 International Journal of Organic Chemistry, 2011, 1, 15-19 doi:10.4236/ijoc.2011.12003 Published Online June 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC One-Pot Three-Component Synthesis of Imidazo[1,5-a]pyridines Abbas Rahmati*, Zahra Khalesi Department of Chemistry, University of Isfahan, Isfahan, Iran E-mail: a.rahmati@sci.ui.ac.ir Received March 3, 2011; revised April 8, 2 011; accepted April 16, 2011 Abstract A one-pot three component condensation synthesis of imidazo[1,5-a]pyridines using of various aromatic al- dehydes and dipyridil ketone with ammonium acetate in the presence of Lithium chloride as catalyst in good yields under microwave irradiation has been described. Keywords: Lithium Chloride, Imidazo[1,5-a]pyridine, Microwave Irradiation 1. Introduction Imidazo[1,5-a]pyridines and their derivatives with a nodal nitrogen atom, an important class of ring fused heterocyclic compounds exhibit a wide spectrum of bio- logical activities [1]. Therefore development of new and efficient synthetic method for the synthesis of these compounds is of importance in both synthetic organic chemistry and medicinal chemistry [2 ]. In addition, oth er applications of these compound in organic light-emitting diodes (OLEDs) [3] and organic thin-layer field effect transistors (FETs) [4] have been reported. The classical method for synthesis of imidazo[1,5-a] pyridines mainly involves the use of 2-pyr i dyla l kylamin e s with acylchlorides [5-15] and/or organic acids deriva- tives [16,17] in two step addition and cyclization. Be- sides these reactions, pereparation of imidazo[1,5-a] pyridines from 2-pyridylalkylamines with α,β-unsuturated compounds [18], and/or aldehydes [19] have been re- ported. Other approaches include the use of imine de- rivatives [20], 2-cyanopyridine [21] and benzotriazoles [22]. Recently, the generation these compounds by a three component condensation reaction using pyridilke- tons, aldehyds and ammonium acetate were reported [23-25]. Most syntheses require harsh reaction cond itions because of the use of highly sensitive reagents. In addi- tion, the syntheses of these heterocycles have been usu- ally carried out in polar organic solvents such as metha- nol, ethanol, DMF and DMSO leading to complex isola- tion and recovery procedures. These processes also gen- erate waste containing catalyst and solvent, which have to be recovered, treated and disposed of. During the course of our studies towards the develop- ment of new routes to the synthesis of nitrogen contain- ing heterocyclic compounds [26,27], we wish to intro- duce an efficient procedure for the synthesis of imi- dazo[1,5-a]pyridine via one-pot three component con- densation of dipyridyl ketone 1 with aldehyde 2 and NH4OAc in the presence of lithium chloride as an inex- pensive mild Lewis acid using microwave irradiation (Scheme 1). 2. Results and Discussion In synthesis of imidazo[1,5-a]pyridines, p-methoxyben- zaldehyde, dipyridyl ketone and ammonium acetate were selected as model reactants during the optimization con- ditions. Initially in order to, in this condensation reaction different solvents such as DMF, EtOH and HOAc in the presence and absen ce of LiCl salt were utili zed ( Ta bl e 1). Studies indicated that among the solvents, HOAc is the best solvent at 3 min (Table 1). However, the results of various times of irradiation show that in all solvents the best yield was obtained in during 3 min by medium power at 300 W. Then, the reactions were performed in the presence of different salts such as Li2SO4, Li2CO3, LiHSO4, Na2SO4, NaBr and NaCl under micr owave irra- diation in HOAc. Among the various salts, LiCl was identified to be the best salts for this three-component reaction in HOAc under the microwave irradiation as illustrated in Tab le 1 (Entry 10). In all cases molar ratio of reactant was used based on previous report [21]. After good consideration we found that these ratios are the best. 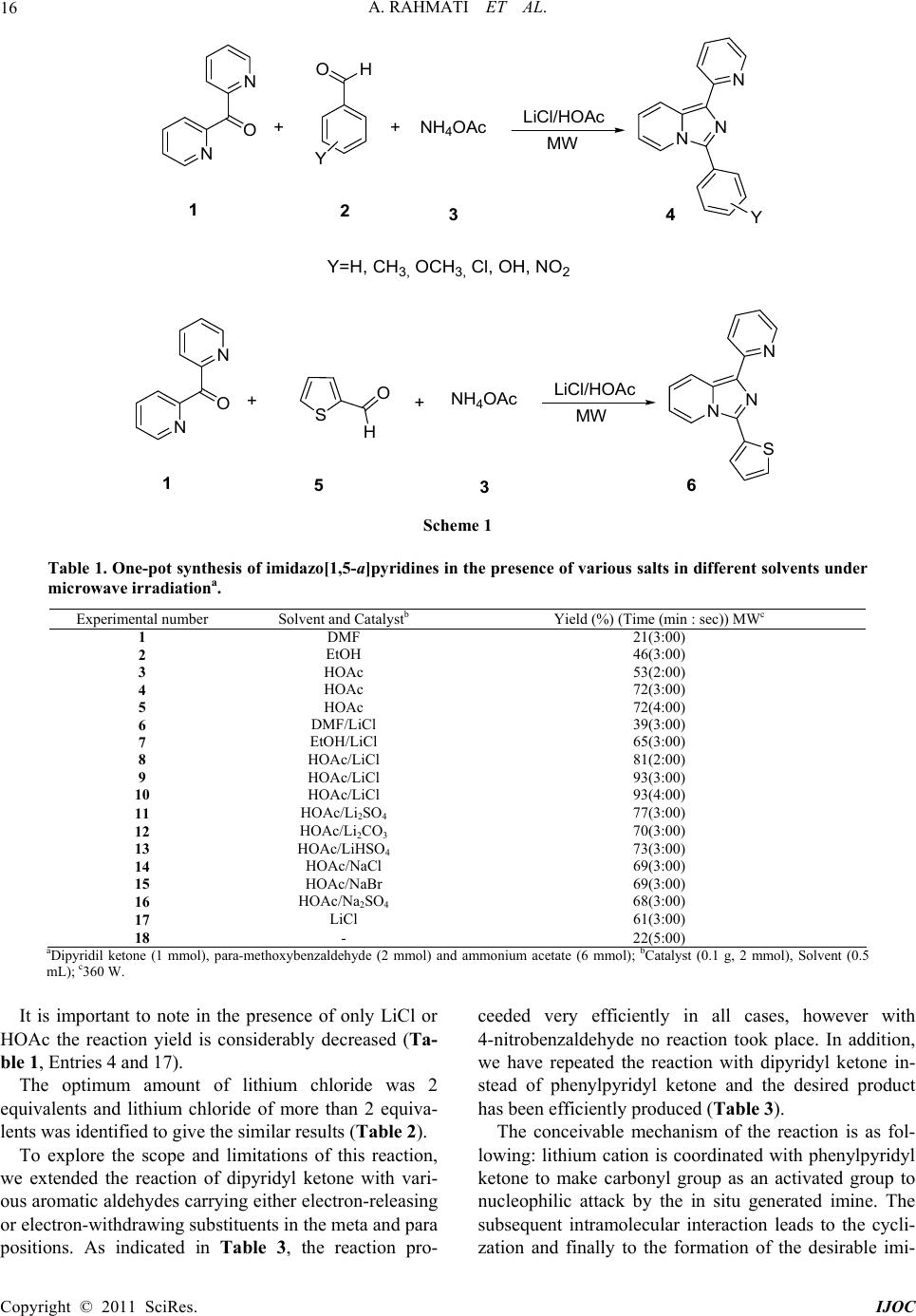 A. RAHMATI ET AL. 16 N O NO H Y ++ 12 NH 4 OAc NN N Y 34 LiCl/HOAc Y=H , CH 3, OCH3, Cl, OH, NO2 N O N ++ 15 NH 4 OAc NN N 3 LiCl/HOA c SO HS 6 MW MW Scheme 1 Table 1. One-pot synthesis of imidazo[1,5-a]pyridines in the presence of various salts in different solvents under microwave irradiationa. Experimental number Solvent and Catalystb Yield (%) (Time (m in : sec)) MWc 1 DMF 21(3:00) 2 EtOH 46(3:00) 3 HOAc 53(2:00) 4 HOAc 72(3:00) 5 HOAc 72(4:00) 6 DMF/LiCl 39(3:00) 7 EtOH/LiCl 65(3:00) 8 HOAc/LiCl 81(2:00) 9 HOAc/LiCl 93(3:00) 10 HOAc/LiCl 93(4:00) 11 HOAc/Li2SO4 77(3:00) 12 HOAc/Li2CO3 70(3:00) 13 HOAc/LiHSO4 73(3:00) 14 HOAc/NaCl 69(3:00) 15 HOAc/NaBr 69(3:00) 16 HOAc/Na2SO4 68(3:00) 17 LiCl 61(3:00) 18 - 22(5:00) aDipyridil ketone (1 mmol), para-methoxybenzaldehyde (2 mmol) and ammonium acetate (6 mmol); bCatalyst (0.1 g, 2 mmol), Solvent (0.5 mL); c360 W. It is important to note in the presence of only LiCl or HOAc the reaction yield is considerably decreased (Ta- ble 1, Entries 4 and 17). The optimum amount of lithium chloride was 2 equivalents and lithium chloride of more than 2 equiva- lents was identified to give the similar results (Table 2). To explore the scope and limitations of this reaction, we extended the reaction of dipyridyl ketone with vari- ous aromatic aldehydes carrying either electron-releasing or electron-withdrawing subs tituen ts in the meta and para positions. As indicated in Table 3, the reaction pro- ceeded very efficiently in all cases, however with 4-nitrobenzaldehyde no reaction took place. In addition, we have repeated the reaction with dipyridyl ketone in- stead of phenylpyridyl ketone and the desired product has been efficiently produced (Table 3). The conceivable mechanism of the reaction is as fol- lowing: lithium cation is coordinated with phenylpyridyl ketone to make carbonyl group as an activated group to nucleophilic attack by the in situ generated imine. The subsequent intramolecular interaction leads to the cycli- zation and finally to the formation of the desirable imi- Copyright © 2011 SciRes. IJOC  A. RAHMATI ET AL.17 Table 2. Optimization of amounts of LiCl in the one-pot synthesis of imidazo[1,5-a]pyridines in HOAc (0.5 mL) under microwave irradiationa. Experimental number Catalyst (mmol) LiCl Time (min) Yield (%) 1 - 3 22 2 0.5 mmol 3 39 3 1 mmol 3 65 4 2 mmol 3 93 5 2 mmol 2 62 5 2 mmol 1 34 6 4 mmol 3 93 aPyridilphenyl ketone (1 mmol), para-methylbenzaldehyde (2 mmol) and ammonium acetate (6 mmol). Table 3. One-pot synthesis of imidazo[1,5-a]pyridines in the presence of lithium chloride as a Lewis acid under solvent-free conditions using microwave irradiation.a Entry Aldehyde Product Yield (%) M.P. (˚C) Found Reportedb 1 O H O 4a 94 119 - 120 120 - 121 2 O H HO 4b 88 195 - 196 195 - 196 3 O H Me 2 N 4c 82 180 - 181 180 - 182 4 O H O 4d 83 94 - 96 92 - 93 5 O H O 2 N 4e 81 91 - 92 96 - 97 6 O H O HO 4f 89 129 - 131 132 - 133 7 S O H 6 71 127 - 130 131 - 132 a Using microwave irradiation HOAc/LiCl at 3 min; bref [25] . dazo[1,5-a]pyridine. (Scheme 2) In conclusion, we have introduced an efficient and en- vironmentally friendly approach for the synthesis of bio- logically active imidazo[1,5-a]pyridines via condensa- tion of dipyridyl ketone with various aromatic aldehydes and ammonium acetate using lithium chloride as a neu- tral Lewis acid catalyst. Corrosiveness safety, less waste and ease of separation are all among desirable factors for the chemical industry which we have considered in our green chemistry approach. 3. Experimental Melting points were measured with an Electrothermal 9100 apparatus and are uncorrected. IR spectra were re- corded with a Shimadzu IR-470 spectrometer. 1H and 13C NMR spectra were recorded with a BRUKER DRX-300 AVANCE spectrometer at 300.13 and 75.47 Copyright © 2011 SciRes. IJOC 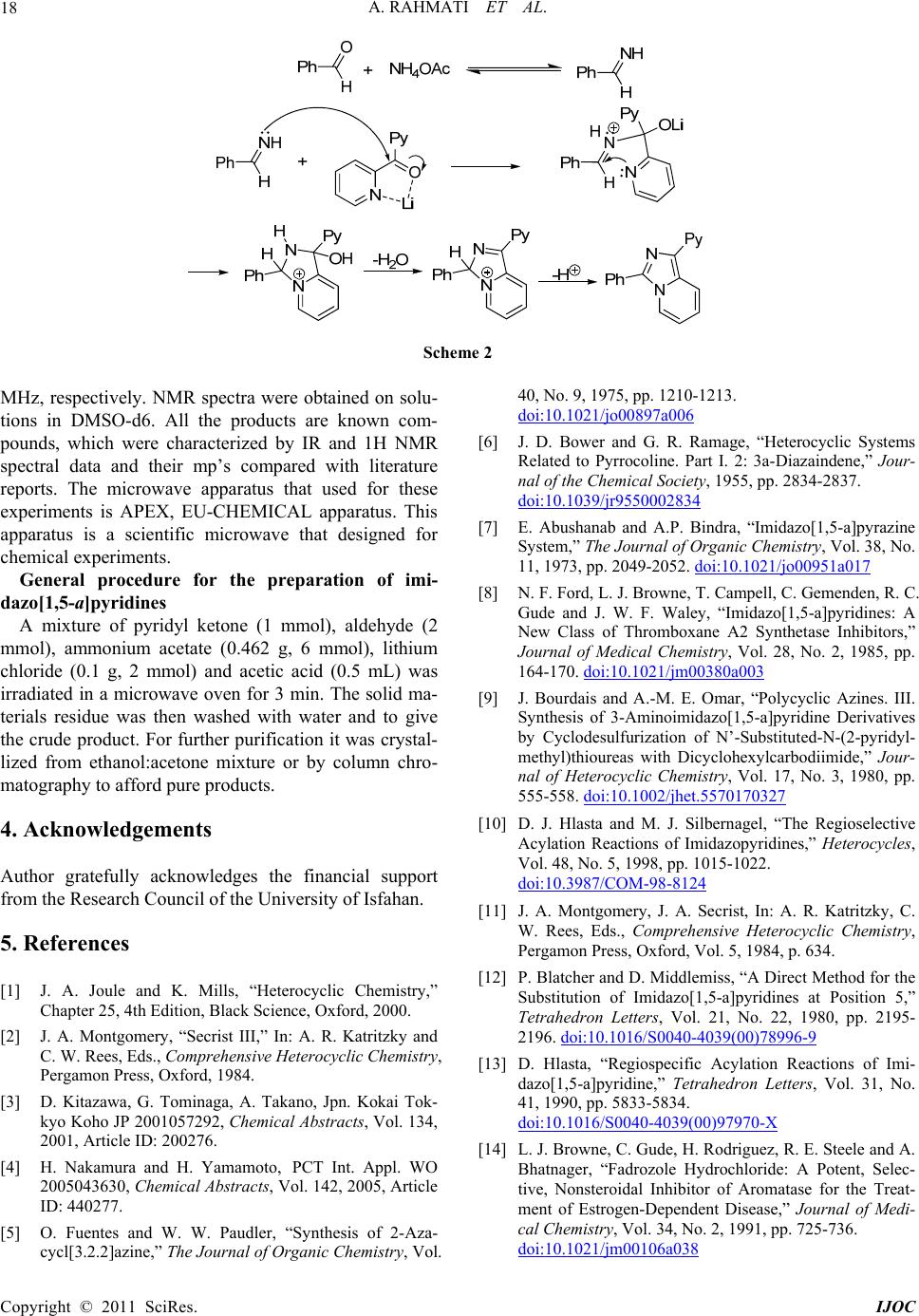 A. RAHMATI ET AL. 18 Scheme 2 MHz, respectively. NMR spectra were obtained on solu- tions in DMSO-d6. All the products are known com- pounds, which were characterized by IR and 1H NMR spectral data and their mp’s compared with literature reports. The microwave apparatus that used for these experiments is APEX, EU-CHEMICAL apparatus. This apparatus is a scientific microwave that designed for chemical experiments. General procedure for the preparation of imi- dazo[1,5-a]pyridines A mixture of pyridyl ketone (1 mmol), aldehyde (2 mmol), ammonium acetate (0.462 g, 6 mmol), lithium chloride (0.1 g, 2 mmol) and acetic acid (0.5 mL) was irradiated in a microwave oven for 3 min. The solid ma- terials residue was then washed with water and to give the crude product. For further purification it was crystal- lized from ethanol:acetone mixture or by column chro- matography t o aff or d p ure p ro duct s. 4. Acknowledgements Author gratefully acknowledges the financial support from the Research Council of the University of Isfahan. 5. References [1] J. A. Joule and K. Mills, “Heterocyclic Chemistry,” Chapter 25, 4th Edition, Black Science, Oxford, 2000. [2] J. A. Montgomery, “Secrist III,” In: A. R. Katritzky and C. W. Rees, Eds., Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxford, 1984. [3] D. Kitazawa, G. Tominaga, A. Takano, Jpn. Kokai Tok- kyo Koho JP 2001057292, Chemical Abstracts, Vol. 134, 2001, Article ID: 200276. [4] H. Nakamura and H. Yamamoto, PCT Int. Appl. WO 2005043630, Chemical Abstracts, Vol. 142, 2005, Article ID: 440277. [5] O. Fuentes and W. W. Paudler, “Synthesis of 2-Aza- cycl[3.2.2]azine,” The Journal of Organic Chemistry, Vol. 40, No. 9, 1975, pp. 1210-1213. doi:10.1021/jo00897a006 [6] J. D. Bower and G. R. Ramage, “Heterocyclic Systems Related to Pyrrocoline. Part I. 2: 3a-Diazaindene,” Jour- nal of the Chemical Society, 1955, pp. 2834-2837. doi:10.1039/jr9550002834 [7] E. Abushanab and A.P. Bindra, “Imidazo[1,5-a]pyrazine System,” The Journal of Organic Chemistry, Vol. 38, No. 11, 1973, pp. 2049-2052. doi:10.1021/jo00951a017 [8] N. F. Ford, L. J. Browne, T. Campell, C. Gemenden, R. C. Gude and J. W. F. Waley, “Imidazo[1,5-a]pyridines: A New Class of Thromboxane A2 Synthetase Inhibitors,” Journal of Medical Chemistry, Vol. 28, No. 2, 1985, pp. 164-170. doi:10.1021/jm00380a003 [9] J. Bourdais and A.-M. E. Omar, “Polycyclic Azines. III. Synthesis of 3-Aminoimidazo[1,5-a]pyridine Derivatives by Cyclodesulfurization of N’-Substituted-N-(2-pyridyl- methyl)thioureas with Dicyclohexylcarbodiimide,” Jour- nal of Heterocyclic Chemistry, Vol. 17, No. 3, 1980, pp. 555-558. doi:10.1002/jhet.5570170327 [10] D. J. Hlasta and M. J. Silbernagel, “The Regioselective Acylation Reactions of Imidazopyridines,” Heterocycles, Vol. 48, No. 5, 1998, pp. 1015-1022. doi:10.3987/COM-98-8124 [11] J. A. Montgomery, J. A. Secrist, In: A. R. Katritzky, C. W. Rees, Eds., Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxford, Vol. 5, 1984, p. 634. [12] P. Blatcher and D. Middlemiss, “A Direct Method for the Substitution of Imidazo[1,5-a]pyridines at Position 5,” Tetrahedron Letters, Vol. 21, No. 22, 1980, pp. 2195- 2196. doi:10.1016/S0040-4039(00)78996-9 [13] D. Hlasta, “Regiospecific Acylation Reactions of Imi- dazo[1,5-a]pyridine,” Tetrahedron Letters, Vol. 31, No. 41, 1990, pp. 5833-5834. doi:10.1016/S0040-4039(00)97970-X [14] L. J. Browne, C. Gude, H. Rodriguez, R. E. Steele and A. Bhatnager, “Fadrozole Hydrochloride: A Potent, Selec- tive, Nonsteroidal Inhibitor of Aromatase for the Treat- ment of Estrogen-Dependent Disease,” Journal of Medi- cal Chemistry, Vol. 34, No. 2, 1991, pp. 725-736. doi:10.1021/jm00106a038 Copyright © 2011 SciRes. IJOC  A. RAHMATI ET AL.19 [15] T. Benincori, E. Brenna and F. Sannicolo, “Studies on Wallach’s Imidaz ole Synthesis,” Journal of the Chemical Society, Perkin Transactions 1, No. 6, 1993, pp. 675-679. doi:10.1039/p19930000675 [16] F. Shibahara, A. Kitagawa, E. Yamaguchi and T. Murai, “Synthesis of 2-Azaindolizines by Using an Iodine-Me- diated Oxidative Desulfurization Promoted Cyclization of N-2-Pyridylmethyl Thioamides and an Investigation of Their Photophysical Properties,” Organic Letters, Vol. 8, No. 24, 2006, pp. 5621-5624. doi:10.1021/ol0623623 [17] D. Kim, L. Wang, J. J. Hale, C. L. Lynch, R. J. Budhu, M. MacCoss, S. G. Mills, L. Malkowitz, S. L. Gould, J. A. DeMartino, M. S. Springer, D. Hazuda, M. Miller, J. Kessler, R. C. Hrin, G. Carver, A. Carella, K. Henry, J. Lineberger, W. A. Schleif and E. A. Eminic, “Potent 1,3,4-Trisubstituted Pyrrolidine CCR5 Receptor Antago- nists: Effects of Fused Heterocycles on Antiviral Activity and Pharmacokinetic Properties,” Bioorganic and Me- dicinal Chemistry Letters, Vol. 15, No. 8, 2005, pp. 2129-2134. doi:10.1016/j.bmcl.2005.02.030 [18] M. E. Bluhm, M. Ciesielski, H. Görls and M. Döring, “Copper-Catalyzed Oxidative Heterocyclization by At- mospheric Oxygen,” Angewandte Chemie International Edition, Vol. 41, No. 16, 2002, pp. 2962-2965. doi:10.1002/1521-3773(20020816)41:16<2962::AID-AN IE2962>3.0.CO;2-6 [19] M. E. Bluhm, C. Folli, D. Pufky, M. Kröger, O. Walter and M. Döring, “3-Aminoiminoacrylate, 3-Aminoacrylate, and 3-Amidoiminomalonate Complexes as Catalysts for the Dimerization of Olefins Organometallics,” Vol. 24, No. 17, 2005, pp. 4139-4152. doi:10.1021/om049075s [20] A. P. Krapcho and J. R. Powell, “Syntheses of 1,3- Disubstituted Imidazo[l,5-a]pyridines,” Tetrahedron Let- ters, Vol. 27, No. 32, 1986, pp. 3713-3714. doi:10.1016/S0040-4039(00)83860-5 [21] F. Palacios, C. Alonso and G. Rubiales, “A New and Efficient Synthesis of Imidazo[1,5-a]pyridine Derivatives by a Tandem Aza-Wittig/Electrocyclic Ring Closure of N-Vinylic Phosphazenes,” Tetrahedron, Vol. 51, No. 12, 1995, pp. 3683-3690. doi:10.1016/0040-4020(95)00083-K [22] A. R. Katritzky, G. Qiu, “Efficient Syntheses of 1-Amido-3-aryl- and 1-Amido-3-alkylimidazo[1,5-a]pyri- dines,” The Journal of Organic Chemistry, Vol. 66, No. 8, 2001, pp. 2862-2864. doi:10.1021/jo0016632 [23] J. Wang, L. Dyers, R. Mason, P. Amoyaw and X. R. Bu, “Highly Efficient and Direct Heterocyclization of Dipyridyl Ketone to N,N-Bidentate Ligands,” The Jour- nal of Organic Chemistry, Vol. 70, No. 6, 2005, pp. 2353-2356. doi:10.1021/jo047853k [24] J. Wang, R. Mason, D. van Derveer, K. Feng and X. R. Bu, “Convenient Preparation of a Novel Class of Imi- dazo[1,5-a]pyridines: Decisive Role by Ammonium Acetate in Chemoselectivity,” The Journal of Organic Chemistry, Vol. 68, No. 13, 2003, pp. 5415-5481. doi:10.1021/jo0342020 [25] S. A. Siddiqui, T. M. Potewar, R. J. Lahoti and K. V. Srinivasan, “From the Methods in Organic Synthesis Da- tabase,” Synthesis, Vol. 127, 2006, pp. 2849-2854. doi:10.1055/s-2006-942522 [26] Rahmati, “Synthesis of 4-Aryl-3-methyl-6-oxo-4,5,6,7-te- trahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile via a One-Pot, Three-Component Reaction,” Tetrahedron Let- ters, Vol. 51, No. 22, 2010, pp. 2967-2970. doi:10.1016/j.tetlet.2010.03.109 [27] A. Rahmati, “A Regio- and Stereoselective Three-Com- ponent Synthesis of 5-(Trifluoromethyl)-4,5,6,7-tetrahy- dro-[1,2,4]triazolo[1,5-a]pyrimidine Derivatives under Solvent-Free Conditions,” Chemical Papers, Vol. 65, No. 4, 2011, pp. 536-541. doi:10.2478/s11696-011-0034-1 Copyright © 2011 SciRes. IJOC
|