Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 20 1 1, 1, 33-36 doi:10.4236/aces.2011.12006 Published Online April 2011 (http://www.scirp.org/journal/aces) Copyright © 2011 SciRes. ACES 33 Preparation of Nano-Modified Polyacrylamide and Its Application on Solid-Liquid Separation in Waste Drilling Mud Fanghui Wang, Jiantao Fan, Hong Zhu*, Kefei Han, Jing Zou, Haiyun Sun Institute of Modern Catalyst, State key Laboratory f Chemical Reso urce Engineering, School of Science, Beijing University of Chemical Technology, Beijing, China E-mail: fhwang@mail.buct.edu.cn Received December 13, 2010; revised January 10, 2011; accepted March 30, 2011 Abstract To satisfy the requirement on solid-liquid separation in high-density waste drilling mud, prepare the na- no-modified polyacrylamide (PAM) flocculant for high density waste drilling mud by in-situ dispersion me- thod, direct dispersion method and simultaneous formation method. The result showed the flocculent effect of nano-modified polyacrylamide prepared by simultaneous formation method was the best. When the con- tent of water glass and acrylamide (AM) were respectively 3% and 15%, reaction temperature was 60˚C and reaction time was 3 h, the performance of product was the best. The water content in filter cake was 24.32% after the waste drilling mud disposed by the optimization flocculant. The flocculent effect of optimization flocculant was superior to that of other flocculant in market. Keywords: In-Situ Dispersion Method, Direct Dispersion Method, Simultaneous Formation Method, Nano-Modified Polyacrylamide, High Density Waste Drilling Mud 1. Introduction The waste drilling fluids was the inevitable industrial waste while Oil and Gas exploration drilling. It had be- come one of the most severe pollution sources, whose effect on environment has been concerned gradually [1-3]. There were many methods [4-6] to dispose the waste drilling mud and drilling wastewater. The solid- liquid separation [7] was the most important and widely application method. At deep well drilling, the component of waste drilling mud was complexity, the density of waste drilling mud was higher and higher, and the waste drilling mud treatment was more and more hard [8]. The flocculant in market had not deal with the high density waste drilling mud. So a new nano-modified polyacry- lamide flocculant for high density waste drilling mud was prepared. 2. Experiment 2.1. Agent Cation polyacrylamide (GP), anion polyacrylamide (GP), silane coupling agent KH570 (GP), homemade nano– SiO2 (40 - 60 nm), water class (modulus = 3.2~2.3), hy- drochloric acid (AR), absolute ethyl alcohol (AR), acry- lamide (AR), Potassium peroxydisulfate (AR), Sodium sulfite (AR), oxalic acid (AR), Nitrogen. High density waste drilling mud, the density is 1.639 g/mL and the solid content is 56.72%. 2.2. Preaparation of Nano-Modified Polyacrylamide 2.2.1. In- Situ Dispersion M e thod (1) Preaparation of nano-modified Cation polyacryla- mide (CPAM) Dissolve respectively 4.2 g Na2SiO3 in 10 ml deio- nized water and 8.0 g CPAM in 380 ml deionized water, then drop the Na2SiO3 solution into CPAM solution and mix equably. Make HCl to adjust the pH of above mix- ture to about 7 and keep the system react for 3 h at room temperature. (2) Preaparation of nano-modified anion polyacryla- mide (APAM) Dissolve respectively 4.2 g Na2SiO3 in 10 ml deio-  F. H. WANG ET AL. Copyright © 2011 SciRes. ACES 34 nized water and 8.0 g APAM in 380 ml deionized water, then drop the Na2SiO3 solution into APAM solution and mix equably. Make HCl to adjust the pH of above mix- ture to about 7 and keep the system react for 3 h at room temperature. 2.2.2. Direct Dispersion Method Disperse 8.0 g homemade nano SiO2 in 50 ml absolute ethyl alcohol, add 2 ml KH570 into above mixture, then keep stirring and reacting for 48h at room temperature. Drying above mixture in the end at 60˚C to gain the nano SiO2 modified by silane coupling agent. Disperse 4.0 g nano SiO2 modified by silane coupling agent in 100 ml deionized water, add 5 g acrylamide into above mixture and removal of oxygen dissolved in the solution by blowing continuously nitrogen. Then add 0.1 g potas- sium persulfate to initiate polymerization and keep the system react for 4 h to gain product. 2.2.3. Simulta neous Formation Metho d Dissolve 4 ml water class into 80 ml deionized water, adjust the pH of the water class solution to 3 - 4 by HCl and react for 1 h at room temperature. Dissolve 1 ml KH570 into 10 ml absolute ethyl alcohol, adjust the pH of the KH570 solution to 3 by oxalic acid and hydrolyze for 1 h at room temperature. Add KH570 solution into water class solution, then add 20 acrylamide into above mixture and removal of oxygen dissolved in the solution by blowing continuously nitrogen. Finally add 0.1 g po- tassium persulfate and 0.1 g sodium sulfite into above system to initiate polymerization. Keep the system react for 4 h to gain product. 2.2.4. Floccule nt Treatment Add 4ml MgCl2 solution (0.2 g/ml) as gel breaker to 200 g waste drilling mud (1:1-fold diluted with water), then add some flocculant to above system and stir, centrifugal separation (4000 r/min) for 10 min. Weight the quality of filter cake (m1) and soild phase after baking (m2). Gain the water content in filter cake by the formula as follows: The water content in filter cake = m1 m2m1. 3. Result and Discussion 3.1. Flocculent Effect of Three Kinds of Preparation Method (1) Nano-modified CPAM flocculant prepared by in-situ dispersion method (2) nano-modified APAM flocculant prepared by in-situ dispersion method (3) nano-modified PAM flocculant prepared by direct dispersion method (4) nano-modified PAM flocculant prepared by simul- taneous formation method From Tables 1-4, we can know that flocculent effect of nano-modified polyacrylamide prepared by simultaneous formation method was the best Among three preparation methods. The optimum conditions for preparing nano- modified polyacrylamide prepared by simultaneous for- mation method was obtained through orthogonal expe- riments. 3.2. Optimization of the Simultaneous Formation Method Content of water class and acrylamide, react temperature and react time were selected and tested by four-factors- three-level orthogonal processing test. The test result is shown in Table 5. From Table 5, we can know the test number 2 was the best. 3.3. Analysis of FTIR Figure 1 was The FTIR spectrum of the sample (test number 2 in Table 5). As can be seen from the Figure 1, the peak of IR spectra of N-H stretching vibration ap- peared at 3419 cm–1 and 3197 cm–1; the peak of IR spec- tra of –CH2– asymmetry stretching vibration and –CH2– symmetric stretching vibration appeared respectively at 2922 cm–1 and 2850 cm–1; the absorption peak appeared at 1652 cm–1and 1417 cm–1 was caused respectively by the stretching vibration of C=O in acylamide group and vibration of saturated C-H. The absorption peak of Si-O-C and Si-O-Si appeared at 1120 cm–1. Table 1. The effect of Content of nano-modified CPAM on the water content in filter cake. Content of flocculant (%) 0.2 0.4 0.60.8 The water content in filter cake (%) 51.05 52.30 46.04 47.62 Table 2. The effect of Content of nano-modified APAM on the water content in filter cake. Content of flocculant (%) 0.2 0.4 0.60.8 The water content in filter cake (%) 56.37 54.47 48.76 50.07 Table 3. The effect of Content of flocculant on the water content in filter cake. Content of flocculant (%) 0.2 0.4 0.6 0.8 The water content in filter cake (%) 35.98 35.24 34.87 35.13 Table 4. The effect of Content of flocculant on the water content in filter cake. Content of flocculant (%) 0.2 0.4 0.6 0.8 The water content in filter cake (%) 33.01 32.55 30.68 31.73 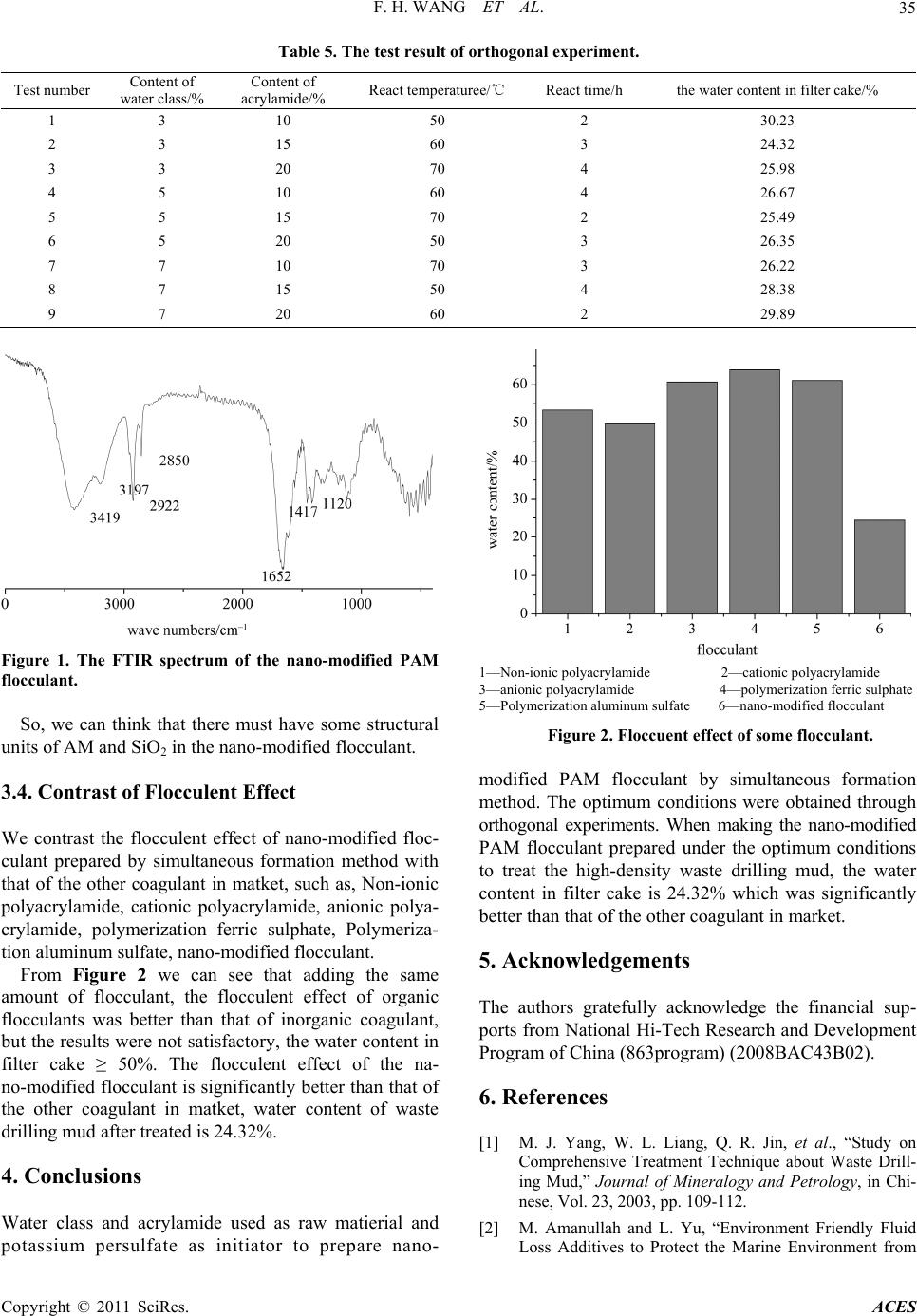 F. H. WANG ET AL. Copyright © 2011 SciRes. ACES 35 Table 5. The test result of orthogonal experiment. Test number Content of water class/% Content of acrylamide/% React temperaturee/℃ React time/h the water content in filter cake/% 1 3 10 50 2 30.23 2 3 15 60 3 24.32 3 3 20 70 4 25.98 4 5 10 60 4 26.67 5 5 15 70 2 25.49 6 5 20 50 3 26.35 7 7 10 70 3 26.22 8 7 15 50 4 28.38 9 7 20 60 2 29.89 Figure 1. The FTIR spectrum of the nano-modified PAM flocculant. So, we can think that there must have some structural units of AM and SiO2 in the nano-modified flocculant. 3.4. Contrast of Flocculent Effect We contrast the flocculent effect of nano-modified floc- culant prepared by simultaneous formation method with that of the other coagulant in matket, such as, Non-ionic polyacrylamide, cationic polyacrylamide, anionic polya- crylamide, polymerization ferric sulphate, Polymeriza- tion aluminum sulfate, nano-modified flocculant. From Figure 2 we can see that adding the same amount of flocculant, the flocculent effect of organic flocculants was better than that of inorganic coagulant, but the results were not satisfactory, the water content in filter cake ≥ 50%. The flocculent effect of the na- no-modified flocculant is significantly better than that of the other coagulant in matket, water content of waste drilling mud after treated is 24.32%. 4. Conclusions Water class and acrylamide used as raw matierial and potassium persulfate as initiator to prepare nano- 1—Non-ionic polyacrylamide 2—cationic polyacrylamide 3—anionic polyacrylamide 4—polymerization ferric sulphate 5—Polymerization aluminum sulfate 6—nano-modified flocculant Figure 2. Floccuent effect of some flocculant. modified PAM flocculant by simultaneous formation method. The optimum conditions were obtained through orthogonal experiments. When making the nano-modified PAM flocculant prepared under the optimum conditions to treat the high-density waste drilling mud, the water content in filter cake is 24.32% which was significantly better than that of the other coagulant in market. 5. Acknowledgements The authors gratefully acknowledge the financial sup- ports from National Hi-Tech Research and Development Program of China (863program) (2008BAC43B02). 6. References [1] M. J. Yang, W. L. Liang, Q. R. Jin, et al., “Study on Comprehensive Treatment Technique about Waste Drill- ing Mud,” Journal of Mineralogy and Petrology, in Chi- nese, Vol. 23, 2003, pp. 109-112. [2] M. Amanullah and L. Yu, “Environment Friendly Fluid Loss Additives to Protect the Marine Environment from  F. H. WANG ET AL. Copyright © 2011 SciRes. ACES 36 the Detrimental Effect of Mud Additives,” Journa l of Pe- troleum Science and Engineering, Vol. 48, No. 3-4, 2005, pp. 199-208. doi:10.1016/j.petrol.2005.06.013 [3] S. Rehan and H. Tahir, “A Fuzzy-Based Methodology for an Aggregative Environmental Risk Assessment: A Case Study of Drilling Waste,” Envi ronmental Modelling & Soft- ware, Vol. 20, No. 1, 2005, pp. 33-46. doi:10.1016/j.envsoft.2003.12.007 [4] C. G. Street and S. E. Guigard, “Treatment of Oil-Based Drilling Waste Using Supercritical Carbon Dioxide,” Jour- nal of Canadian Petroleum Technology, Vol. 48, No. 6, 2009, pp. 26-29. [5] J. A. Veil, “Drilling Waste Management,” Journal of Petroleum Technology, Vol. 54, 2002, pp. 50-52. [6] M. P. Carignan, C. B. Lake and T. Menzies, “Assessment of Two Thermally Treated Drill Mud Wastes for Landfill Containment Applications,” Waste Management and Re- search, Vol. 25, No. 5, 2007, pp. 394-401. doi:10.1177/0734242X07073652 [7] J. G. Zhang, J. Nie and H. Deng, “An Experimental Study on the Treatment of Waste Drilling Fluid by Solid-Liquid Separation,” Envi ronmental Protection of Oi l & Gas Fields, in Chinese, Vol. 11, 2001, pp. 20-32. [8] X. J. Guo and H. Ma, “Application and Study of High Density Drilling Fluid Technology,” Drilling Fluid and Completion Fluid, in Chinese, Vol. 26, 2009, pp. 8-10. |

