2005, pp. 1985-2000. doi:10.1002/pola.20669 [16] J. Vartiainen, T. Tammelin, J. Pere, U. Tapper and A. Harlin, “Biohybrid Barrier Films from Fluidized Pectin and Nanoclay,” Carbohydrate Polymers, Vol. 82, No. 3, October 2010, pp. 989-996. doi:10.1016/j.carbpol.2010.06.031 [17] G. Kumar, J. F. Bristow, P. J. Smith and G. F. Payne, “Enzymatic Gelation of the Natural Polymer Chitosan,” Polymer, Vol. 41, No. 6, March 2000, pp. 2157-2168. doi:10.1016/S0032-3861(99)00360-2 [18] S. F. Wang, L. Shen, Y. J. Tong, L. Chen, I. Y. Phang, P. Q. Lim and T. X. Liu, “Biopolymer Chitosan/Mont- mo- rillonite Nanocomposites: Preparation and Characteriza- tion,” Polymer Degradation and Stability, Vol. 90, No. 1, October 2005, pp. 123-131. doi:10.1016/j.polymdegradstab.2005.03.001 [19] M. M. Malwitz, S. Lin-Gibson, E. K. Hobble, P. D. But- ler and G. Schmidt, “Orientation of Platelets in Multi- layered Nanocomposite Polymer Films,” Journal of Po- lymer Science Part B: Polymer Physics, Vol. 41, No. 24, December 2003, pp. 3237-3248. [20] G. L. Clark and A. F. Smith, “X-Ray Diffraction Studies of Chitin, Chitosan and Derivatives,” Journal of Physical Chemistry, Vol. 40, No. 7, 1936, pp. 863-879. doi:10.1021/j150376a001 [21] M. Darder, M. Colilla and E. Ruiz-Hitzky, “Biopoly- mer-Clay Nanocomposites Based on Chitosan Interca- lated in Montmorillonite,” Chemistry of Materials, Vol. 15, No. 20, October 2003, pp. 3774-3780. doi:10.1021/cm0343047 [22] M. Darder, M. Colilla and E. Ruiz-Hitzky, “Chitosan- Clay Nanocomposites: Application as Electrochemical Sensors,” Applied Clay Science, Vol. 28, No. 1-4, pp. 199- 208. [23] H.-C. Liang, W.-H. Chang, K.-J. Lin and H.-W. Sung, “Genipin-Crosslinked Gelatin Microspheres as a Drug Carrier for Intramuscular Administration: In vitro and in vivo studies,” Journal of Biomedical Materials Research Part A, Vol. 65A, No. 2, May 2003, pp. 271-282. doi:10.1002/jbm.a.10476 [24] J. D. Wind, S. M. Sirard, D. R. Paul, P. F. Green, K. P. Johnston and W. J. Koros, “Relaxation Dynamics of CO2 Diffusion, Sorption, and Polymer Swelling for Plasticized Polyimide Membranes,” Macromolecules, Vol. 36, No. 17, August 2003, pp. 6442-6448. doi:10.1021/ma034359u [25] C. Thellen, C. Orroth, D. Froio, D. Ziegler, J. Lucciarini, R. Farrell, N. A. D’souza and J. A. Ratto, “Influence of Montmorillonite Layered Silicate on Plasticized Poly (L-Lactide) Blown Films,” Polymer, Vol. 46, No. 25, November 2005, pp. 11716-11727. doi:10.1016/j.polymer.2005.09.057 [26] C. M. Roland and C. A. Aronson, “Crystallization of Polydimethylsiloxane End-Linked Networks,” Polymer Bulletin, Vol. 45, No. 4-5, December 2000, pp. 439-445. doi:10.1007/s002890070019 [27] O. O. Christopher and M. Lerner, “Nanocomposites and Intercalation Compound, Encyclopedia of Physical Sci- ence and Technology,” 3rd Edition, Academic Press, San Diego, 2001. [28] Z. Ke and B. Yongping, “Improve the Gas Barrier Prop- erty of PET Film with Montmorillonite by In Situ Inter- layer Poly merization,” Materials Letters, Vol. 59, No. 27, November 2005, pp. 3348-3351.  Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films Copyright © 2011 SciRes. MSA 354 doi:10.1016/j.matlet.2005.05.070 [29] J. Lange and Y. Wyser, “Recent Innovations in Barrier Technologies for Plastic Packaging—A Review,” Pack- aging Technology and Science, Vol. 16, No. 4, July/Au- gust 2003, pp. 149-158. [30] S. S. Ray, K. Yamada, M. Okamoto and K. Ueda, “New Polylactide-Layered Silicate Nanocomposites. 2. Concur- rent Improvements of Material Properties, Biodegradabil- ity and Melt Rheology,” Polymer, Vol. 44, No. 3, Febru- ary 2003, pp. 857-866. [31] J. H. Chang, Y. Uk-An and G. S. Sur, “Poly(Lactic Acid) Nanocomposites with Various Organoclays. I. Thermo- mechanical Properties, Morphology, and Gas Permeabili- ty,” Journal of Polymer Science Part B: Polymer Physics, Vol. 41, No. 1, January 2003, pp. 94-103. doi:10.1002/polb.10349 [32] K. C. Gupta and F. H. Jabrail, “Glutaraldehyde and Glyoxal Cross-Linked Chitosan Microspheres for Con- trolled Delivery of Centchroman,” Carbohydrate Re- search, Vol. 341, No. 6, May 2006, pp. 744-756. doi:10.1016/j.carres.2006.02.003 |

