 Atmospheric and Climate Sciences, 2011, 1, 19-32 doi: 10.4236/acs.2011.12003 Published Online April 2011 (http://www.SciRP.org/journal/acs) Copyright © 2011 SciRes. ACS An Examination of the Effects of Aero sol Chemical Composition and Size on Radiative Properties of Multi-Component Aerosols Shaocai Yu*, Yang Zhang Department of Marine, Earth, and Atmospheric Sciences, North Carolina State University, Raleigh, USA E-mail:yu.shaocai@epa.gov Received April 11, 2011; revised April 18, 2011; accepted April 19, 2011 Abstract The sensitivity of aerosol radiative properties (i.e., scattering coefficient, extinction coefficient, single scatter albedo, and asymmetry factor) and radiation transmission to aerosol composition, size distributions, and rela- tive humidity (RH) is examined in this paper. Mie calculations and radiation calculations using a tropo- spheric visible radiation model are performed. The aerosol systems considered include inorganic and organic ions (e.g., Cl-, Br-, 3 NO , , Na+, 2 4 SO 4 NH , K+, Ca2+, Mg2+, HCOO-, CH3COO -, CH3CH2COO -, CH3COCOO-, OOCCOO2-, MSA1-), and water-insoluble inorganic and organic compounds e.g., (black carbon, n-alkanes, SiO2, Al2O3, Fe2O3 and other organic compounds). The partial molar refraction method and the volume-average method are used to calculate the real and imaginary parts of refractive index of real aerosols, respectively. The sensitivity simulations show that extinction coefficient increases by 70% when RH varies from 0 to 80%. Both extinction coefficient and asymmetry factor increase by ~48% when real part varies from 1.40 to 1.65. Scattering coefficient and single scattering albedo decrease by 18% and 24%, re- spectively, when the imaginary part varies from –0.005 to –0.1. Scattering and extinction coefficients in- crease by factors of 118 and 123, respectively, when the geometric mean radius varies from 0.05 to 0.3 m. Scattering and extinction coefficients and asymmetry factor increase by factors of 389, 334, and 5.4, respec- tively, when geometric standard deviation varies from 1.2 to 3.0. The sensitivity simulations using a tropo- spheric visible radiation model show that the radiation transmission is very sensitive to the change in geo- metric mean radius and standard deviation; other factors are insignificant. Keywords: Radiative Properties, Sensitivity Study, Aerosol Composition, Aerosol Size Distribution, Multi-Component Aerosols 1. Introduction Atmospheric aerosols may influence the Earth’s radia- tion balance directly by backscattering and absorption of solar radiation, and indirectly by increasing cloud condensation nuclei (CCN) concentrations, which in turn increase cloud droplet concentrations and thus backscattering of solar radiation [1-3]. The IPCC [4] concluded that increasing concentrations of the long-lived greenhouse gases have led to a combined radiative forcing +2.63 [±0.26] Wm–2, and the total direct aerosol radiative forcing is estimated to be –0.5 [±0.4] Wm–2, with a medium-low level of scientific understanding, while the radiative forcing due to the cloud albedo effect (also referred to as the first indirect effect), is estimated to be –0.7 [–1.1, +0.4] W m–2, with a low level of scientific understanding. Evaluation of aerosol direct radiative forcing is complicated by the fact that aerosols are highly and non-uniformly distrib- uted over the Earth and comprise a variety of chemical species, and their abundance varies with particle size, location and time. As indicated by Penner et al. [5], one of central scientific questions related to the direct radia- tive influence of aerosols is how the aerosol composi- tion and size distributions affect the optical depth and radiative properties of aerosols, including dependence on relative humidity. Up to today the sensitivity of di- *Now at AMAD, NERL, U.S. EPA, RTP, NC 27711.  20 S. C. YU ET AL. rect aerosol forcing to chemical composition, size dis- tribution and relative humidity on a global scale has been tested with a “reference box model” [1] and a GCM model [6-8] Most of these studies except for Ja- cobson (2001) on direct aerosol forcing only focused on anthropogenic sulfate aerosols. The objectives of this paper are 1) to accurately calculate the refractive index of aerosol particles with the known chemical composi- tion of atmospheric aerosol; 2) to theoretically evaluate the sensitivity of aerosol radiative properties and radia- tion transmission in the visible range to refractive index, size distributions, and relative humidity (RH) using a box model that includes Mie and radiative transfer cal- culation. Since the aerosol particle refractive index is determined by its chemical composition, the depend- ence of radiative properties of aerosol particles on the refractive index can indicate the effects of chemical composition. Since most of the light scattering and ex- tinction are caused by particles in the accumulation mode size range (0.1 - 1.0 m, diameter), and these particles are neither removed effectively by impaction nor by diffusion, the accumulation mode particles are the most important one in terms of aerosol radiative forcing. In this study the sensitivity of aerosol radiative properties to size distribution is examined on the basis of the calculation of the particle radiative properties for the accumulation mode only. 2. Model Formulation 2.1. Atmospheric Aerosol Composition and Size Distribution Atmospheric aerosol particles are composed of complex mixtures of natural and anthropogenic chemical species that include 1) water-soluble inorganic and organic compounds such as sulfate, nitrate, formate and acetate, 2) water-insoluble inorganic and organic compounds such as black carbon, Al2O3 and n-alkanes, and 3) water itself. Soluble individual anion and cation concentrations of atmospheric aerosol are typically measured by Ion Chromatography (IC), and elements such as Al and Pb are determined by partially induced X ray emission (PIXE). On the other hand, the concentrations of insolu- ble high molecular weight organic compounds in aero- sols are measured by gas-chromatography-masss pec- trometer (GS/MS) method [9]. The IC and PIXE meth- ods provide no information on the concentrations of spe- cific salts or classes of inorganic and organic salts. The GC/MS method can quantify the concentrations of indi- vidual organic compounds in atmospheric particles. However, only about 10% of the total organic mass can be typically identified by the GC/MS method [9]. In gen- eral, the water-soluble materials within atmospheric aerosol particles are expected to be a mixture of different chemicals and the water soluble parts of aerosol particles are considered to be a mixture of electrolytes together with any other water-soluble material. There are possible interactions between those ions that do not commonly exist between chemical components, especially at high RH conditions [10]. For example, in a mixture of KNO3 and NaCl, there is a possible interaction between K+ and Na+. It is therefore reasonable to consider water-soluble parts of aerosol particles as a mixed solute, and aerosol particles at a dry state composed of mixed solute and insoluble substances. Since the composition of the aero- sol particles depends on the sources and subsequent transformation while airborne, it is possible to separate aerosols into urban, rural continental and marine aerosols in a first approximation [11]. Table 1 provides the esti- mates of refractive index of chemical components for the three atmospheric aerosol types. The concentrations of inorganic compounds (Cl-, Br-, 3, NO2 4 SO , Na+, 4 NH , K+, Ca2+, Mg2+, SiO2, Al2O3, and Fe2O3), total concentrations of organics, and total mass concentrations of aerosol particles for three aerosol types were taken from the estimates of Pueschel [11]. For organics, over 80 individual organic compounds found in aerosol parti- cles were identified and quantified previously [9]. In this study, the concentrations of soluble organic compounds (HCOO-, CH3COO-, CH3CH2COO-, CH3COCOO-, 2 CCOOOO , Methane sulfonic acid (MSA)) were taken from the estimates of Yu [2]. Chylek et al. [12] showed that the average black carbon (BC) atmospheric concen- tration in the continental air was 0.23 ± 0.04 g/m3 compared with 0.03 ± 0.01 g/m3 for the maritime air in the measurements over the southern Nova Scotia. Huntzicker et al. [13] indicated that the average BC con- centration for 26 cities in the United State was 3.8 g/m3. In this study, the BC concentrations used for urban, con- tinental, and marine aerosols are 3.8, 0.23 and 0.06 g/m3, respectively. The other organic compound con- centrations listed in Table 1 were taken from the esti- mates of Roggie et al. [9]. Table 2 lists the physical chemical and optical properties of various pure salts in atmospheric particles. For the aerosol model computation, a lognormal distribution function is suitable to charac- terize the size distribution of atmospheric aerosols [11]. Particles in the accumulation mode (0.1 - 1.0 m, aero- dynamic diameter) are the most important one in terms of aerosol radiative forcing. Table 3 lists the typical size distributions for three types of aerosol for the accumula- tion mode at a dry state compiled from literatures. As shown, the total number concentration, the geometric mean diameter (Dg), and the geometric standard devia- tion (g) for the accumulation mode are in the ranges of Copyright © 2011 SciRes. ACS 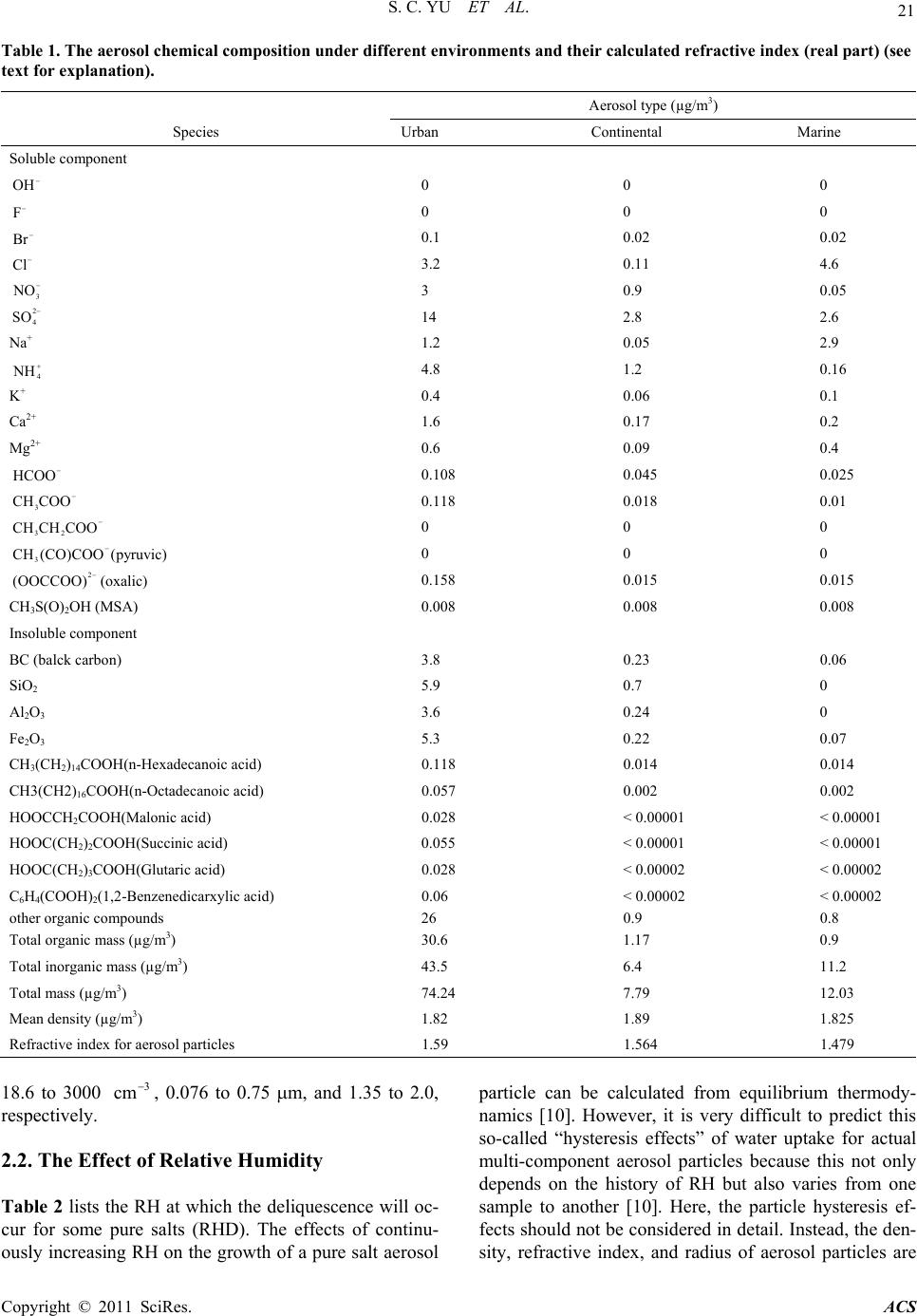 S. C. YU ET AL. Copyright © 2011 SciRes. ACS 21 Table 1. The aerosol chemical composition under different environments and their calculated refractive index (real part) (see text for explanation). Aerosol type (µg/m3) Species Urban Continental Marine Soluble component OH 0 0 0 F 0 0 0 Br 0.1 0.02 0.02 Cl 3.2 0.11 4.6 3 NO 2 3 0.9 0.05 4 SO 14 2.8 2.6 Na+ 1.2 0.05 2.9 4 NH 4.8 1.2 0.16 K+ 0.4 0.06 0.1 Ca2+ 1.6 0.17 0.2 Mg2+ 0.6 0.09 0.4 HCOO 0.108 0.045 0.025 3 CH COO 0.118 0.018 0.01 32 CH CHCOO 0 0 0 3 CH (CO)COO (pyruvic) 2 0 0 0 (OOCCOO) (oxalic) 0.158 0.015 0.015 CH3S(O)2OH (MSA) 0.008 0.008 0.008 Insoluble component BC (balck carbon) 3.8 0.23 0.06 SiO2 5.9 0.7 0 Al2O3 3.6 0.24 0 Fe2O3 5.3 0.22 0.07 CH3(CH2)14COOH(n-Hexadecanoic acid) 0.118 0.014 0.014 CH3(CH2)16COOH(n-Octadecanoic acid) 0.057 0.002 0.002 HOOCCH2COOH(Malonic acid) 0.028 < 0.00001 < 0.00001 HOOC(CH2)2COOH(Succinic acid) 0.055 < 0.00001 < 0.00001 HOOC(CH2)3COOH(Glutaric acid) 0.028 < 0.00002 < 0.00002 C6H4(COOH)2(1,2-Benzenedicarxylic acid) 0.06 < 0.00002 < 0.00002 other organic compounds 26 0.9 0.8 Total organic mass (µg/m3) 30.6 1.17 0.9 Total inorganic mass (µg/m3) 43.5 6.4 11.2 Total mass (µg/m3) 74.24 7.79 12.03 Mean density (µg/m3) 1.82 1.89 1.825 Refractive index for aerosol particles 1.59 1.564 1.479 18.6 to 3000 , 0.076 to 0.75 m, and 1.35 to 2.0, respectively. 3 cm 2.2. The Effect of Relative Humidity Table 2 lists the RH at which the deliquescence will oc- cur for some pure salts (RHD). The effects of continu- particle can be calculated from equilibrium thermody- namics [10]. However, it is very difficult to predict this so-called “hysteresis effects” of water uptake for actual multi-component aerosol particles because this not only depends on the history of RH but also varies from one sample to another [10]. Here, the particle hysteresis ef- fects should not be considered in detail. Instead, the den- ously increasing RH on the growth of a pure salt aerosol sity, refractive index, and radius of aerosol particles are  S. C. YU ET AL. 22 ffernt salts in atmospheric aerosol particles. RHD is the Index RHD(%)Salts Refractive Index Density RHD (%) Table 2. The physical-chemical and optical properties of di RH of deliquescence (see text for explanations). e Salts Refractive Density 3 (g cm) 3 (g cm) NH4O 1.Mg(CH)2(H2O)4 CH3CO 174 3COO 1.491 1.454 NH4Br 1.712 2.429 MgBr 3.724 .423 0 (H2O)5 1.456 3 SO4 1.521 0 )2(H2O)6 4 1.473 )2 .433 2O) .710 4 2O)6 1.460 3 2 3(H2O)2 OO)2 4 2O)2 4 OO 8 6 8 3(H2O)10 5.3 .320 4(H2O)2 3 4 .240 2O)10 O) H H2)14COOH 32 16COOH 1.acid 2 450 2 MgCO3 NH4HCO3 11.580 1.717 2.958 NH4Cl 1.642 1.527 8MgCO31.730 NH4F 1.009 MgCl2 1.675 2.320 NH4NO 1.725 62 MgCl2(H2O)6 1.495 1.569 (NH ) 4 2 NH4HSO 1.769 8Mg(NO3 MgSO4 1.636 1.780 40 1.560 2.660 Ca(CH COO 3 Ca(Br) 1.550 MgSO4(H2O)7 11.680 3.353 MgSO4(H 1.523 2.445 CaCO 3 CaCO3(H 1.658 2 KBr 1.559 2.750 8 1.771 KCO3 2 K2CO 1.531 2.428 4 CaCl 2 CaCl2(H2O)6 1.520 2.150 31.380 2.043 43 1.417 1.710 KHCO 3 KCl 1.482 2.170 Ca(HC 1.510 2.015 1.490 1.984 88 Ca(NO )(H O) 3 22 CaSO 1.465 1.896 KF 1.363 2.480 4 CaSO4(H2O)2 1.505 2.610 KF(H1.352 2.454 1.521 2.320 K2SO 1.494 2.662 NaCH3C1.464 1.528 7KHSO 4 Pb 1.480 2.322 8 NaBr 1.641 3.203 52.010 11.344 Na CO3 2 Na2CO 1.535 2.532 90 BC 2.000 2.250 1.405 1.440 O 3 SiC 1.223 NaHCO31.500 2.159 2.654 3.217 NaCl 1.544 2.165 7SiO2 H2SO 1.487 2 NaF 1.336 2.558 1.405 NaNO 1.587 2.261 74.5 PbCl 2 Fe O3 2.199 5.850 Na SO 2 4 Na2SO4(H 1.484 2.680 82 Al2O3 3.220 5 1.394 2.680 84 1.768 3.965 NaHSO (H 4 2 CH3CHO 1.460 2.476 52 PbO 2.510 8.000 1.332 0.783 H2O 1.333 1.000 CH3(CH )14COO 2 CH (CH ) 1.433 0.853 CH(C 3 formic 1.434 0.853 422 0.941 1.371 1.220 HOOCCH COOH 1.619 acetic acid 1.372 1.049 HOOC(CH2)2COOH 1.1.572 pyruvic acid 1.428 1.227 HOOC(CH2)3COOH 1.419 1.424 oxalic acid 1.900 C6H4(COOH)2 1.431 1.462 Other organics 1.550 1.400 ction of RH in whiche hysteresis effects” are partially taken into account for onsidered as a unique func th “ three aerosol types using the experimental data of Hänel [10]: 1 0 011 ww wRH (1) RH Copyright © 2011 SciRes. ACS 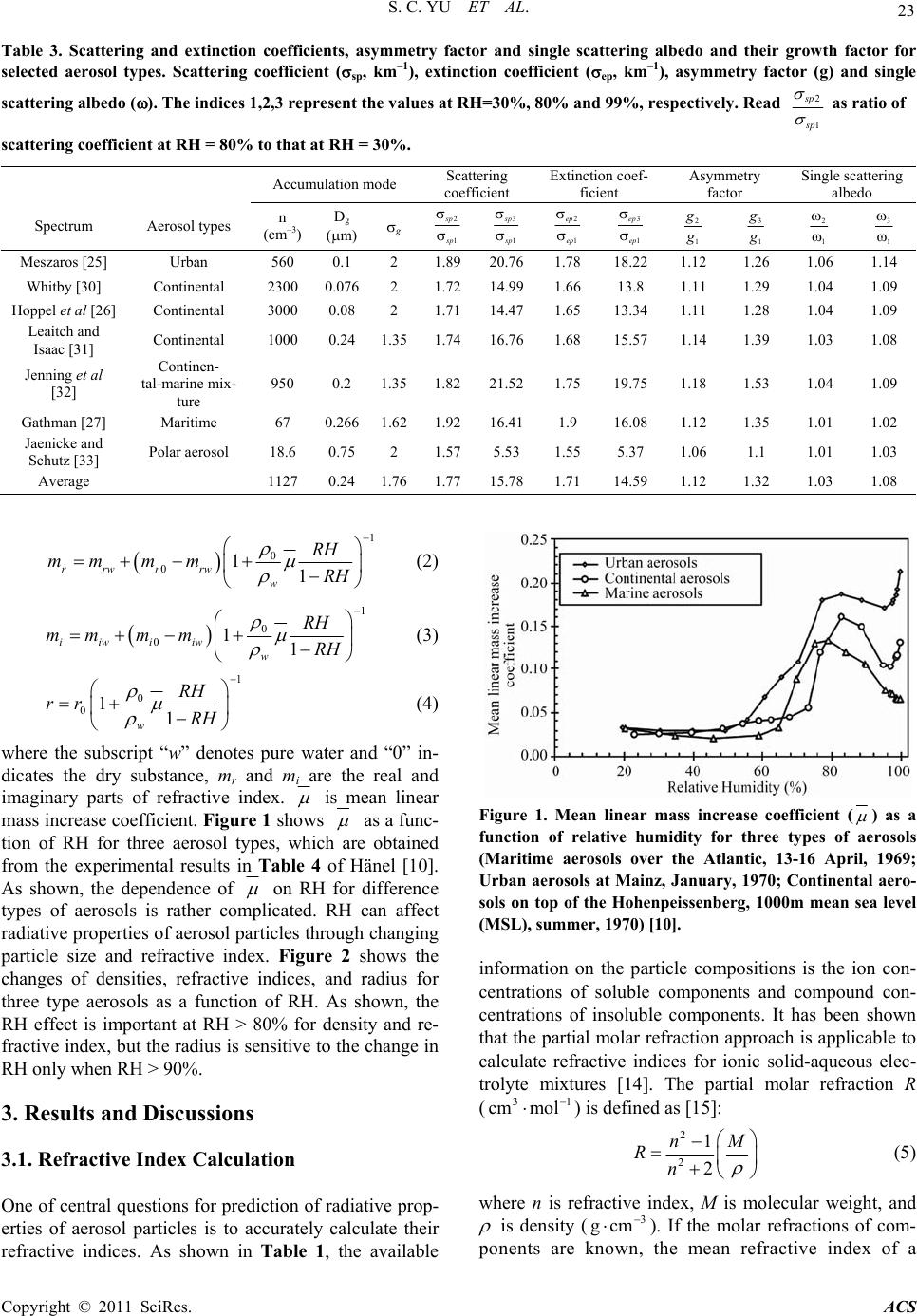 S. C. YU ET AL. 23 Table 3. Scattering and extinction coefficients, ctor and single scattering albedo and their growth factor for selected aerosol types. Scattering coefficient (sp ction coefficient (ep, km–1), asymmetry factor (g) and single attering albedo (). The indices 1,2,3 represent the values at RH=30%, 80% and 99%, respectively. Read asymmetry fa , km–1), extin sc 2 1 p p scattering coefficient at RH = 80% to that at RH = 30%. as ratio of coefficient ficient factor Sin albedo Accumulation mode Scattering Extinction coef-Asymmetry gle scattering Spectrum Aerosol types n –3 Dg g (cm) (m) 2 1 p p 3 1 p p 2 1ep ep 3 1ep ep 2 1 3 1 2 1 3 1 Meszar Urn 1.1.78.221.12.26 1.0614 os [25]ba560 0.1 2 89 20.76 18 1 1. W 2300 0 1.72 14.991.66 13.8 1.11 1.29 1.04 1.09 Hoppel 2 1.71 14.471.65 13.341.11 1.28 1.04 1.09 Cl 1. ta 1. G] 0. Schutz [33] 1127 1. hitby [30] et al [26] Continental Continental .076 0.08 2 3000 Leaitch and Isaac [31] ontinenta1000 0.24 351.74 16.761.68 15.571.14 1.39 1.03 1.08 Jenning et al [32] athman [27 Continen- l-marine mix- ture 950 0.2 351.82 21.521.75 19.751.18 1.53 1.04 1.09 Maritime Polar aerosol 67 266 1.621.92 16.411.9 16.081.12 1.35 1.01 1.02 Jaenicke and 18.6 0.75 2 1.57 5.53 1.55 5.37 1.06 1.1 1.01 1.03 Average 0.24 1.761.77 15.7871 14.591.12 1.32 1.03 1.08 1 0 011 rrwr rw w R mRH H mm m (2 ) 1 0 011 iiwi iw w RH mmm mRH (3) 1 0 011 w RH rr RH where the subscript “w” denotes pure water and “0” in- dicates the dry substance, mr imaginary parts of refractive index. (4) and mi are the real and is mean linear mass increase coefficient. Figure 1 shows as a func- tion of RH for three aerosol types, which are obtained from the experimental results in Table 4 of Hänel [10]. As shown, the dependence of on RH r difference types of aerosols is rather complicated. RH can affect radiative properties of aerosol particles through changing particle size and refractive ind. Figure 2 shows the changes of densities, refractive indices, and radius for three type aerosols as a function of RH. As shown, the RH effect is important at RH > 80% for density and re- fractive index, but the radius is sensitive to the change in RH only when RH > 90%. 3. Results and Discussions fo ex .1. Refractive Index Calculation ne of central questions for prediction of radiative prop- ly calculate their refractive indices. As shown in Table 1, the available 3 O erties of aerosol particles is to accurate Figure 1. Mean linear mass increase coefficient ( ) as a function of relative humidity for three typesols (Maritime aerosols over the Atlantic, 13-16 April, 1969; Urban aerosols at Mainz, January, 1970; Continental aero- sols on top of the Hohenpeissenberg, 1000m mean sea level (MSL), summer, 1970) [10]. partial molar refraction R of aeros information on the particle compositions is the ion con- centrations of soluble components and compound con- centrations of insoluble components. It has been shown that the partial molar refraction approach is applicable to calculate refractive indices for ionic solid-aqueous elec- trolyte mixtures [14]. The 31 (cm mol ) is defined as [15]: 2 2 1 2 nM Rn (5) where n is refractive index, M is molecular weight, and is density (3 gcm ). If the molar refractions of com- ponents are known, the mean refractive index of a Copyright © 2011 SciRes. ACS  S. C. YU ET AL. 24 Ta ar refraction of aerosol chemicMH [29 Species refrtion (Ri, cm–3) ble 4. The partial molal components. ] is Moelwyn-Hughes [29]. artial molar Ri/Mi Reference P ac Soluble components 1 H+ 0 0 MH [29] 2 4 .148 [14] 39 0.237 10 K+ 3. 11 Ca2+ 1. 12 M2+ 0. 13 oxalic) 3S(O)2OH (MSA) 2O 3.71 component 2 7.43 ] 2 8.4 .082 telson [14] ] 2)14COOH (n-Hexadecanoic acid) 2)16COOH (n-Octadecanoic acid) CH2COOH (Malonic acid) C(CH2)2COOH (Succinic acid) 7 OH 4.43 0.261 MH [29] 3 F 2.17 0.114 MH [29] Br 11.84 0Stelson 5 Cl8. Stelson [14] 6 3 NO 10.19 0.164 MH [29] 7 2 SO 4 + 0. 13.45 0.14 Stelson [14] 8 Na93 0.04 Stelson [14] 9 4 NH 4.89 0.271 Stelson [14] 03 0.078 Stelson [14] 93 0.048 Stelson [14] g HCOO 03 0.001 Stelson [14] 7.27 0.161 This work 14 3 CH COO 12.94 0.219 This work 15 32 CH CHCOO 17.59 0.241 This work 16 3 CH(CO)COO(pyruvic) 2 17.65 0.203 This work 17 (OOCCOO) (14.53 0.165 This work 18 CH16.82 0.175 This work 19 H insoluble 0.206 Stelson [14] 20 BC 2.11 0.176 This work 21 SiO0.124 Stelson [14] 22 AlO3 2 Fe O 10.62 0.104 Stelson [14 23 23 PbO 22.210.139 Stelson [14] 410 S 25 Pb 9.24 0.045 Stelson [14 26 CH3(CH 78 0.305 This work 27 CH3(CH 87.29 0.307 This work 28 HOOC17.24 0.166 This work 29 HOO24.2 0.205 This work 30 HOOC(CH2)3COOH (Glutaric acid) 28.40.216 This work 31 C6H4(COOH)2 39.99 0.241 This work 32 other organic compound 50 0.24 This work mediumn b cae calculated as follows [15]: 1 2 12 R 1 (6) V nR V for an aerosol particle: C i i i R M R VAV (7) R is the partialr refraction oent i in 1 where i ol molaf compon 3 cm m Mi is thear weightnt i , 1 mol molecul of compone in g , [Ci] is tcentration oent i in he conf compon 3 mg , and [AV] iaerosol volus the me in 3 gm . predicted The aerosol volume can be either measured or by: Ci i AV where i is the density of component i in g cm–3. Table 4 (8) Copyright © 2011 SciRes. ACS 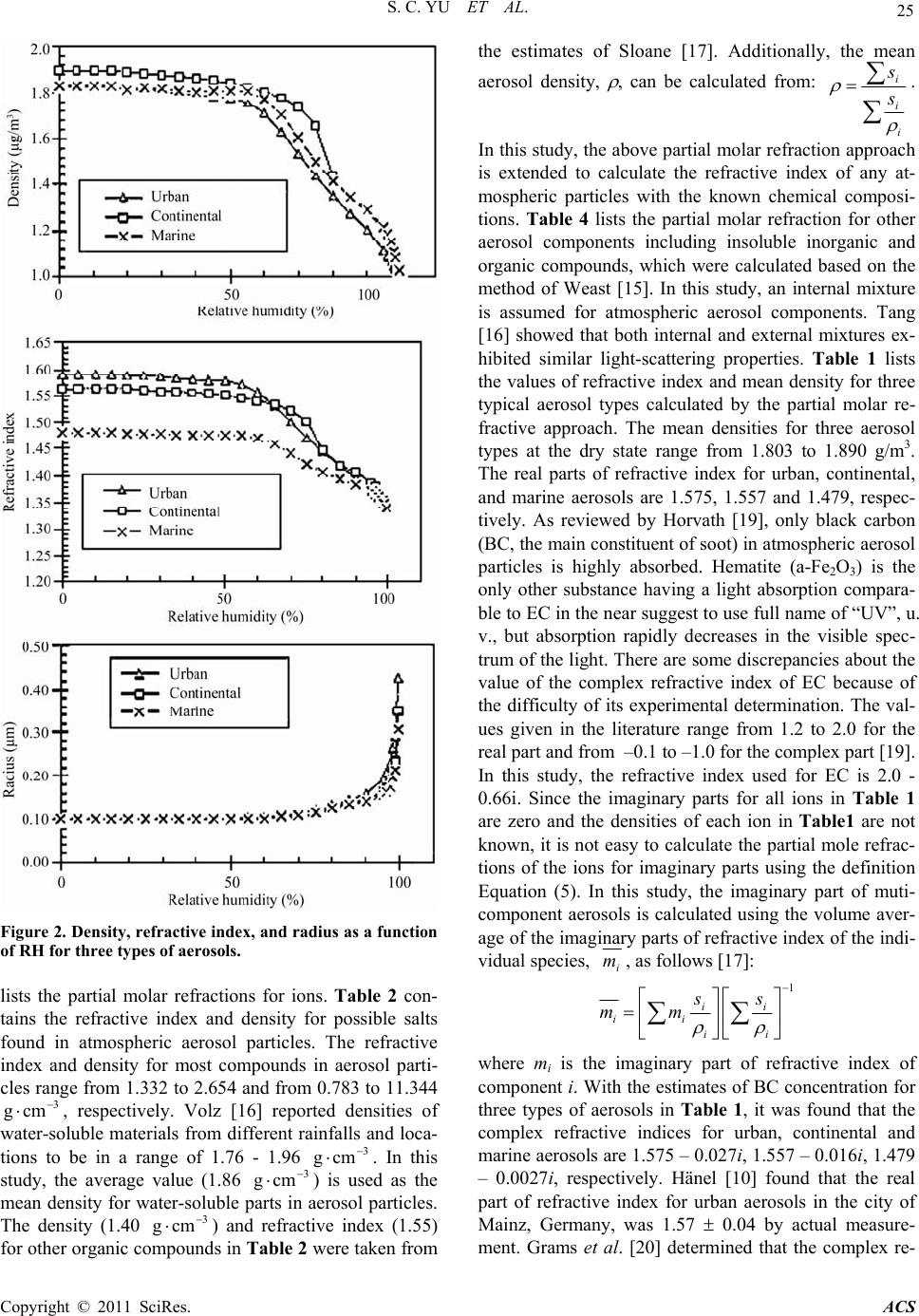 S. C. YU ET AL. 25 Figure 2. Density, refractive index, and radius as a function of RH for three types of aerosols. lists the partial molar refractions for ions. Table 2 con- tains the refractive index and density for possible salts found in atmospheric aerosol particles. The refractive index and density for most compounds in aerosol parti- cles range from 1.332 to 2.654 and from 0.783 to 11.344 , respectively. Volz [16] reported densities of luble materials from different rainfalls and loca- be in a range of 1.76 - 1.96. In this e average value (1.86 as the nsity for water-soluble particles. ty (1.40 ) andx (1.55) organic comin ken from lated from: 3 gcm water-so tions to study, th mean de The densi for other 3 gcm 3) is used rts in aerosol p e inde were ta gcm a refractiv Table 2 3 gcm pounds the estimates of Sloane [17]. Additionally, the mean erosol density, , can be calcua i i i . tions. T ed bas th inter lar re- ree aeroso types at the dry state range from 1.803 to 1.890 g/m3. In this study, the above partial molar refraction approach is extended to calculate the refractive index of any at- mospheric particles with the known chemical composi- able 4 lists the partial molar refraction for other aerosol components including insoluble inorganic and organic compounds, which were calculated on the method of Weast [15]. In this study, an internal mixture is assumed for atmospheric aerosol components. Tang [16] showed that bonal and external mixtures ex- hibited similar light-scattering properties. Table 1 lists the values of refractive index and mean density for three l motypical aerosol types calculated by the partia fractive approach. The mean densities for thl The real parts of refractive index for urban, continental, and marine aerosols are 1.575, 1.557 and 1.479, respec- tively. As reviewed by Horvath [19], only black carbon (BC, the main constituent of soot) in atmospheric aerosol particles is highly absorbed. Hematite (a-Fe2O3) is the only other substance having a light absorption compara- ble to EC in the near suggest to use full name of “UV”, u. v., but absorption rapidly decreases in the visible spec- trum of the light. There are some discrepancies about the value of the complex refractive index of EC because of the difficulty of its experimental determination. The val- ues given in the literature range from 1.2 to 2.0 for the real part and from –0.1 to –1.0 for the complex part [19]. In this study, the refractive index used for EC is 2.0 - 0.66i. Since the imaginary parts for all ions in Table 1 are zero and the densities of each ion in Table 1 are not known, it is not easy to calculate the partial mole refrac- tions of the ions for imaginary parts using the definition Equation (5). In this study, the imaginary part of muti- component aerosols is calculated using the volume aver- age of the imaginary parts of refractive index of the indi- vidual species, i m, as follows [17]: 1 ii ii ii ss mm where mi is the imaginary part of refractive index of component i. With the estimates of BC concentration for three types of aerosols in Table 1, it was found that the complex refractive indices for urban, continental and marine aerosols are 1.575 – 0.027i, 1.557 – 0.016i, 1.479 – .0027i, respectively. Hänel [10] found that the real part of refractive index for urban aerosols in the city of Mainz, Germany, was 1.57 0.04 by actual measure- ment. Grams et al. [20] determined that the complex re- Copyright © 2011 SciRes. ACS  S. C. YU ET AL. 26 ent this study are very close to these actual measurements. These complex re- fractive indices for three types of aerosole used in the calculation hereafter. d from 1.55 to 1.90 (average fficients, on value the aerosol diative properties. The scattering and extinction coeffi- factor de- rease by 6% with real part increasing from 1.4 to 1.65. fractive index for urban aerosols in the city of Boulder, Colorado O, was 1.55 – 0.044i on the basis of light scat- tering measurems. The results from s will b 3.2. The Sensitivity to Relative Humidity A Mie theory computer code developed by Dave [21] is used in this study to compute aerosol radiative properties. All aerosol particles are assumed to be spherical in shape in the calculation. Ta ble 3 shows the values of the parti- cle light scattering and extinction coefficients calculated with the above assumption at RHs of 30%, 80% and 99% for different particle size distributions of several aerosol types at a wavelength of 580 nm. The wavelength, 580 nm, is chosen based on the recommendation by Horvath [19] to give the maximum perception of an object under the daylight conditions. As shown, the growth factors for an RH range of 30 to 80% RH range from 1.57 to 1.92 average 1.77 0.12) an( 1.77 0.11) for scattering and extinction coe espectively. This is in agreement with the criterir of the hydroscopic growth factor (1.7 0.3) (which is defined as the ratio of the light scattering coefficient by an aerosol at an RH of 80% to that at 30%) derived from the direct nephelometer measurement [22]. This value has been utilized to date as the first estimate in climate change modeling studies [1]. It is interesting to note that Hegg et al. [23] obtained substantially larger values of the hygroscopic growth (see Table 1 of Hegg et al. [23]) for the same size distribution as those in Table 3. Hegg et al. [23] attributed it to be influenced by the position of the initial dry aerosol size distribution relative to the ef- fective light scattering droplet size range. The main dif- ferences in our calculation results and those of Hegg et al. [23] lie in different values of the refractive index and mean linear mass increase coefficient used for aerosol particles. The consistence of our results in Table 3 with the range of hygroscopic growth factor (1.5 to 1.8) from the direct measurements of Charlson et al. [22] indicates that our calculation for the effects of RH on scattering coefficient is reasonable. At a high RH such as 99%, the growth factors are much higher and more variable than values at lower RHs as shown in Table 4. The growth factors range from 1.06 to 1.18 (average 1.12 0.04) and from 1.10 to 1.53 (average 1.32 0.13) for asymmetry factor in the RH range of 30% to 80% and 30% to 99%, respectively. The growth factors range from 1.01 to 1.06 (average 1.03 0.02) and from 1.02 to 1.14 (average 1.08 0.04) for the single scattering albedo in the RH range of 30% to 80% and 30% to 99%, respectively. The single scattering albedo is not sensitive to RH. At a high RH such as 99%, the single scatter albedo is close to 1.0. The single scattering albedo and asymmetry factor are insensitive to changes in RH. This is in agreement with those of Pilinis et al. [24]. Both scattering and extinction coefficients are more sensitive to changes in RH than single scatter albedo and asymmetry factor. 3.3. The Sensitivity to Refractive Index In the following sensitivity studies, the parameters used for three types of aerosols are assumed to be (1) urban aerosols of Meszaros [25], N=560 cmm, Dg=0.100 m, g =2.0, m=1.590 – 0.027i, RH = 80%; (2) conti- nental aerosols of Hoppel et al. [24], N = 3000 cm–3, Dg = 0.080 m, g = 2.0, m = 1.564 – 0.016i, RH = 80%; (3) marine aerosols of Gathman [27], N = 67 cm–3, Dg=0.266 m, g = 1.622, m = 1.479 – 0.003i, RH = 80%. Note that the values of radius, refractive index in the above assumptions are for a dry state. RH is set to be 80% in the Mie calculation. Table 5 lists the ranges and aver- aged values of the change factors for the effects of real and imaginary parts of refractive index on ra cients increase by about 48% and asymmetry c But the single scattering albedo is insensitive to the changes in real part. Figures 3(a) and (b) show extinc- tion coefficient and single scatter albedo as a function of real part of refractive index for three types of aerosols at a wavelength of 580 nm. Table 5 shows that the scatter- ing coefficient and single scattering albedo decrease by 18% and 24% when imaginary part varies from –0.005 to 0.10, respectively. The extinction coefficient and asym- metry factor are insensitive to the change in the imagi- nary part. As expected, extinction coefficient and asym- metry factor increase slightly as imaginary part in- creases. 3.4. The Sensitivity to Size Distributions As shown in Table 5, scattering and extinction coeffi- cients are very sensitive to changes in geometric mean radius. The scattering and extinction coefficients increase by factors of 118 and 123, respectively, whereas the asymmetry factor only increases by 17% and the single scattering albedo decreases by 0.8%, when the geometric mean radius varies from 0.05 to 0.3 m. Figures 4(a) and (b) show the sensitivity tests for the case of marine aerosols at different wavelengths. Table 5 lists the ranges and averaged changes of radiative properties for three types of aerosols when geometric standard devia- tion (g) varies from 1.2 to 3.0. The scattering and Copyright © 2011 SciRes. ACS  S. C. YU ET AL. Copyright © 2011 SciRes. ACS 27 Figure 3. The radiative properties at 580 nm as a function of real and imaginary parts of refractive index for three types of aerosols at a dry state. Table 5. The change factors for radiative properties of aerosols as a function of real part, imaginary part, geometric mean radius (rg) and geometric standard deviation (g). The values in parenthesis are the average change factors. Real part from 1.40 to 1.65 Imaginary part from –0.005 to –0.10rg from 0.05 to 0.3 mm g from 1.20 to 3.0 Scattering coefficient 1.34 - 1.65 (1.49) 0.80 - 0.84 (0.82) 51.5 - 248.5 (118.8) 153 - 753 (389) extinction coefficient 1.32 - 1.65 (1.47) 1.01 - 1.15 (1.07) 59.2 - 249.3 (123.7) 155 - 607 (334) 1.00 - 1.01 (1.01) 0.79 - 0.73 (0.76) 0.87 - 1.00 (0.92) 0.99 - 1.24 (1.1) Asymmetry factor 0.92 - 0.95 (0.94) 1.03 - 1.01 (1.02) 1.09 - 1.32 (1.17) 3.1 - 8.3 (5.4) Single scattering albedo extinction coefficients and asymmetry factor are very sensitive to the change in geometric standard deviation. aerosol size distribution when geometric mean radius varies from 0.1 to 0.4 m at N0 = 560 cm–3 The scatteng and ex factors of 89.3 and 3 stries fhis change gease octor by a fact of rease ong albedo 1d sensitivity symmetry factor and single scattering albedo to changes and g = 2.0 metriion 0 . e light sca of aar- ependent e, win- urring betw7 m iius wavelen, aeroan have large scattering and extinction coefficients if their ri 3 tinction coefficients inc 34, respectively, when rease by geometric and when geo to 3.0 at N = 5 andard deviation varom 1.2 to 3.0. Tof Since th results in the incrf asymmetry faor ticle is d 5.4 and the incf single scatteriby cies occ 0%. Figures 4(c) an(d) show the of for a light c standard deviat 60 cm–3 and r= 0.15 varies from 1.2 m, respectively g ttering efficiencyn individual p on the particle sizth peak efficie een ~0.2 and 0.n particle rad gth of 580 nmsol particles c a in g values for urban aerosols at different wavelengths. Figure 5(a) and (b) show the changes of the shape of size distributions grow into this efficient light scattering size range. Figure 5 indicates that both cases can result  28 S. C. YU ET AL. Figure 4. The radiative properties at 580 nm as a function of geometric mean radius (for Gathma’s maritime aerosols (a, b), and geometric standard deviation (for Meszaros’ urban aer osols (c , d)). (a) (b) Figure 5. The size distribution of aerosol number concentration as a function of geometric mean radius (a) and standard de- viation (b). Copyright © 2011 SciRes. ACS  S. C. YU ET AL. 29 in more particles in the efficient light scattering size range (with radii of 0.2 to 0.7 m). It is not surprised to find that the scattering and extinction coefficients are very sensitive to the changes in geometric mean radius and geometric standard deviation. 3.5. The Sensitivity of Wavelength Dependence of Radiative Properties The wavelength dependence of aerosol radiative properties is very sensitive to geometric mean radius. When the geometric mean radius is small, both single scattering albedo and asymmetry factor decrease with increasing wavelengths, but when the comes larger than a value, both single scat he latter case, the Angstrom law will not be light scattering effi- iency of an individual particle is a nonlinear function of t wavtive index is wavelength ependent, the wavelength dependence of aerosol radia- metric standard deviation is weak. For small geometric mean radius and small geometric standard deviation, both asymmetry factor and single scattering albedo in- crease with decreasing wavelengths, however, for large values of geometric mean radius and geometric standard deviation, both asymmetry factor and single scattering albedo increase with increasing wavelengths, especially for single scattering albedo, as shown in Figure 4. 3.6. Radiation Transmission Since human-induced aerosols are likely to greatly in- fluence future regional climate change instead of global amine the sensitivity nsmission changes at tributions, and RH. In this study, the radiation transmis- nm is amined under the following constant conditions: date = lbedo = 0.15, air pres- ure = 940 mb, Latitude = 35.63˚, longitude = 82.33˚, UT radiative transfer model for different aerosol types assuming atit .90, zenith ang geometric mean radius be- tering albedo climate change, it is of interest to ex of the aerosol-induced radiation tra and asymmetry factor increase with increasing wave- engths. For t a local or regional scale to aerosol composition, size dis- l applicable. The values of geometric mean radii at the crossing point are different for asymmetry factor and single scattering albedo as shown in Figures 4(a) and (b), and are also determined by the geometric standard devia- tion as analyzed below. Since the c particle size and depends on the particle size and ligh elength tested, and the refrac d tive properties will strongly rely on the size distribution and refractive index. For the wavelength dependence of refractive index, available data were closely matched by setting n() = n( = 0.598 m) – 0.03( – 0.598), where is the wavelength in m. As shown, the wavelength dependence of refractive index is weak. As shown in Figure 4, the wavelength dependence of asymmetry fac- tor and single scattering albedo strongly relies on the geometric mean radius and geometric standard deviation. But the wavelength dependence of scattering and extinct- tion coefficients on the geometric mean radius and geo- sion is calculated for the assumed aerosol layer with a depth of 2 kilometers using the Madronich’s Tropo- spheric Ultraviolet-Visible Radiation Transfer Model (TUVRTM) [28]. The optical depth 2 1 d z e z zz is calculated by assuming a constant extinction coefficient within the aerosol layer. The sensitivity of aero- sol-induced radiation transmission changes at 580 Table 6. The radiation transmission at 580 nm calculated by a an aerosol layer of 2 km. The date is 7/1/1995, O3 = 278 DU, l 13.31˚. Accumulation mode ex 7/01/1995, O3 = 278 DU, ground a s = 17.90, solar zenith angle = 13.31, the aerosol layer = 2 km. Three aerosol radiative properties (optical depth, asymmetry factor, and single scattering albedo) are needed in the TUVRTM model to calculate the aero- sol-induced radiation transmission changes. In this sec- tion, the sensitivity of the aerosol-induced radiation transmission change to RH, refractive index, and size distribution is studied based on previous calculation re- ude = 35.63˚, longitude = –82.33˚, UT = 17le = Transmission at 580 nm Spectrum Aerosol types n (cm–3) Dg (μm) σg T1 (RH=30)T2 (RH=80) T1 (RH=99) T2/T1 T 3/T1 Meszaros [23] Urban 560 0.1 Whitby [28] Continental 2300 0.076 Hoppel et al [24] Continental 3000 0.08 Leaitch and Isaac [29] Continental 1000 0.24 Jenning et al [30] Continental- marine mixture 950 0.2 Gathman [25] Maritime 67 0.266 Jaenicke and Schutz [31] Polar aerosol 18.6 0.75 Background without aerosol layer 0 0 2 2 2 1.3 1.3 1.6 2 0 0.908 0.908 0.906 1 1 0.906 0.906 0.890 1 0.98 0.904 0.895 0.840 0.99 0.93 5 0.897 0.895 0.844 1 0.94 50.904 0.903 0.876 1 0.97 20.911 0.911 0.911 1 1 0.910 0.911 0.911 1 1 0.911 0.911 0.911 Copyright © 2011 SciRes. ACS  S. C. YU ET AL. 30 nm for three types of aerosols.* The average is calculated only for ban and continental aerosols. Table 7. The change factors for radiation transmission at 580 as a function of relative humidity and radiative properties ur Aerosol type Parameter Urban Continental Marine average* Relative humidity from 0 to 95% 0.999 0.993 1 0.996 Real part fro 1.40 to 1.65 0.992 0.995 Imaginary part from –0.005 to –0.100.9 Numbeom 0.958 .30 μm 0.467 2 0.99 .0 0.934 1 0.997 m0.998 0.979 1 67 0.998 0.973 r concentrations fr 50 to 4000 cm-3 0.94 0.977 77 0.9 rg from 0.05 to 0 σ from 1.2 to 3 0.02 0.83 0.244 0.883 g Figure 6. The radiation transmission at 580 nm across an aerosol layer with a 2-km in depth as a function of RH, real and imaginary parts, number conce ntration, and size distribution for three types of aerosols. Copyright © 2011 SciRes. ACS  S. C. YU ET AL. Copyright © 2011 SciRes. ACS 31 sults of aerosol radiative properties for the three types of aerosols. Table 6 lists the radiation transmissions at 580 nm for different aerosol types at RHs of 30%, 80% and 99%. Figure 6 shows the sensitivity of radiation trans- mission to RH, refraction index, number concentrations and size distributions for the three types of aerosols. Ta- ble 7 lists the change factors for radiation transmission for three types of aerosols. Note that the radiation trans- mission at 580 nm is 0.911 without the aerosol layer un- der the assumed atmospheric conditions. It is interesting to note that the radiation transmission is not sensitive to the changes in the above parameters if the total aerosol number concentration is small as it for maritime aerosols of Gathman [27] (total number concentration is only 67 cm–3 as indicated in Table 7). In this case, the radiation transmission only decreases by 0.4% and 0.5% when RH varies from 0% to 95% and the real part varies from 1.40 to 1.65, respectively. The radiation transmission is sensi- tive to the change in imaginary part and number concen- trations with the decrease of visible radiation transmis- sion by 2.7% and 4.2% when the imaginary part varies from –0.005 to –0.1 and number concentration from 50 to 4000 cm–3, respectively. The radiation transmission is very sensitive to the changes in geometric mean radius and geometric standard deviation. The radiation trans- mission decreases by 76% when geometric mean radius varies from 0.05 to 0.3 m and decreases by 12% when geometric standard deviation varies from 1.2 to 3.0. This is in agreement with the strong dependence of scattering and extinction coefficients on geometric mean radius and geometric standard deviation. It should be emphasized that the radiation transmission also strongly depends on the solar zenith angle, latitude and longitude, and ozone concentrations. 4. Conclusions In this work, the partial molar refraction method is used to accurately calculate the real part of refractive index of aerosol particles with actual measured chemical compo- sitions including soluble inorganic and organic ions and dius and geometric standard deviation of a particle size distribution. The radiation transmission decreases by 76% and 12% when geometric mean radius varies from 0.05 to 0.3 m and geometric standard deviation varies from 1.2 to 3.0, respectively. Other sensitivities for the radiation transmissions are insignificant. The comparison between theoretical calculation and actual measurement will be necessary in the future work. 5. References [1] R. J. Charlson, S.E. Schwartz, J. M. Hales, R. D. Cess, J. A. Coakley, J. E. Hansen and D. J. Hofmann, “Climate Forcing by Anthropogenic Aerosols,” Science, Vol. 225, 1992, pp. 423-430. doi:10.1126/science.255.5043.423 insoluble inorganic and organic substances. It is found hat the complex refractive indices for urban, continental t and marine aerosols are 1.575 – 0.027i, 1.557 – 0.016i and 1.479 – 0.003i, respectively. The scattering and ex- tinction coefficients are sensitive to changes in RH, while both single scattering albedo and asymmetry factor are insensitive to change in RH. The extinction coeffi- cient and asymmetry factor are sensitive to changes in real part of refractive index. The scattering coefficient and single scattering albedo are sensitive to the imagi- nary part changes. The aerosol radiative properties are very sensitive to the change in both geometric mean ra- [2] S. C. Yu, “The Role of Organic Acids (Formic, Acetic, Pyruvic and Oxalic) in the Formation of Cloud Conden- sation Nuclei (CCN): A Review,” Atmospheric Research, Vol. 53, 2000, pp. 185-217. doi:10.1016/S0169-8095(00)00037-5 [3] S. C. Yu, V. K. Saxena and Z. Zhao, “A Comparison of Signals of Regional Aerosol-Induced Forcing in Eastern China and the Southeastern United States,” Geophysical Research Letters, Vol. 28, 2001, pp. 713-716. doi:10.1029/2000GL011834 [4] Intergovernmental Panel on Climate Change (IPCC), “Climate Change 2007: The Physical Science Basis,” Contribution of Working Group I to the Fourth Assess- ment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York, 2007. [5] J. E. Penner, R. J. Charlson, J. M. Hales, N. S. Laulainen, R. Leifer, T. Novakov, J. Ogren, L. F. Radke, S. E. Schwartz and L. Travis, “Quantifying and Minimizing Uncertainty of Climate Forcing by Anthropogenic Aero- sols,” Bulletin of the American Meteorological Society, Vol. 75, 1994, pp. 375-400. doi:10.1175/1520-0477(1994)075<0375:QAMUOC>2.0. CO;2 [6] O. Boucher and T. L. Anderson, “General Circulation Model Assessment of The Sensitivity of Direct Climate Forcing by Anthropogenic Sulfate Aerosols to Aerosol Size and Chemistry,” Journal of Geophysical Research, Vol. 100, 1995, pp. 26117-26134. doi:10.1029/95JD02531 [7] M. Z. Jacobson, “Global Direct Radiative Forcing Due to Multicomponent Anthropogenic and Natural Aerosols,” Journal of Geophysical Research, Vol. 106, 2001, pp. 1551-1568. doi:10.1029/2000JD900514 [8] D. Koch, S. Bauer, A. Del Genio, G. Faluvegi, J. R. McConnell, S. Menon, R. L. Miller, D. Rind, R. Ruedy, G. A. Schmidt and D. Shindell, “Coupled Aero- sol-Chemistry-Climate Twentieth Century Transient Model Investigation: Trends in Short-Lived Species and Climate Responses,” Journal of Climate, 2011. [9] W. F. Rogge, M. A. Mazurek, L. M. Hildemann, G. R. Cass and B. R. T. Simoneit, “Quantification of Urban  32 S. C. YU ET AL. Organic Aerosols at a Molecular Level: Identification, Abundance and Seasonal Variation,” Atmospheric Envi- ronment, Vol. 27, 1993, pp. 1309-1330. [10] G. Hänel, “The Properties of Atmospheric Aerosol Parti- cles as Functions of Relative Humidity at Thermody- namic Equilibrium with the Surrounding Air,” Advances in Geophysics, Vol. 19, 1976, pp. 73-188. doi:10.1016/S0065-2687(08)60142-9 [11] R. F. Pueschel, “Atmospheric Aerosols,” In: Singh H. B.,Van Nostrand Reinhold, Eds., Composition, Chemistry and Climate of the Atmsopher, 1995, pp. 120-175. [12] P. Chylek and J. Wong, “Effect of absorbing aerosols on global radiation budget, Geophysical Research Letters, Vol. 22, No. 8, 1995, pp. 929-931. doi:10.1029/95GL00800 [13] J. J. Huntzicker, R. L. Johnson, J. J. Shah and R. A. Cary, “Analysis of Organic and Elemental Carbon in Ambient Aerosols by a Thermal-Optical Method,” In: Particulate a Particle Trap Impac- mpler, Environmental Science & Tech- nology, Vol. 35, No. 24, 1982, pp. 4857-4867. Carbon: Atmospheric Life Using tor/Denuder Sa [14] A. W. Stelson, “Urban Aerosol Refractive Index Predic- tion by Partial Molar Refraction Approach,” Environ- mental Science & Technology, Vol. 24, 1990, pp. 1676-1679. doi:10.1021/es00081a008 [15] R. C. Weast, CRC Handbook of Chemical and Physics, Cleveland, OH, 1988. [16] F. Volz, “Infrared Absorption by Atmsopheric Aerosol Substances,” Journal of Geophysical Research, Vol. 77, 1972, pp. 1017-1031. doi:10.1029/JC077i006p01017 [17] C. S. Sloane, “Optical properties of aerosols of mixed composition,” Atmospheric Environment, Vol. 18, 1984, pp. 871-878. doi:10.1016/0004-6981(84)90273-7 [18] I. N. Tang, W. T. Wong and H. R. Munkelwiz, “The Relative Importance of Atmospheric Sulfates and Nitrates in Visibility Reduction,” Atmospheric Environment, Vol. 15, 1981, pp. 2463-2471. doi:10.1016/0004-6981(81)90062-7 [19] H. Horvath, “Atmospheric Light Absorption - a Review,” Atmospheric Environment, Vol. 27, 1993, pp. 293-317. [20] G. W. Grams, I. H. Blifford, D. A. Gillette and P. B. Rus- sell, “Complex Index of Refraction of Airborne Soil Par- ticles,” Journal of Applied Meteorology, Vol. 13, 1974, pp. 459-471. doi:10.1175/1520-0450(1974)013<0459:CIOROA>2.0.C O;2 [21] J. V. Dave, “Subroutines for Comp of Electomagnetic Radiation Scatter uting the Parameters ed by a Sphere,” 0302 IBM Journal of Research and Development, Vol. 13, 1969, pp. 302-312. doi:10.1147/rd.133. on, “Observa- tical Study idity on Light Scattering by [22] R. J. Charlson, D. S. Covert and T. V. Lars tions of the effect of humidity on light scattering by aerosols,” In: Ruhnke T. H. and Deepak A., Eds., Hygro- scopic Aerosols, pp. 35-44, Hampton, VA, 1984. [23] D. Hegg, T. Larson and P. F. Yuen, “A Theore of the Effect of Relative Hum Tropospheric Aerosols,” Journal of Geophysical Re- search, Vol. 98, 1993, pp. 18435-18439. doi:10.1029/93JD01928 [24] C. Pilinis, S. N. Pandis and J. H. Seifeld, “Sensitivity of Direct Climate Forcing by Atmospheric Aerosols to Aerosol Size and Composition,” Journal of Geophysical Research, Vol. 100, 1995, pp. 18739-18754. doi:10.1029/95JD02119 [25] A. Meszaros, “On the Concentration and Size Distribu- tion of Atmospheric Sulfate Particles under Rural Condi- tions,” Atmospheric Environm 2425-2428. ent, Vol. 12, 1978, pp. doi:10.1016/0004-6981(78)90286-X W. A. [26] Hoppel, R. Larson and M. A. Vietti, “Aerosol Size Distributions at a Site on the East Coast of the United States,” Atmospheric Environment, Vol. 18, 1984, pp. 1613-1621. doi:10.1016/0004-6981(84)90383-4 [27] S. C. Gathman, “Optical Properties of the Maritime Aerosols as Predicted by the Navy A tical Engineering, Vol. 22 erosol Model,” Op- , 1983, pp. 56-62. ag, Amsterdam, [28] S. Madronich, “Tropospheric Photochemistry and Its Response to UV Changes,” In: M. L. Chanin, Ed., The role of the stratosphere in global change, NATO-ASI Se- ries, Vol. 18, pp. 437-461, Springer-Verl 1993. [29] E. A. Moelwyn-Hughes, Physical Chemistry, 2nd rev. ed., Pergamon Press, New York, 1961. [30] K. T. Whitby, “The Physical Characteristics of Sulfur Aerosols,” Atmospheric Environment, Vol. 12, 1978, pp. 135-159. doi:10.1016/0004-6981(78)90196-8 [31] W. R. Leaitch and G. A. Isaac, “Tropospheric Aerosol Size Distributions from 1982 to 1988 over Eastern North America,” Atmospheric Environment, Vol. 25, 1991, pp. 601-619. [32] S. G. Jennings, C. D. O’Dowd, T. C. O’Connor and F. M. McGovern, “Physical Characteristics of Ambient Aerosol at Mace Head,” Atmospheric Environment, Vol. 25A, 1991, pp. 557-562. [33] R. Jaenicke and L. Schiitz, “Arctic Aerosol in Surface Air,” ldoyaras 86, 1982235-241. Copyright © 2011 SciRes. ACS
|