Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 20 1 1, 1, 77-82 doi:10.4236/aces.2011.12013 Published Online April 2011 (http://www.scirp.org/journal/aces) Copyright © 2011 SciRes. ACES Amphiphilic Block Copolymer for Adsorption of Organic Contaminants Jae-Woo Choi1, Kyung-Youl Baek2, Kie-Yong Cho2, Natalia Valer’yevna Shim1,3, Sang-Hyup Lee1* 1Water Environment Center, Korea Institute of Science and Technology, Seoul, South Korea 2Hybrid Materials Research Center, Korea Institute of Science and Technology, Seoul, South Korea 3University of Science and Technology, Daejeon, South Kor e a E-mail:yisanghyup@kist.re.kr Received December 24, 2010; January 16, 2011; accepted April 2, 2011 Abstract In this study, new polymeric adsorbents, 2 types of polystyrene-block-poly (N-isopropylacrylamide) (PSN, structure of hydrophobic core and hydrophilic shell), were developed and applied for removal of organic pollutants from wastewater. Encapsulation of organic pollutants by the polystyrene-block-poly (N-isopropy- lacrylamide) (PSN) resulted in increasing hydrophobicity of the polystyrene with abundant hydrophobic spaces within the amphiphilic block copolymer. The encapsulation mechanism of BTEX by PSN was inves- tigated and found to be mainly attributable to the Van der Waals interactions between the aromatic ring of BTEX and the hydrophobic core of PSN. Polystyrene-block-poly (N-isopropylacrylamide) showed good po- tential as a novel and cost effective adsorbent for application to wastewater treatment, which can be simply regenerated and reused using an external temperature changing system. Keywords: BTEX, Encapsulation, External Temperature Changing System, Polymeric Adsorbent, Van der Waals Force 1. Introduction Organic contaminants naturally persist in the environ- ment, biodegrade slowly, cause significant damage to natural water systems and consequently present prob- lems to human health. To remove such toxic organic substances, water treatment methods such as adsorp- tion, biodegradation, chemical oxidation (using agents such as ozone, hydrogen peroxide or chlorine dioxide), incineration and solvent extraction have been studied. Among these practical methods, adsorption has been widely used as an effective method for removal of or- ganic contaminants from wastewater. Various adsor- bents, including activated carbon, natural materials and organoclays, have been investigated for removal of organic contaminants [1-11]. Although activated car- bon has high capacity of absorbing toxic organic sub- stances and can be easily modified by chemical treat- ment to increase its adsorption capacity, it also has several disadvantages [12,13]. Powdered activated carbon is hard to separate from an aquatic system when it becomes exhausted or the effluent reaches the legal discharge level. Furthermore, the regeneration of ex- hausted activated carbon by chemical and thermal procedures is also expensive and leads to loss of the adsorbent [14-16]. In addition, biodegradation, che- mical oxidation and incineration treatments become exceedingly expensive when low effluent concentra- tions are required, and produce by-products that can also cause pollution. Solvent extraction is also expen- sive and may lead to contamination of groundwater. Therefore, alternative advanced technologies have to be investigated, and polymer adsorbents offer advan- tages over traditional carbon adsorbents since they can be simply regenerated by washing with a chemical so- lution, such as acid or alkali, under ambient conditions [17-19]. In this study, new polymeric adsorbents, 2 types of polystyrene-block-poly (N-isopropylacrylamide) (PSN, structure of hydrophobic core and hydrophilic shell), were developed and applied for the removal of organic pollutants from wastewater. When this novel material is discharged into water, micelles form as a unit molecule. Through hydrophobic binding, the core-organic com-  J.-W. CHOI ET AL. Copyright © 2011 SciRes. ACES 78 plexes are effective in adsorbing a variety of organic pollutants from water. It was found that the total mole cular weight of the PSN and the hydrophobic to hydro- philic ratio influenced the encapsulation and removal of BTEX from wastewater. 2. Materials and Methods 2.1. Chemicals and Materials Carboxyl acid group terminated trithiocarbonate as a chain transfer agent (CTA) and polystyrene-block-poly (N-isopropylacrylamide) (PSN) as an adsorbent were synthesized by previously reported literature (Figure 1) [20]. Chemicals used for BTEX encapsulation experi- ment were benzene, toluene, ethylbenzene, xylene and methanol, all of them were reagent grade and purchased from Sigma-Aldrich. 2.2. Polystyrene-Block-Poly (N-Isopropylacrylamide) Analysis Polystyrene-block-poly (N-isopropylacrylamide) was characterized via size exclusion chromatography (SEC), comprised of a Shodex GPC LF-804 column, CTS 30 column oven, Youngrin refractive index (RI) detector and HITACHI L-6000 pump, with dimethylformaide (DMF) used as the eluent, at a flow rate of 1.0 ml/min, to analyze for the molecular weight and polydispersity in- dex (PDI). Thermo gravimetric analysis (TGA) and dif- ferential scanning calorimetry (DSC) were used to measure the weight changes as a function of the temper- ature and the temperatures and heat flows associated with thermal transitions in the polystyrene-block-poly N-isopropylacrylamide), respectively. The conditions used for the TGA and DSC were temperature ranges of 27 - 1000℃ and –70 - 600℃, respectively, with a sensi- tivity and heating rate of 0.5 - 100 mcal/sec and 0.1 - 200℃/min, respectively. 2.3. Encapsulation Procedure The encapsulation reaction was carried out in 25 ml screw-cap vials, with Teflon-backed septa, and stirred on a rotary shaker. Sample aliquots of 1 ml were withdrawn from the reactor at regular time intervals using a syringe. Multiple experiments were carried out for a given set of conditions. Preliminary experiments indicated that the adsorption equilibrium of BTEX was reached at around 24 h, with no appreciable decrease in the adsorbate bulk concentration for time periods of up to 2 days. Con- trolled experiments, where there were no reactions with the adsorbents, were also conducted to confirm that the decreases in the BTEX concentrations were actually caused by adsorption onto the adsorbents rather than by adsorption onto the walls of the glass bottles or from volatilization. 2.4. Encapsulation Analysis BTEX standards; 20, 10, 5, 1 and 0.1 mg·L-1, were pre- pared in deionized water (18 MΏ·cm) by diluting a 1000 mg·L-1 BTEX solution prepared in methanol. Replicate aliquots of 1 ml were placed in 10 ml Teflon-sealed screw- caps vials for GC analysis. The standard solutions were also run to check for the effects. Separate standard solu- tions of benzene, toluene, ethylbenzene and xylene; 20, 10, 5, 1 and 0.1 mg·L-1, were also prepared for GC analyses by appropriate dilution of 1000 mg·L-1 solutions of each compound. Glass bottle experiments were carried out by bringing 10 ml aliquots of the stock solution into contact with 20 mg·L-1 for each BTEX solution and 0.05 g of each adsorbent polystyrene-block-poly (N-isopropylacrylamide) (PSN) in glass bottles sealed with Teflon- sealed screw-caps. The bottles were then shaken for 24 h on a temperature controlled shaker at 20℃ to attain equili- brium. Gas chromatograph (Varian STAR 3400 CX; Purge & Trap) equipped with a flame ionization detector (FID) was used for analysis of the equilibrium concentra- tion. 3. Results and Discussion 3.1. Polystyrene-Block-Poly (N-Isopropylacrylamide) Characterization In this study, 2 types of polystyrene-block-poly (N-iso- HO SS O S C 11 H 23 Styrene CTA-COOH AIBN in bulk at 80 o C SS S C 11 H 23 HO O mNIPAM AIBN in DMF at 80 o C ON HHO O m SS S C 11 H 23 ON H n Polystyrene precursorPolystyrene-block-PolyNIPAM Figure 1. Synthesis of polystyrene-block-poly(N-isopropylacrylamide) (PSN), using the situational reversible addition fragmentation chain transfer polymerization (RAFT) of styrene and N-isopropylacrylamide, from the carboxyl acid group terminated trithiocarbonate s and AIBN initiating system. 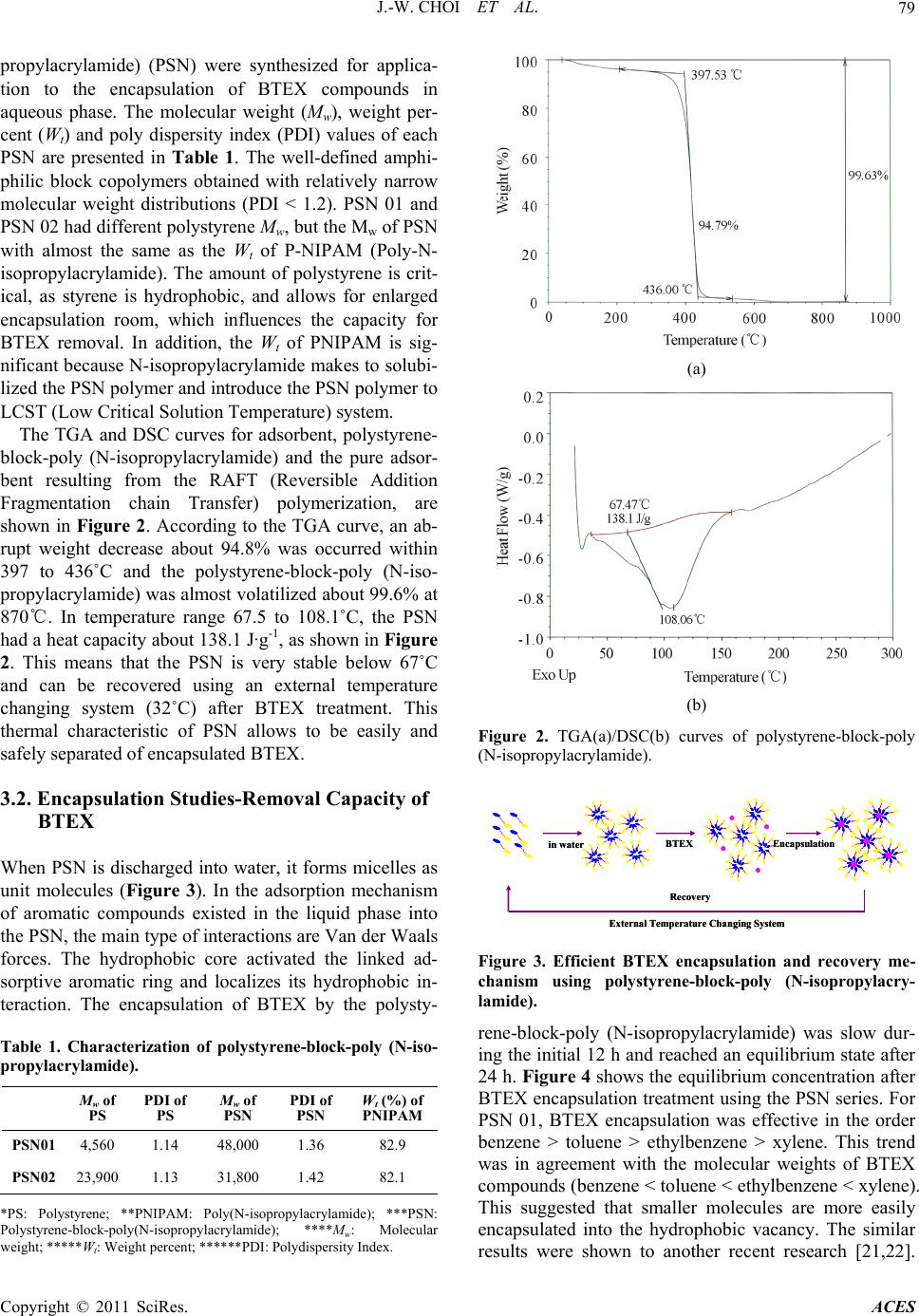 J.-W. CHOI ET AL. Copyright © 2011 SciRes. ACES 79 propylacrylamide) (PSN) were synthesized for applica- tion to the encapsulation of BTEX compounds in aqueous phase. The molecular weight (Mw), weight per- cent (Wt) and poly dispersity index (PDI) values of each PSN are presented in Table 1. The well-defined amphi- philic block copolymers obtained with relatively narrow molecular weight distributions (PDI < 1.2). PSN 01 and PSN 02 had different polystyrene Mw, but the Mw of PSN with almost the same as the Wt of P-NIPAM (Poly-N- isopropylacrylamide). The amount of polystyrene is crit- ical, as styrene is hydrophobic, and allows for enlarged encapsulation room, which influences the capacity for BTEX removal. In addition, the Wt of PNIPAM is sig- nificant because N-isopropylacrylamide makes to solubi- lized the PSN polymer and introduce the PSN polymer to LCST (Low Critical Solution Temperature) system. The TGA and DSC curves for adsorbent, polystyrene- block-poly (N-isopropylacrylamide) and the pure adsor- bent resulting from the RAFT (Reversible Addition Fragmentation chain Transfer) polymerization, are shown in Figure 2. According to the TGA curve, an ab- rupt weight decrease about 94.8% was occurred within 397 to 436˚C and the polystyrene-block-poly (N-iso- propylacrylamide) was almost volatilized about 99.6% at 870℃. In temperature range 67.5 to 108.1˚C, the PSN had a heat capacity about 138.1 J·g-1, as shown in Figure 2. This means that the PSN is very stable below 67˚C and can be recovered using an external temperature changing system (32˚C) after BTEX treatment. This thermal characteristic of PSN allows to be easily and safely separated of encapsulated BTEX. 3.2. Encapsulation Studies-Removal Capacity of BTEX When PSN is discharged into water, it forms micelles as unit molecules (Figure 3). In the adsorption mechanism of aromatic compounds existed in the liquid phase into the PSN, the main type of interactions are Van der Waals forces. The hydrophobic core activated the linked ad- sorptive aromatic ring and localizes its hydrophobic in- teraction. The encapsulation of BTEX by the polysty- Table 1. Characterization of polystyrene-block-poly (N-iso- propylacrylamide). Mw of PS PDI of PS Mw of PSN PDI of PSN Wt (%) of PNIPAM PSN01 4,560 1.14 48,000 1.36 82.9 PSN02 23,900 1.13 31,800 1.42 82.1 *PS: Polystyrene; **PNIPAM: Poly(N-isopropylacrylamide); ***PSN: Polystyrene-block-poly(N-isopropylacrylamide); ****Mw: Molecular weight; *****Wt: Weight percent; ******PDI: Polydispersity Index. (a) (b) Figure 2. TGA(a)/DSC(b) curves of polystyrene-block-poly (N-isopropylacrylamide). in waterEncapsulationBTEX External Temperature Changing System Recovery in waterEncapsulationBTEX External Temperature Changing System Recovery Figure 3. Efficient BTEX encapsulation and recovery me- chanism using polystyrene-block-poly (N-isopropylacry- lamide). rene-block-poly (N-isopropylacrylamide) was slow dur- ing the initial 12 h and reached an equilibrium state after 24 h. Figure 4 shows the equilibrium concentration after BTEX encapsulation treatment using the PSN series. For PSN 01, BTEX encapsulation was effective in the order benzene > toluene > ethylbenzene > xylene. This trend was in agreement with the molecular weights of BTEX compounds (benzene < toluene < ethylbenzene < xylene). This suggested that smaller molecules are more easily encapsulated into the hydrophobic vacancy. The similar results were shown to another recent research [21,22].  J.-W. CHOI ET AL. Copyright © 2011 SciRes. ACES 80 benzenetoluene ethylbenzenexylene Equilibrium Concentration (mgL-1) 0 2 4 6 8 10 PSN 01 PSN 02 benzenetoluene ethylbenzenexylene Equilibrium Concentration (mgL-1) 0 2 4 6 8 10 PSN 01 PSN 02 Figure 4. The equilibrium concentration after BTEX en- capsulation treatment by the polystyrene-block-poly (N- isopropylacrylamide). All the equilibrium concentrations on the PSN 02 adsor- bents were lower than those on PSN 01. Consequently, in this study, PSN 02 was found to be a more effective ad- sorbent than PSN 01 for equilibrium concentrations of all BTEX compounds via encapsulation. The amount of encapsulation, distribution ratio and percent removal, qe (mg·g-1), KD (mL·g-1) and R (%) in equilibrium concen- tration, respectively, were calculated as follows: 0ee qCCVM (1) 0Dee K CCCVM (2) 00 %100 e RCCC (3) where, C0 is the initial concentration of the BTEX solu- tion (mg·L-1), Ce the equilibrium concentration of BTEX (mg·L-1), V the volume of BTEX solution (l) and M the mass of PSN adsorbent (g). In Table 2, the sequence of distribution ratio is PSN 02 > PSN 01, which is related to the Mw of PS (23 900 > 4 560). Conversely, the order of removal capacity for the tested polymers was PSN 02 < PSN 01, and was dependent on the Mw of PSN (31 800 < 48 000). The result indicates that an amphiphilic block copolymer with a lower polystyrene molecular weight and higher PSN molecular weight would have a greater binding affinity. In other words, the Van der Waals inte- ractions between the aromatic ring of BTEX compounds and the hydrophobic core of PSN contribute to the en- capsulation of BTEX into polymer. 3.3. Encapsulation Studies-Competition in Mixed BTEX Solution Solutions containing either a single compound and mixed compounds were analyzed to verify the difference for amounts of encapsulation. Different results were ob- served and it became possible because of competition between the different organic compounds within the Table 2. Values of equilibrium encapsulation, qe, distribu- tion ratio, KD, and percent removal, R (%), by each polys- tyrene- block-poly (N-i sopropylacrylamide). AdsorbentsAdsorbates qe (mg·g-1) KD (ml·g-1)R (%) PSN 01 benzene 3.8791 6,417 97.0 toluene 3.6421 2,035 91.1 ethylbenzene 3.1648 757.9 79.1 xylene 3.0588 649.9 76.5 PSN 02 benzene < 3.9 7,800 80.5 toluene 3.8438 4,920 80.8 ethylbenzene < 3.9 7,800 80.5 xylene < 3.8 3,800 81.0 mixture adsorbed by PSN 01 (Figure 5). Lesser amounts of BTEX adsorption were observed due to the competi- tion between the BTEX within mixed solutions. The ad- sorption of BTEX was; thus, observed to proceed in the order xylene < ethylbenzene < toluene < benzene. The tendency for adsorption of organic chemical compounds (BTEX) is generally explained as being associated with increasing solubility and decreasing molecular weight [23,24]. For the observed uptake of BTEX, it appears that the PSN 01 amphiphilic block copolymer exhibited different removal capacities (R, %) in the order ben- zene > toluene > ethylbenzene > xylene (Table 3). The results of present study showed that polystyrene- block-poly (N-isopropylacrylamide) is an effective ad- sorbent for BTEX treatment, with potential application as a novel and cost effective adsorbent for organic pollu- tants, which can also be simply regenerated and reused with LCST characteristics. Taking into consideration not only the encapsulation of BTEX, but also their decom- position, further works is in progress to improve the en- capsulation capacity of the amphiphilic block copolymer benzenetolueneethylbenzene xylene Equilibrium concentration (mg L-1) 0 2 4 6 8 10 12 14 B,T,E,X compounds Total BTE X benzenetolueneethylbenzene xylene Equilibrium concentration (mg L-1) 0 2 4 6 8 10 12 14 B,T,E,X compounds Total BTE X Figure 5. Equilibrium concentration after BTEX encapsu- lation treatment by the PSN 01 amphiphilic block copoly- mer.  J.-W. CHOI ET AL. Copyright © 2011 SciRes. ACES 81 Table 3. Different equilibrium encapsulation values, qe, distribution ratio, KD, and percent removal, R(%), into PSN01 when total BTEX was discharged. benzene tolueneethylbenzene xylene qe (mg·g-1) 3.7876 2.8411 2.1251 2.0949 KD (mL·g-1) 3,566 490 227 220 R (%) 94.7 71.0 53.1 52.4 and on the introduction of a photocatalytic amphiphilic block copolymer. 4. Conclusions Novel polymeric adsorbents, 2 types of polystyrene- block-poly (N-isopropylacrylamide) (PSN), were de- veloped and could be applied for removal of dissolved organic pollutants from wastewater. When this novel material is discharged into water, micelles form as a unit molecule. Encapsulation of organic contaminants by the polystyrene-block-poly (N-isopropylacrylamide) (PSN) resulted in increased hydrophobicity of the po- lystyrene, with abundant hydrophobic spaces within the amphiphilic adsorbents. This means that the core- organic complexes are effective in adsorption of vari- ous organic pollutants by hydrophobic binding. The encapsulation mechanism of aromatic compounds by PSN was investigated and found to be mainly attribut- able to the Van der Waals interactions between the aromatic ring of BTEX and the hydrophobic core of PSN is the main kinetics of BTEX removal. Polysty- rene-block-poly (N-isopropy-lacrylamide) showed good potential as a new cost effective adsorbent for applica- tion to wastewater treatment, which can be simply re- generated and reused by changing temperature of ex- ternal solution. 5. Acknowledgement Authors acknowledge that this study was granted by project No 022-081-044 from the Next-generation Core Environmental Technology Development Project by the Ministry of Environment in Korea. 6. References [1] A. Bembnowska, R. Pelech and E. Milchert, “Absorption from Aqueous Solutions of Chlorinated Organic Com- pounds onto Activated Carbon,” Journal of Colloid and Interface Science, Vol. 265, 2003, pp. 276-282. doi:10.1016/S0021-9797(03)00532-0 [2] Y. Matsui, Y. Fukuda, T. Inoue and T. Matsushita, “Ef- fect of Natural Organic Matter on Powdered Activated Carbon Adsorption of Trace Contaminants: Characteris- tics and Mechanism of Competitive Adsorption,” Water Research, Vol. 37, 2003, pp. 4413-4424. doi:10.1016/S0043-1354(03)00423-8 [3] S. Nouri, F. Haghseresht and G. Q. M. Lu, “Comparison of Adsorption Capacity of p-Cresol & p-Nitrophenol by Activated Carbon in single and Double Solute,” Adsorp- tion, Vol. 8, 2002, pp. 215-223. doi:10.1023/A:1021260501001 [4] M. F. R. Pereira, S. F. Soares, J. J. M. Orfao and J. L. Figueiredo, “Adsorption of Dyes on Activated Carbons: Influence of Surface Chemical Groups,” Carbon, Vol. 41, 2003, pp. 811-821. doi:10.1016/S0008-6223(02)00406-2 [5] R. Crisafully, M. A. L. Milhome, R. M. Cavalcante, E. R. Silveira, D. D. Keukeleire and R. F. Nascimento, “Re- moval of Some Polycyclic Aromatic Hydrocarbons from Petrochemical Wastewater Using Low-Cost Adsorbents of Natural Origin,” Bioresource Technology, Vol. 99, 2008, pp. 4515-4519. doi:10.1016/j.biortech.2007.08.041 [6] J. M. Li, X. G. Meng, C. W. Hu and J. Du, “Adsorption of Phenol, p-Chlorophenol and p-Nitrophenol onto Func- tional Chitosan,” Bioresource Technology, Vol. 100, 2009, pp. 1168-1173. doi:10.1016/j.biortech.2008.09.015 [7] T. Robinson, B. Chandran and P. Nigam, “Removal of Dyes from a Synthetic Textile Dye Effluent by Biosorp- tion on Apple Pomace and Wheat Straw,” Water Re- search, Vol. 36, 2002, pp. 2824-2830. doi:10.1016/S0043-1354(01)00521-8 [8] M. X. Loukidou, K. A. Matis and A. I. Zouboulis and M. Liakopoulou-Kyriakidou, “Removal of As (V) from Wastewaters by Chemically Modified Fungal Biomass,” Water Research, Vol. 37, No. 18, 2003, pp. 4544-4552. doi:10.1016/S0043-1354(03)00415-9 [9] S. Y. Lee, S. J. Kim, S. Y. Chung and C. H. Jeong, “Sorption of Hydrophobic Organic Compounds onto Or- ganoclays,” Chemosphere, Vol. 55, 2004, pp. 781-785. doi:10.1016/j.chemosphere.2003.11.007 [10] R. Celis, H. M. Carmen and J. Cornejo, “Heavy Metal Adsorption by Functionalized Clays,” Environmental Science & Technology, Vol. 34, 2000, pp. 4593-4599. doi:10.1021/es000013c [11] O. Abollino, M. Aceto, M. Malandrino, C. Sarzanini and E. Mentasti, “Adsorption of Heavy Metals on Na-Mont- morillonite Effect of pH and Organic Substances,” Water Research, Vol. 37, 2003, pp. 1619-1627. doi:10.1016/S0043-1354(02)00524-9 [12] M. Kruk and M. Jaroniec, “Determination of the Specific Surface Area and the Pore Size of Microporous Carbons from Adsorption Potential Distributions,” Langmuir, Vol. 14, 1999, pp. 1442-1448. doi:10.1021/la980789f [13] S. Babel and T. A. Kurniawan, “Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review,” Journal of Hazardous Mater ials, Vol. 97, 2003, pp. 219-243. doi:10.1016/S0304-3894(02)00263-7 [14] M. C. Diez, M. L. Mora and S. Videla, “Adsorption of Phenolic Compounds and Color from bleached Kraft Mill Effluent Using Allophanic Compounds,” Water Research, Vol. 33, 1999, pp. 125-130. doi:10.1016/S0043-1354(98)00161-4  J.-W. CHOI ET AL. Copyright © 2011 SciRes. ACES 82 [15] Z. Qinglin and T. C. Karl, “Adsorption of Organic Pollu- tants from Effluents of a Kraft Pulp Mill on Activated Carbon and Polymer Resin,” Advances in Environmental Research, Vol. 3, 2001, pp. 251-258. [16] J. H. Qu, “Research Progress of Novel Adsorption Pro- cesses in Water Purification: A Review,” Journal of En- vironmental Sciences, Vol. 20, 2008, pp. 1-13. doi:10.1016/S1001-0742(08)60001-7 [17] B. G. Broddevall, “Purification of Wastewater from sul- phate Pulp Bleaching Plants,” US Patent 3, 969,990, 1976. [18] A. K. Chowdhury, “Process for Treatment of Caustic Liquors,” US Patent 4, 350, 599, 1982. [19] A. A. Atia, A. M. Donia, S. A. Abou-El-Enein and A. M. Yousif, “Studies on Uptake Behavior of Copper(II) and Lead(II) by Amine Chelating Resins with Different Tex- tural Properties,” Separation and Purification Technology, Vol. 33, 2003, pp. 295-301. doi:10.1016/S1383-5866(03)00089-3 [20] J. T. Lai, D. Filla and R. Shea, “Functional Polymers from Novel Carboxyl-Terminated Trithiocarbonates as Highly Efficient RAFT Agents,” Macromolecules, Vol. 35, 2002, pp. 6754-6756. doi:10.1021/ma020362m [21] L. Tušek, K. Stana-Kleinschek, V. Ribitsch and S. Strnel, “Spectrophotometric and Electrokinetic Characterization of Dye Sorption on PA6,” Materials Science Forum, Vol. 456, 2004, pp. 455-458. doi:10.4028/www.scientific.net/MSF.455-456.455 [22] M. Marsili, N. Witkowski, O. Pulci, O. Pluchery, P. L. Silvestrelli, R. Del Sole and Y. Borensztein, “Adsorption of Small Hydrocarbon Molecules on Si Surfaces: Ethy- lene on Si(001),” Physical Review B, Vol. 77, 2008, pp. 125337-125344. doi:10.1103/PhysRevB.77.125337 [23] F. DeSilva, “Acrivated Carbon Filtration,” Water Quality Products Magazine, 2000. [24] L. K. Wang, R. P. Leonard, D. W. Goupil and M. H. Wang, “Adsorption of Dissolved Organics from Industri- al Effluents on to Activated Carbon,” Journal of Chemi- cal Technology and Biotechnology, Vol. 25, 2007, pp. 491-502. |

