 Journal of Biosciences and Medicines, 2014, 2, 43-55 Published Online May 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.23007 How to cite this paper: Shigwedha, N., et al. (2014) Probiotical Cell Fragments (PCFs) as “Novel Nutraceutical Ingredients”. Journal of Biosciences and Medicines, 2, 43-55. http://dx.doi.org/10.4236/jbm.2014.23007 Probiotical Cell Fragments (PCFs) as “Novel Nutraceutical Ingredients” Nditange Shigwedha1,2,3, Liubov Sichel3, Li Jia4, Lanwei Zhang1 1School of Food Science and Engineering, Harbin Institute of Technology, Harbin, PR China 2Department of Food Science and Technology, University of Namibia, Windhoek, Namibia 3Pure Research Products LLC, Boulder, USA 4Wyeth Nutrition, Suzhou, China Email: ndita nge@gmail.co m, l sichel1@g mail.co m, jialicad@ gmail.co m, zhanglw@hit.edu.cn Received January 2014 Abstract Probiotical cell fragments (PCFs) are structural components of the probiotic cell lysate(s) and ex- hibit similar beneficial effects on the host as live probiotic bacteria. With cell fragment technology (CFT™), the structural fragments are isolated and purified from live probiotic cells. While ob- served to be strain-dependent as in the case of live probiotics, orally administered PCFs demon- strated a broad spectrum of immune modulation functions; anti-allergy; anti-inflammation; an- ti-bacterial and anti-viral properties; anti-mutagenic; and radioprotective and detoxification abil- ities in humans and animals. The PCFs mechanisms of action include events of motifs of cell wall peptidoglyc ans (PGs), DNA motifs, nucleotide containing components, lipoteichoic acids (LTAs), surface layer (S-layer) proteins, and cellular carbohydrates. Different immunological in vivo-in vi- tro tests have shown that PCFs, essentially, have the ability to stimulate the macrophages, and in- duce cytokines such as interleukins (ILs), tumor necrosis factors (TNFs), interferons (IFNs), and natural killer (NK) cells. PCFs may be used as ingredients for foods and beverages or as nutritional supplements with long term stability and shelf-life up to 5 years. PCFs may also be used as health restorative ingredients in cosmetic products. The outcome of probiotics CFT™ stands as an advan- tage to the food and pharmaceutical industries, regarding the formulation of unique products with unadulterated sensory characteristics of origin. Hence, PCFs are being characterized here as “nov- el nutraceutical ingredients” for health maintenance in both humans and animals. Keywords Probiotical Cell Fragments (PCFs), P ar ap r ob i oti c s, I m munobi otics, I mm unomod u lator , Nutr aceu t i cal I ngred ients, Lactic Acid Bacteria (LAB) 1. Introduction Probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host [1]. In contrast, paraprobiotics are defined as non-viable microbial cells (intact or broken) or crude cell ex- tracts which when administered (orally or topically) in adequate amounts, confer a benefit on the human or animal 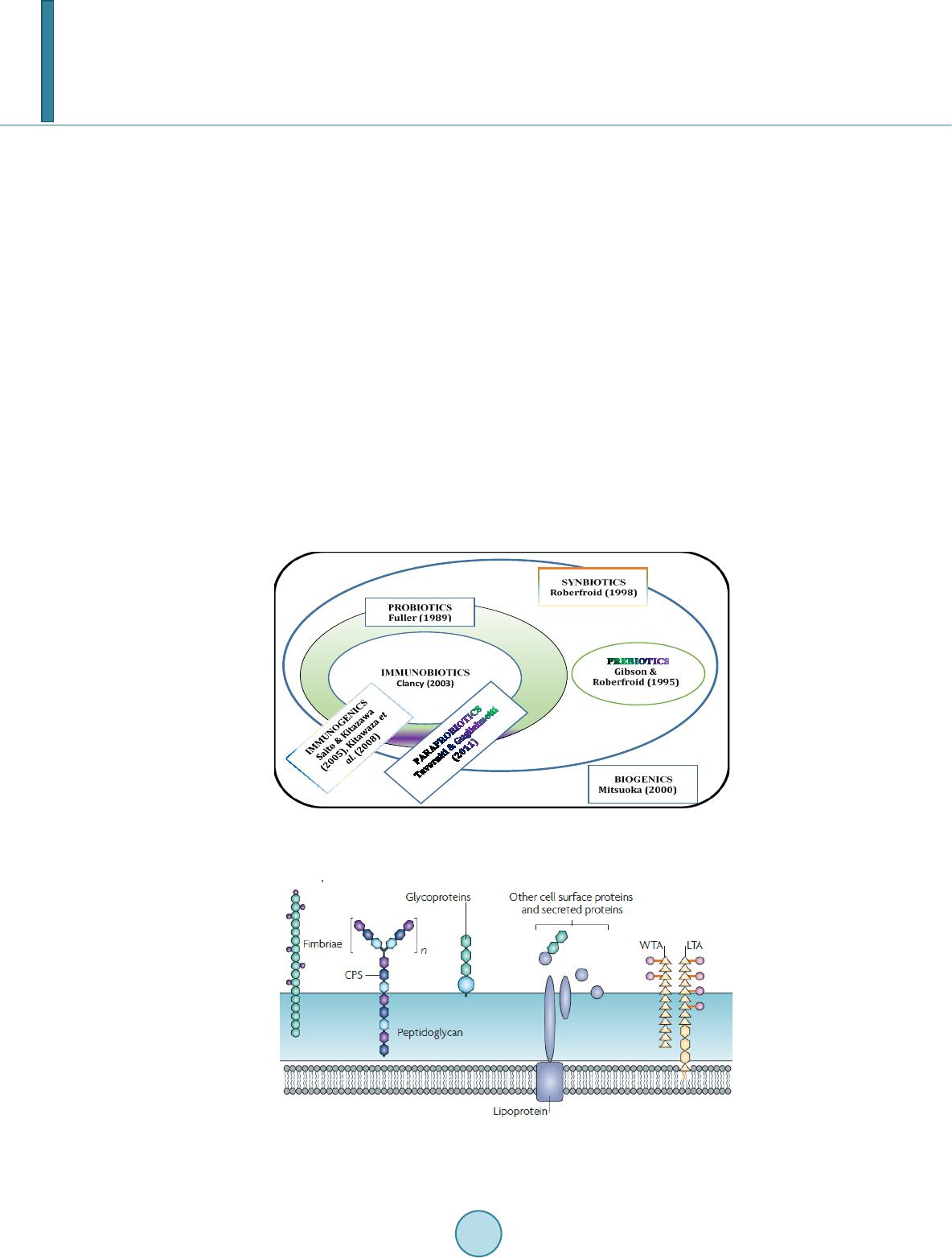 N. Shigwedha et al. consumer [2]. The prefix “para” has been preferred for its interpretation of “alongside of” or “atypical”, which can simultaneously display similarity to and difference from the traditional probiotic definition. When isolated and purified, the dynamic cellular structures from probiotics or paraprobiotics (including their derivatives or metabolites) can be categorized into different fragments. These active fragments, metabolites, or derivatives are expressed as immunogenics (Figure 1) [3]-[5]. Immunogenics include intracellular and extracellular bacterial components with immunoregulatory abilities, such as peptidoglycan (PG), lipoteichoic acid (LTA), extracellular phosphopolysaccharides, and DNA/RNA [4]. Paraprobiotics in particular, contain many structural fragments with physiological functions, such as cell wall PGs, teichoic acids (TAs), nucleotide containing components of DNA and/or RNA motifs, surface layer (S-layer) proteins, secreted proteins, and various cell wall-associated polysaccharides (CPSs) as shown in Figure 2. Among these fragments of which some are immunogenics, the expression “probiotical cell fragments” or PCFs is assigned to them (see Figure 3). 2. Background of PCFs as the Potent Immune Response Modifiers By the use of cell fragment technology (CFT™), the structural fragments can be isolated and purified from the renowned live probiotic cells. For example, cell wall of lactobacilli contains several distinctive structural ele- ment s, consisting of the multilayered PG sacculus, namely, muramyl-peptides (MPs), muramyl dipeptides (MDPs), and muramyl polypeptides (MPPs), which are seeded with TAs , cellular polysaccharides, and some other proteins. TAs are the sec o nd main constituents of the cell walls and usually contain repeating units of po- lyribitol phosphate or polyglycerol phosphate (Glc) covalently anchored to PG (see Figure 2). The TAs hold Figure 1. Diagram representation of the interactions between the modern concepts of probiotic and related terms (Modified from reference [5]. Figure 2. Example of isolated PCFs within a structure of gram (+) para- probiotics (Adapted from reference [6]). 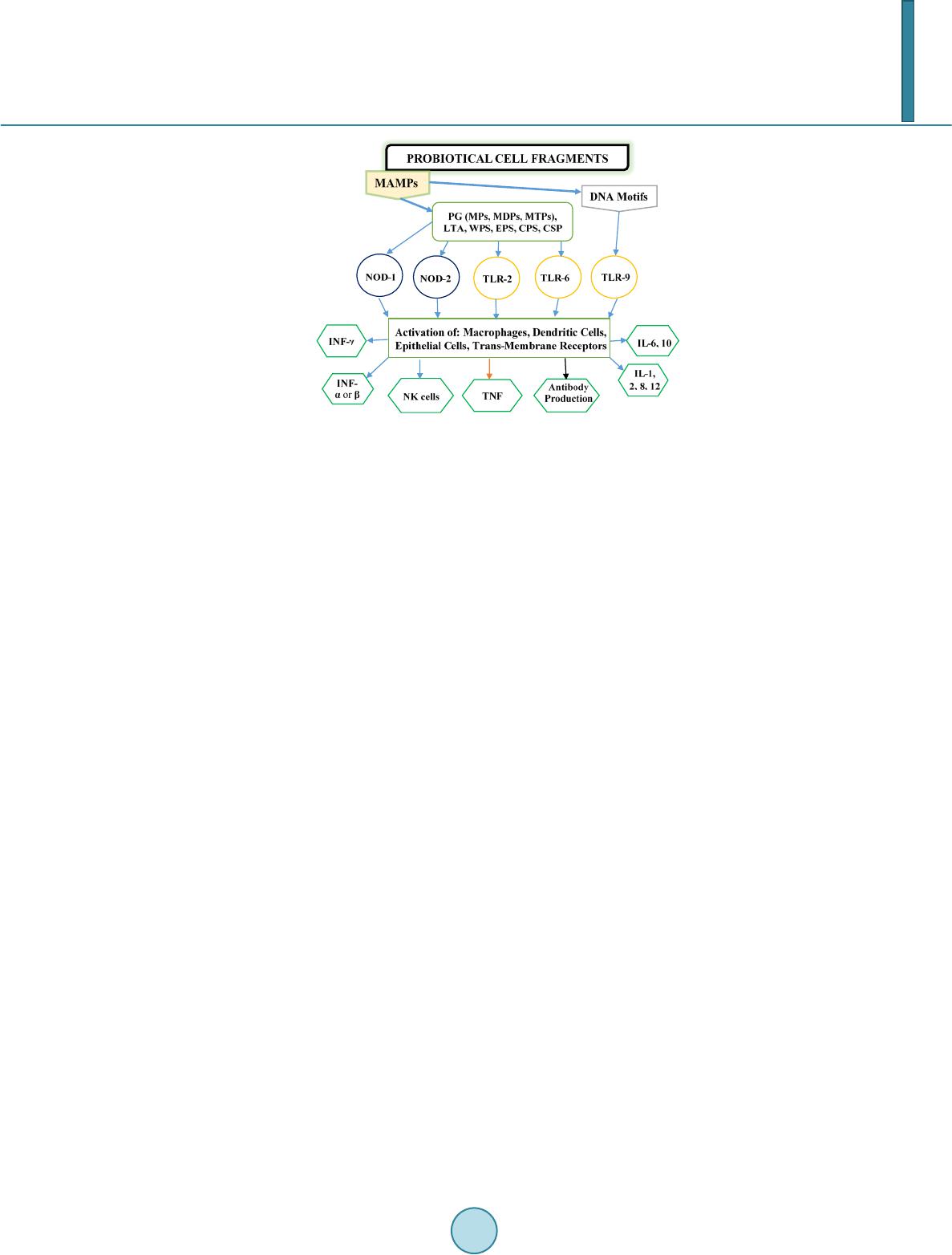 N. Shigwedha et al. Figure 3. Immunoactivat ion mechanisms of immunogenic PCFs; PG is peptidoglycan, MPs are muramyl peptides; MDPs are muramyl dipeptides; MTPs are muramyl tripeptides; LTA is lipoteichoic acid; WPS is wall polysaccharides; EPS is exopoly- saccharides; CPS is capsular polysaccharides, and CSP is cel- lular surface proteins. 2 types of polymers: wall-teichoic acids (WTAs) and lipoteichoic acids (LTAs) [7]. Interestingly, only LTAs are immunogenics, which have been identified as a key factor for triggering the synergistic induction of interleukin (IL)-10 production [8]. The fragments of genome immunogenic DNA motifs have been found to induce immu- noglobulin secretion and B-cell proliferation, or inhibited IL-8 secretion by HT-29 cells stimulated with tumor necrosis factor-α (TNF-α) [5]. In addition, genome DNA motifs from immunobiotic lactic acid bacteria (LAB) induce the immunoactivation of gut-associated lymphoid tissue (GALT). The term immunobiotics refers to probiotic strains that are benefi- cially able to regulate the mucosal immune system [9]. It has been also established that the nucleotide-binding oligomerization domain-containing protein-1 (NOD-1), NOD-2, toll-like receptor-2 (TLR-2), and TLR-9 are able to identify or recognize the DNA motifs and cell wall components of dietary LAB, thereby contributing to immunoregulation in the GALT [3]. With respect to immunoactivation mechanisms caused by immunogenic PCFs, mechanisms mediated by different TLRs and NOD-like receptors (NLRs) as pattern recognition receptors (PRRs) have recently been uncovered, and found to be part of the complex innate immune system in both hu- mans and animals (see Figure 3). The polysaccharides within paraprobiotic cell wall possess 3 types: PG glycan strand (known as wall poly- saccharides or WPS), exopolysaccharides (EPS), and capsular polysaccharides or CPS [7]. Among them, only WPS and EPS are immunogenics. Overall, MPs, MDPs, MTPs, LTAs, WPS, EPS, and some surface proteins are immunogenics which have been clinically proven to interact with different PRRs from intestinal epithelial cells (IECs) and mucosal immune cells, thereby modulating the mucosal immune system. It is necessary to emphasize that there are 3 classes of PRRs which spot and suspect viral components: 1) retinoic acid-inducible gene I like receptors (RLRs), 2) TLRs, and 3) NLRs [5]. Remarkably, carbohydrates and glycolipids (which exist in many microbes) are also known to activate epithelial cells through interaction with TLRs [10]. 3. PCFs as MAMPs and PRRs as Host Receptors As delineated in Figure 3, the PCFs may serve as microbe-associated molecular patterns (MAMPs) because they activate the corresponding PRRs of the innate immunity. The PRRs recognize the PCFs as MAMPs, which are naturally popular and conserved among many probiotics, often being located on the bacterial surface or se- creted molecules [11]. Thus far, several MAMPs of immunobiotics that can be connected to the famous host- responses have been identified [6], and in many cases, these effector molecules are associated with the bacterial cell wall [7]. The most notable PRRs include TLRs, the NLRs, the RIG-I-like RNA helicases, the C-type lectin receptors, and cytosolic DNA sensors [5]. These receptors brilliantly fine tune signals that specifically recognize 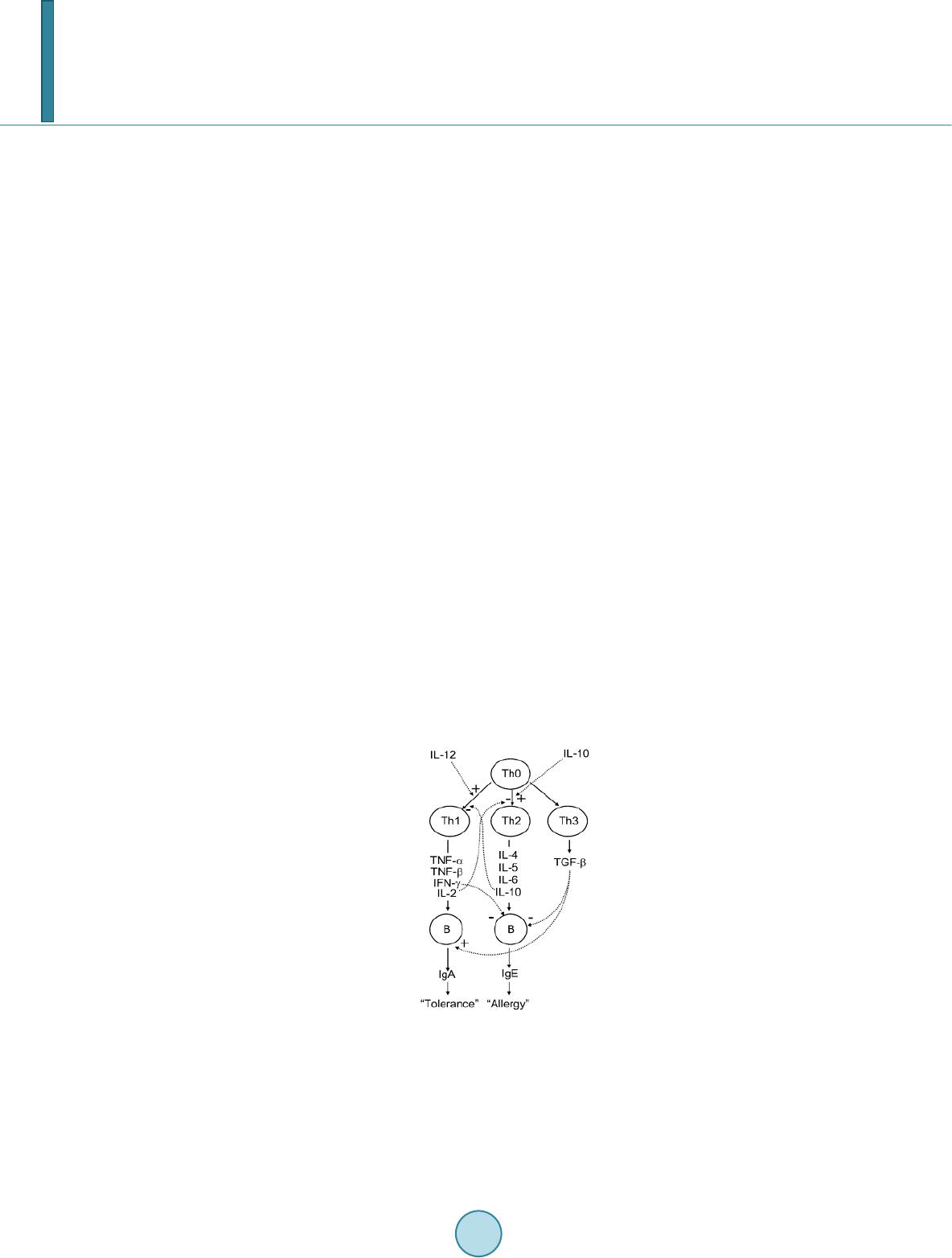 N. Shigwedha et al. the quantity and quality of conserved moieties associated with MAMPs including carbohydrates, lipids, proteins, lipoproteins, bacteriocins, and nucleic acids. Following binding of the moieties to PRRs, activation of the innate immune system leads to diverse cellular responses including the induction of interferon regulatory factors (IRFs), activator protein-1 (AP-1), and nuclear factor-kappa B (NF-κB) which all regulate the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and type I interferon (IFN) [12]. TLRs are trans-membrane proteins present at the cell surface or on the membrane of endocytic vesicles of both IECs and intestinal dendritic cells (DCs). Interestingly, PCFs encounter both intestinal DCs and IECs which are key players in both innate and adaptive immunity by means of their PRRs. In addition to TLRs, the NLRs also recognize MAMPs and are known to transmit signals on the communication with PCFs (see NOD-1 and NOD-2 in Figure 3). The PCFs are, therefore, be able to modulate the production of cytokines and regulate the immune system. Besides, the PCFs also activate the corresponding receptors of adaptive immunity towards allergy tolerance [13] (see Figure 4). With regards to immunostimulatory DNA motifs, it has been reported that cytosine-guanosine dinucleotides (CpG) -DNA derived from bacteria stimulates B lymphocytes [5] and lead to a huge surge of interest in immu- nostimulatory DNA. As part of the PCFs, immunostimulatory DNA motifs induce interferon IFN-α and IFN-γ and are also strong activator of natural killer (NK) cells. The TLR-9 has been long recognized as a receptor mo- lecule for CpG-DNAs, [14] and as a result, our understanding of the ef fe ct of DNA motifs within the PCFs on the immune system has also advanced through the TLR-9 (see Figure 3). The TLRs have been considered to be ideal for natural defense mechanisms, and for each TLR, there are unique identification molecules, which are bacterial modulins [15] [16]. In general, the TLRs are able to recognize bacterial modulins having MAMPs or patho ge n -associated molecular patterns (PAMPs) and exert the cytokine-mediated regulation over the immune response [17]. 4. History of Safe Use of PCFs The PCFs are borne of a half-century research by the European scientists [18], and their value to new and inno- vative probiotic lysates has a shared long history of more than a decade in Russia, Europe, Japan, and United States of America. Practically, all the strains used in CFT™ are considered commensal microbiota, with no pa- thogenic potential, and most have passed the evaluation of the European Union (EU) novel food regulations. LAB and the yeast Saccharomyces are the well-known probiotics. Strains of the genera Lactobacillus and Bifi- dobacterium are the utmost quantified with safety, and a number of them have gained a generally recognized as Figure 4. Development of the “allergic” [Type 2 T helper cells (Th2)] or “tolerant” (Th1) phenotype. IL-12 stimulates a shift toward the tolerant phenotype, whereas IL-10 stimulates the provision of the allergic phenotype. Th3 cells, through the production of transforming growth factor-β (TGF- β), further fuel the trend toward tolerance. On the other hand, Immunoglobulin A (IgA) supports allergy elimination; IgE may activates mast cells and cause allergic symptoms. Adapted from reference [13].  N. Shigwedha et al. safe (GRAS) status. Other genera of Streptococcus, Bacillus or Enterococcus cover numerous opportunistic pa- thogens. Clinical and experimental analyses have drawn scrutiny to many cases of prevention and treatment of gastrointestinal (GI) disorders, allergies, colonic cancer, wound healings, sinus, flu, and other acute and chronic infections/diseases or daily discomforts. A majority of these could be expressively resolved safely with the use of paraprobiotics or PCFs. Most of the PCF formulations have been tested and found to be effective in the pre- vention and treatment of all the previous stated concerns, including the stomach colonization by pathogens in humans, immune deficiency, as well as infectious lesions of bacterial, viral and fungal etiology [18]. The me- chanisms of action for PCFs on some of the essential health conditions are described separately below. Overall, the established PCFs are considered as highly safe. 5. Gastrointestinal Digestion and Absorption It is necessary to point out that the GI ecosystem features dynamic and reciprocal interactions among its com- mensal microbiota, the epithelium, and the immune system. As probiotics and/or paraprobiotics are part of the gut flora (either naturally or by supplementation), an understanding of the mechanisms whereby PCFs compel their immune modulating effects on the host requires an outline of what position PCFs occupy in the gut flora. It is also required to understand how the commensal microbiota within the stomach interact and/or support the immune system of the own host. Moreover, systemic induction of minor inflammations is believed to be consi- derable in PCF effects against atopic eczema-dermatitis syndrome. Similarly, application for PCFs aimed at en- hancing the host immune response, such as those used for the prevention of gastroenteritis or traveler’s diarrhea, also seem to require special PCF formulations with a slight immunostimulatory activity. In contrast, for inflam- matory bowel disease (IBD) treatment, the PCFs that can correct the pro-inflammatory pathology may be for- mulated a bit differently, taking the damaged epithelial barrier into account. The combinations of both viable probiotic cells and non-viable PCFs are preferred in this case. It is remarkably clear in this article that PCFs may be used as “novel nutraceutical ingredients” with the intentions of promoting excellent health, preventing and treating of infectious diseases, anti-allergies, anti-tumor or anti-colon cancer, anti-inflammatory diseases, wounds healing, and many more. Along these lines, PCFs look to establish a consensus in the development of novel nutraceutical products that are of beneficial to food and pharmaceutical industries. 5.1. PCFs as a Source of Essential and Nonessential Amino Acids The PCFs represent a source not only of muramyl peptides with biological activities but also their derived vital amino acids and organic nitrogen. The MPs, MDPs, and MTPs with biological activities (shown in Figure 3) are of significant importance as they are well characterized within PG of paraprobiotical cell wall. It has been re- ported that the stem peptides in those muramyl peptides contain 4 alternating D- and L-amino acids with bio- logical activities [19]. The 1st amino acid of the MTPs is L-alanine, the 2nd and 4th amino acids are D-glutamine and D-alanine, respectively. L-lysine is the 3rd amino acid in most Gram (+) bacteria, whereas, in some Gram (+) bacilli and the majority of Gram (–) bacteria, it is m eso -diaminopimelic acid (meso-DAP) [19]. The consensus sequence of the amino acids in lactobacilli is L-Ala/D-Glu/(L-Lys or meso-D AP) /D -Al a/ D-Ala [6]. Moreover, D-asparagine is often used as a cross-bridge between L-lysine and D-alanine and the rest may also be amidated [6]. It is necessary to acknowledge that a depletion of L-argin i ne has been implicated in the pathogenesis. Ap- parently, the use of L-Arg by trophozoites dramatically reduces the availability of this amino acid to synthesize NO, a compound that inhibits growth, encystation and excystation of Gia rdia [20]. Besides, low luminal con- centration of L-Arg has been reported to reduce enterocyte movements, thus providing the parasite with a more stable environment for colonization [20] [21]. 5.2. Mechanisms of Action As “novel nutraceutical ingredients,” PCFs are equally subjected to both digestion and absorption processes in the GI tract. They can first interact within the intestinal tract and subsequently exert their targeted needful activi- ties in various peripheral organs subsequent to absorption. For the PCFs to become bioactive, they must be pro- vided in sufficient amount during GI digestion and then regulated before and after absorption. It means that GI processes regulate the supply of digested PCFs including their amino acids and/or nitrogen into the body, and consequently, their metabolic utilization. As indicated earlier on, the key PCFs include the PGs, TAs, WPS, EPS,  N. Shigwedha et al. CPS, and different surface proteins. PG has 3 distinguished peptides: MPs, MDPs, and MTPs. These 3 PG fragments, for example, must first be hydrolyzed by lysozyme or remodeled through autolysis action in the gut before they could be detected by PRRs [6]. In addition to that, the PGs are ligands for TLR-2, with CD14 as a co-receptor [6]. On the other hand, the principal components of TAs are WTAs and LTAs. Naturally, the first digestion stage of TAs and other cellular surface proteins or secreted proteins entails pepsin hydrolysis and acid secretion in the stomach. Moreover, most of the digested PCFs are expected to be readily absorbable in an intact form as soon as they are emptied into the duodenum. However, some of the fragments are expected to precipi- tate in the stomach and then gradually released into the small intestine in the form of degraded fragments. In the duodenum, these cell fragments would be further subjected to pancreatic enzymes. To our knowledge, the PCFs have been found in the intestinal lumen (in intact and/or degraded formats), whe reby they activated the TLRs, leading to a proliferation-inducing ligand (APRIL) and the production of B cell activating factor (BAFF) [22]. These 2 cytokines are also known for promoting all kinds of IgA class-switching responses in the intestines [22]. As the intestinal tail contains numerous peptidases such as pep- sin, elastase, trypsin, chymotrypsin, and carboxypeptidase, the PGs are expected to be degraded into MTPs, MDPs, MPs, other smaller peptides, and amino acids, which would be eventually absorbed. Absorption of the digested PCFs from the intestine is anticipated to be specific too. In contrast, some PGs may be relatively resis- tant to the proteolytic digestive enzymes and thus, may also reach their core targets intactly. To be specific, pro- line or lysine substance containing MPs are likely to be highly resistant to proteolytic degradation. MDPs, on the other hand, are reported to have been taken up by the intestinal peptide transporter PEPT1 (known as SLC15A1) in intact form [6]. In comparison to the mechanisms of action outlined in Figure 3, other research also described that the acid-digested CPS induces production of higher IFN-γ and lower IL-4 in mice splenocytes than in water treated mice [23]. This discovery confirms the role of gastric acid in providing a variety of different PCFs as nu- trients that are made readily available to the body for absorption. That is to say; the digested PCFs can be ab- sorbed by Peyer’s patches through microfold (M) cells in the intestinal tract and modulate antigen-presenting cells (APCs) such as DCs, through the TLRs or NLRs. That may result in a selective enhancement of Th1 cell proliferation, and the subsequent production of IFN-γ and IL-2 (see Figure 4), which are dynamic and powerful cytokines for cell-mediated immune responses [23]. The point is that, IFN-γ does not only activates macrophag- es and NK cells, but also selectively inhibits the proliferation of Th2 cells, which produce cytokines such as IL-4 and a few others (see Figure 4). Interestingly, an apical intestine alkaline phosphatase (IAP) enzyme has been found to reduce lipopolysaccharide (LPS)-TLR-4 signaling by detoxifying LPS [6]. Most of the PCFs including the bioactive MPs may operate directly on their spectacular targets existing in the GI tract. The MDPs and MTPs may also cross the intestine and reach the peripheral target sites. Apart from in- testinal peptide transporter PEPT1 or as branded as SLC15A1, other specific transport arrangements, such as trans -cytosis (which is implicated in macromolecules and microorganisms transport by M cells) [19] and endo- cytosis (of the viral envelope proteins, DNA and RNA, outside of cells and in cytoplasmic vacuoles after pha- gocytosis) have been described [24]. The absorption and transportation of digested PCFs into the Peyer’s patches through the M cells would be processed further by the professional APCs (DCs or macrophages) before entering the mesenteric lymph nodes. Alternatively, luminal PCFs may enter lamina propria either via M cells (primarily particulate antigen) overlying the Peyer’s patch through the enterocytes (soluble antigen), or poten- tially via capture by traversing DCs as per interpretation of reference [25]. The mesenteric lymph nodes and Peyer’s patches are believed to be the 2 main inductive sites (see Figure 5). The Peyer’s patches are situated mainly on the antimesenteric side of the small intestine and include a number of B cell rich zones with germinal centers called follicles. So adjacent to these in the parafollicular areas, there are T cell rich zones [25]. After intra- cellularly processing and handling of the digested PCFs, the immune cells travel from lamina propria and Peyer’s patches to mesenteric lymph nodes from where they can enter the bloodstream via the thoracic duct (see Figure 5). However, during PCFs processing and through interaction within immunocompetent cells, most of the PCFs are expected to exert the immunomodulatory effects by inducing the right cytokine production of immunocom- petent cells. In essence, this appears to demonstrate how the PCFs may be useful in preventing cytokine me- diated GI and/or respiratory diseases. Cytokines are known by their influence to stimulate or suppress cell func- tions [25]. Furthermore, degraded PCFs can also be found in the blood in degraded format. The enzymatic pro- teolysis may produce other bioactive MPs or simple peptides in the body too. However, it should be noted that the specific MPs, MDPs, MTPs, or other peptides may be formed by proteolytic bacterial species, and they are likely to be less effective in their original format if not prepared intra-cellularly. 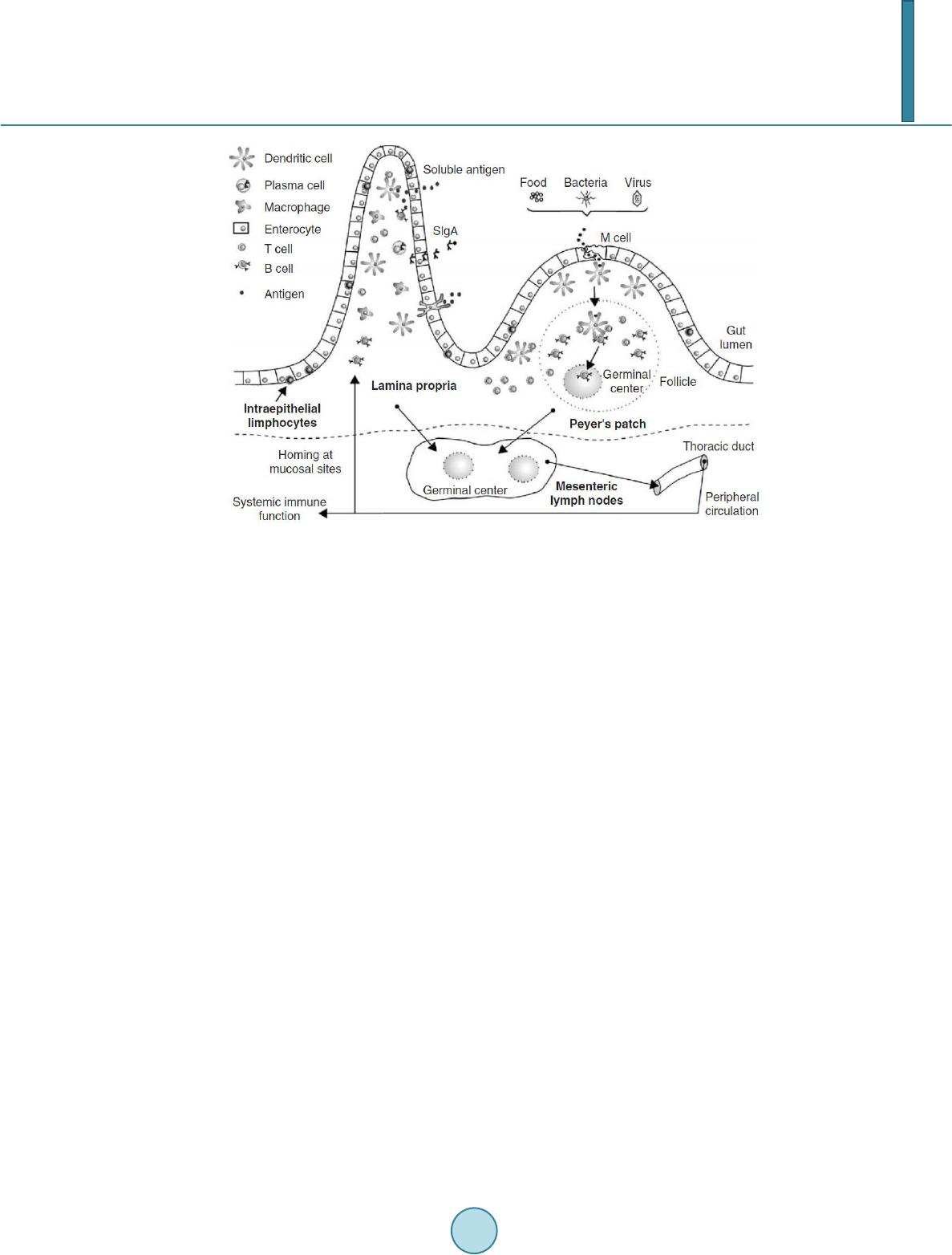 N. Shigwedha et al. Figure 5. Schematic representation of the gut-associated lymphoid tissue (GALT) (Adapted from reference [25]. 6. Body Defense Enhancement The PCFs are associated with diffe r e nt antimicrobial activities. As it can be observed in Figure 3, the PG in- cludes MPs, MDPs, and MTPs, which all confer passive defense on human gut against a wide range of patho- genic microbes. In addition, paraprobiotics contain other immunological components, such as growth factors, S-layer proteins, cytoplasmic extracts inc l ud in g LTAs, CpG-DNA motifs, WPS, EPS, and other molecules, which can modulate the immune system. Such anti-infective fragments can be presented as “novel nutraceutical ingredients” for functional foods because they boost specific and nonspecific body defenses. 6.1. Anti-Microbial and Anti-Viral Activities The S-layer proteins of paraprobiotics have been found to inhibit the adhesion of enteropathogenic bacteria such as Clostridium perfringens [26], Escherichia coli O157:H7, Salmonella Typhimurium [27], and Clostridium dif- ficile, Shigella sp., and Salmonella sp. [28] to mucus and/or intestinal epithelial cells in a competitive way. The literature reveals that if the S-layer proteins of lactobacilli are damaged or removed, the adhesion activity to the host epithelial cells is compromised [29]. They can also displace, inhibit the binding of enterobacterial toxins to their receptors, especially to mucous and Caco-2 cell surface, competitively [29]. While the CpG-DNA motifs are known to activate epithelial cells through interaction with TLRs (see Figure 3) for sinus, influenza, menin- goencephalitis, and virus infections, unspecified DNA motifs have demonstrated a higher level of antagonistic activity against bacterial and viral infections [18]. PCFs contain a number of immunostimulatory DNA motifs. Moreover, paraprobiotics have been found to contain about 2939 to 3381 different host defense peptides (HDPs) [30]. In general, the HDPs are known to influence the host response to infections in several ways, in- cluding direct antimicrobial killing, promoting wounds healing, production of cytokines, modulation of chemo- kines, enhance angiogenesis [31], limiting inflammation, anti-sepsis, enhancing vaccine responses, and regulat- ing metabolism [32]. We also believe that HDPs are deeply involved in the activation of neutrophils. Activated neutrophils are reported to have a persistent presence with a continued recruitment and activation throughout the interrupted healing process in chronic wounds as well [33]. Equally important, the bacteriocins from various probiotics have attracted considerable attention in the area of food preservation [34] and may be characterized into “novel nutraceutical ingredients” too, because they can inhibit foodborne pathogenic and spoilage bacteria. 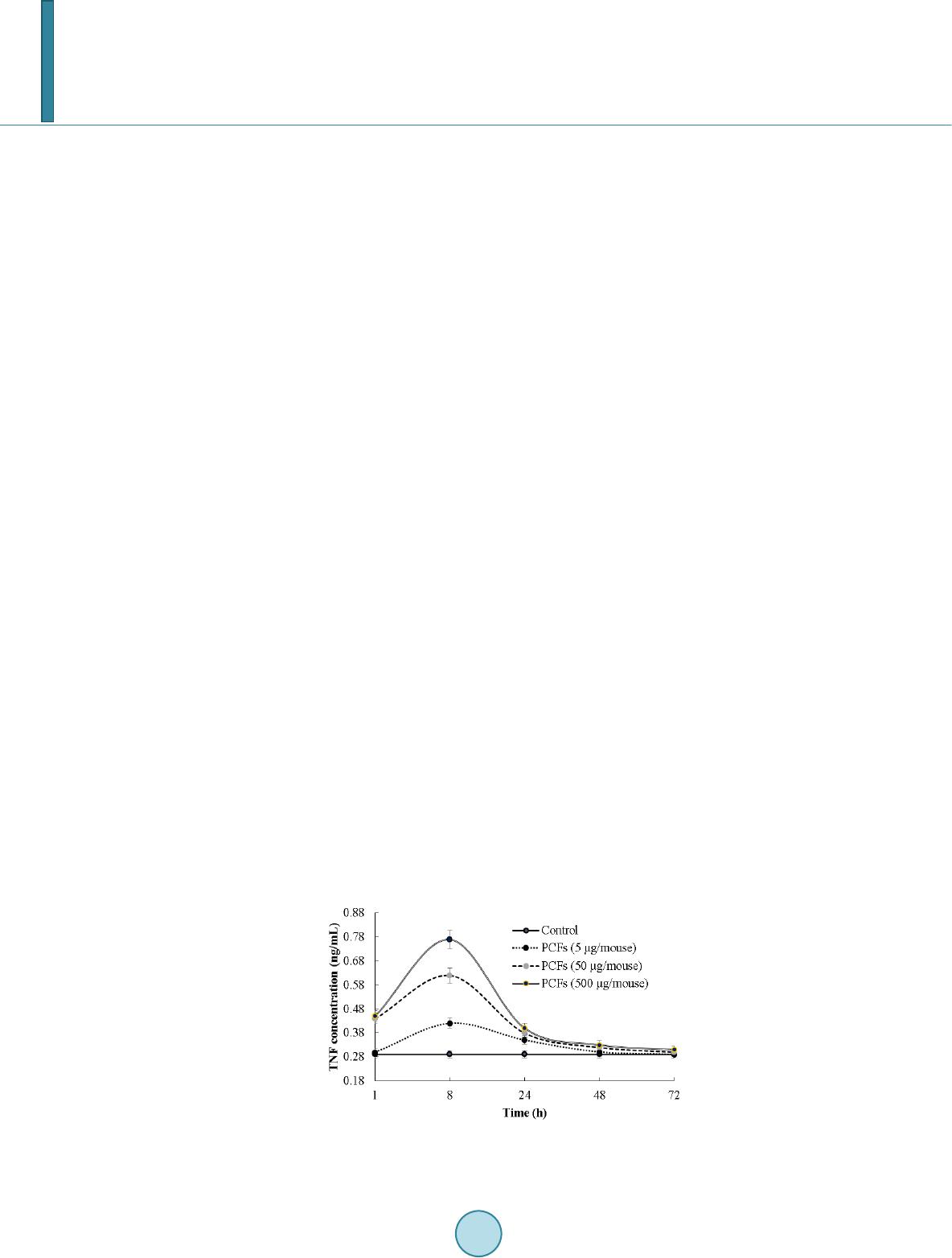 N. Shigwedha et al. 6.2. Anti-Allergic, Anti-Mutagenic, Anti-Inflammatory, and Anti-Infectious Activities Immunostimulatory sequences of DNA (ISS-DNA) and their synthetic sequences of oligodeoxynucleotides (ODNs) are the 2 identified structures of immunostimulatory DNAs [35]. They are derived from immunobiotic LAB and deeply involved in host defenses against tumor, inflammations, and other infections. Elsewhere, the DNA motifs of CpG-ODN have been reported to suppress allergen-specific acidophilic respiratory tract inflam- mation in murine models of bronchial asthma and also improves airway hyperreactivity [36]. Generally, the DNA motifs are known to be involved in the immunostimulatory events and are still gaining an advanced un- derstanding of their competence in regulating immune networks in the intestine through cytokines induction (see Figure 3). Someday, the production of PCFs as “novel nutraceutical ingredients” would be playing a significant role in the development of immunogenic foodstuffs, immunogenic feeds, and immunogenic drugs. These would have immediate effects to the prevention and protection of the host against all kinds of allergies, infect ious-, au- toimmune-, and inflammatory-disease s. Although it seems to be accepted that EPSs could not yield significant commercial products on their own [37], they are still being recommended for health-promoting effects, such as immu ne-modulating activities, anti-tumor, promising cholesterol-lowering activity, and prebiotic activity [38]. 6.3. Immunomodulating Effects Various PGs, HDPs or their peptides have been found to act as immunomodulating compounds. By employing the probiotic CFTTM, MPs, MDPs, MTPs were found to enhance phagocytosis and modulate the proliferation and/or differentiation of human peripheral blood lymphocytes [18]. For example, pure MDPs of L. rhamnosus V have been shown to stimulate the immune system predominantly by phagocytosis. This instance demonstrates that each peptide may have various biological activities. The mechanisms by which the PCFs exert their immu- nomodulatory effects, including individual thousands of HDPs [30], are stipulated in Figure 3. It can be clearly seen that the immunomodulatory effects are all related to the NLRs and TLRs. Our experimental and clinical data also confirmed that PCFs are strongly involved in the regulation of Th1/Th2 paths of the immune response as per Figure 4. Efficiency of the PCFs is predetermined by their ability to have an impact on different links of innate and adaptive immunity, both specific and non-specific links, thus be able to control and coordinate the immu ne response by Th1 or Th2 pathways, depending upon the immune status of the body. Most of research confirmed that stimulation with paraprobiotics as MAMPs, certainly activated the macro- phages, DCs, epithelial cells, and trans-membrane receptors, thereby, induces an increase in the production level of a variety of cytokines, chemokines, and costimulatory molecules (Figure 3). They notify the host to breach in the mucosal barrier and turn the immune response at the site of infection [25]. Most of the cytokines (ILs, IFNs, TNFs are produced from either adherent T lymphocytes (macrophages and DCs) or non-adherent T lymphocytes. Depending on the exact combination and timing of clear signals that are coming from the epithelium and/or res- ident effector cells. For example, the DCs grow and respond differentially whereby their cytokines control the direction and types of the immune response to be initiated [25]. In our study, orally administered PCFs demon- strated the ability to induce the production of TNF and NK cells, as well as to regulate the synthesis of IL-4, IL-10, IL-12, and IFNs (like Figure 3, Figure 4, Figure 6 & Figure 7). It should be known also that the in vivo results of cytokine production upon ingestion of probiotics has been found to vary greatly in both humans and Figure 6. Murine serum TNF dynamics after oral administra- tion of PCFs in mice.  N. Shigwedha et al. Figure 7. Interferon activity of PCFs in mice. animals. They seemed to depend on the kinds of strain used and the experimental settings. TLR-9 is one of the PRRs which is highly involved in the recognition of un-methylated CpG DNAs, plentifully existent in bacterial genomes (see Figure 3). Bacterial DNA motifs induces immunoglobulin secretion and triggers B-cell proliferation [39]. Compared to Figure 3, another research has also reported that the cytoplasmic fractions of paraprobiotics, which is mainly LTA, induce macrophages to release IL-6 and TNF-α, and increase the number of IgA (+) cells in the lamina propria of the small intestine [39]. In addition to DNA motifs, different peptides as part of PCFs have been found to inhibit the proliferation of Staphylococcus aureus and protected mice from infection of herpes and me- ningo enc e p ha li tis by stimulating T lymphocytes proliferation and phagocytosis cells, in vivo [18]. It is, therefore, thought that the continuous exposure of the PCFs to problematical antigens reduces the immune responses and normalizes the production of IgA and IgG antibodies by enhancing suppressor T cells. Consequently, these im- muno modula ting PCFs are qualified as health-corrective ingredients for functional foods, nutritional supple- ments, distinctive drugs, cosmetics, animal health products, and so forth. 7. Putative Protective Effects on Chronic Diseases and Infections 7.1. Anti-Ca rc in og en ic Activities and Anti-Malaria Possibility PCFs have demonstrated anti-proliferative activities with regards to the preclusion of cancer. Many experimen- tal studies have suggested different mechanisms about the intake of most components found in the PCFs and their chemo-preventive role in various forms of cancer. For example, the anti-cancer effects of cellular compo- nents of lactobacilli have been attributed to: 1) binding of genotoxic carcinogens; 2) interaction with the immune system; 3) induction of apoptosis exerting anti-proliferative activity and differentiation of cancer cells; 4) buta- nol extract of B. adolescentis dose dependently precluded the development of colon cancer cells (Caco-2, HT-29, and SW480); 5) concentrated supernatants of L. plantarum precluded the rise of human promyelocytic cell line (HL-60 cells); 6) cell-bound EPS of L. acidophilus exerted anti-tumor effects on HT-29 cells; 7) probiotical cell walls and/or cytoplasm extracts indicated significant anti-proliferative activities against cancer [40]. However, it should be acknowledged here that research on the anti-carcinogenic effects using PCFs is challenging due to the diverse etiologies of cancers. Interestingly, PCFs of L. delbrueckii subsp. bulgaricus LB86 VCIM-B5788 have been approved in Ukraine, and the formulation is still being used there as a supportive medication for anti-cancer treatments [18]. The impression that paraprobiotic DNA motifs may exhibit strong anti-tumor effects against tumors of the same type was first made by reference [41] in 1984. Both the CpG-ODN and non-CpG -ODN DNA-motifs are each found to play a different role, depending on the paraprobiotic strains used. However, there is enough evi- dence that unmethylated CpG-DNA sequences and ODNs are all involved in the prevention and treatment of cancers, allergies, infectious diseases, and inflammatory diseases [42]. Immunosuppressive ODNs, on the other hand, have been shown to suppress the symptoms of multiple sclerosis, sepsis, endotoxic shock, and the forma- tion and proliferation of tumor cells, because they suppress the activity of CD8+ T cells and B cells [35]. There- fore, paraprobiotics with immunosuppressive oligodeoxynucleotide mutated in CpG sequence (ISM-ODN) are indebted for their identified cures. The literature revealed that the ISM-ODN binds strongly to high-mobility  N. Shigwedha et al. group box (HMGB) and prevent activation of the immune system [35]. Thus far, several AT-ODNs that have been discovered made an immense influence in the medical field and others are still being considered in drug developments for malaria [35] [43], an infectious disease. EPS has been found to protect mice from severe influenza viral infections [23]. In addition, EPSs have relia- ble immunostimulatory effects such as anti-tumorigenesis, activation of macrophages, induction of cytokines, and B-cell mitogenic activities [23]. Consequently, PCFs seem to be effective in braking the replication of the cancer cells; however, they (PCFs) do not bother the normal tissues. Equally important, the mitochondria has been found to play a vital protagonist in the thriving and dying of cancer cells [40] [44]. Ever since that discov- ery, the amount of mitochondrial membrane potential (△ᴪm) loss has then started gaining popularity worldwide as this is known to be associated with the initiation of apoptotic mitochondrial pathway on cancer cells [40]. While the mechanisms of △ᴪm loss are still complicated, the exposure of PCFs to cancer cells is required to breakdown the △ᴪm through the modulation of mitochondrial signal pathway. Our research has shown that oral administration of PCFs in mice manifested a high level of the TNFs within 8 h of ingestion (Figure 6). In addi- tion, the production level of critical IFNs wa s raised 2.5 - 3 times as compared to the control (Figure 7). Overall, the PCFs demonstrated the ability to induce the production of NK cells, TNFs, and to regulate the synthesis of IL-4, IL-10, IL-12, IFNs, and neutrophils [18]. Fascinatingly, higher dosages of PCFs did not always result in higher effectiveness. 7.2. Putative Anti-Parasitogenic Activity The oral administration of paraprobiotic L. casei or its spent culture supernatant has been shown to reduce Tri- chinella spiralis infection in the animal intestine from 32 - 44% [20]. In the same study, a reduction of T. spira- lis larvae in muscle tissues was also observed. The protective mechanisms of action appear to depend on the co- lonization of the intestine by cell fragments of lactobacilli, macrophage processing of the PCFs or probiotic cell lysates, and activation of T and B-cells through IL-2 production. The active components of PCFs are the prime objects in exerting the health benefits to the host, and their gut penetrability is straightforward as elucidated in Figure 5. The reality here is that the PCFs are being transported and absorbed through the intestine. With re- gards to anti-parasitogenic activity, the PCFs may act on the pathogenic parasites for humans by the production of IL-10 from CD4+ T-cells. However, further investigations by using PCFs as nutraceutical ingredients are still needed and be validated in vivo. 8. Use of PCFs as Heath Corrective Ingredients The use of paraprobiotics in the prevention and treatment of a dramatic list of acute and chronic diseases, im- mune deficiencies, as well as infectious diseases such as viral (flu, hepatitis C), bacterial (bronchitis), asthma, fungal etiology, chronic fatigue, fibromyalgia, and colonic cancer etiology has been featuring lately in gastroen- terology at an increasing rate, indisputably. To date, the cellular fragments that make paraprobiotics and indeed, the PCFs particularly suitable for diverse industrial applications have been recently evolving while others con- tinue to be carefully investigated. These include their GRAS status, dairy fermenting properties as starter culture supplements and/or stabilizers, immunomodulating properties, ability to evoke mucosal and systemic immune responses against associated antigens, coaggregation with pathogens and competitive exclusion, decreasing the luminal pH, provision of specific compounds such as bacteriocins or HDPs, adhesion to mucus and/or intestinal epithelial cells in a competitive nature against enteropathogenic bacteria, and many more. PCFs may be marketed in many different formats. In the future, it will be attractive to see paraprobiotics in the form of PCFs and packaged as: 1) Nutritional supplements, and can be included in liquid foods , powder foods, gels, creams, gummies, pills and sprays; 2) specific drugs that are intended to relieve or mitigate, cure, or prevent infections and diseases; 3) food additives intended for beverages, ready-to-eat foods, and functional foods; 4) direct-feed lysates meant to be fed to animals that produce the foods and/or those which requiring support for health issues (including pets); 5) starter-culture stabilizers for many nourishers; 6) cosmetics meant for retarding the significant effects of aging, and so on. It is, however, expected that small amounts of PCFs may be added to foods and labeled as such, because these natural nutraceutical ingredients have a track record for improving health. The shelf stability of one unique product with PCFs known as “Del-Immune V®” has been measured and found to be as from 3-5 years [18]. In foods (for example, bread, ice-cream or cheese) or beverages, if the PCFs  N. Shigwedha et al. are to be incorporated, they are expected to last for much longer time when compared to their counterpart viable probiotics which require refrigeration while at the same time to be consumed in an adequate amount to take ad- vantage of the most testified health benefits. At this point, it is essential to stress that, the form in which the PCFs may be formulated and consumed is an economical advantage to the host. With the PCFs, product prepa- ration, storage, transit time, and expiration date are secondary factors in how much live probiotic cells are miss- ing during ingestion or application. For instance, improper handling and storage can kill viable bacteria in yo- gurts, and it is difficult to trace or know how the product was handled prior to purchase or use. With a PCFs-yogurt, these kinds of concerns are not valid to anybody to utilize such a product. The same principle ap- plies to many other possible PCF formulations, depending on the selected strains and the intentions. High post-fermentation acidification is another controversial situation that affects the sensory quality of yogurt; that is characterized by a good flavor, smooth texture and suitable viscosity. With PCF-yogurt, post-fermentation aci- dification would not be a serious issue and the actual sensory characteristics be retained as time goes. 9. Concluding Remarks It is encouraging to demonstrate the need of the PCFs as “novel nutraceutical ingredients” in the paraprobiotic prevention and treatment cases. The kinds of paraprobiotic treatments would depend on the selected bacterial strain, host response, and the intended purpose. For example, the preferred PCFs for patients with common al- lergic diseases (food allergy, bronchitis, hay fever, and asthma) are somehow different to that of rectal cancer, gastroenteritis or IBD patients. In allergic diseases, one of the highest target of PCFs (extracts of L. rhamnosus V) is DCs due to their abilit y to polarize T cell responses. Allergic diseases often result from exaggerated Th2 type immune responses. For the prevention of allergic diseases per se, the PCFs positively modulate T cell pola- rization into an increase in Th1 cell and CD4+CD25+ regulatory T cell responses, primarily by modulating DC functions. Other conclusions embrace that the PCFs accelerate normalization of humoral immunity, increase re- sistance to bacterial and viral infections, increase the activity of NK cells and phagocytosis, increase production of IL-1, IL-2, and TNFs, significantly increase in T-lymphocytes or T cells production, normalize the contents of IgA and IgGs, prevent side effects after antibiotics and cyclophosphamide usage, induce cytosuppression, and significantly reduce the toxic side effects. The combined effects of PCFs appear to be specific in their individual actions whereby making the completion of physicochemical- and/or radio-therapy possible without disruption. In addition, the PCFs offer many advantages for use as active health corrective ingredients in functional foods, beverages, starter cultures, nutritional supplements, specific drugs, animal feeds or health products, cosmetics, chronic wound treatments, and many more including prospects of formulating the anti-malaria drugs. Acknowledgements This work was financially supported by the China Postdoctoral Science Foundation (Grant No. 2013M541397). References [1] FAO/WHO (2002) Guidelines for the Evaluation of Probiotics in Food. http://www.who.int/entity/foodsafety/publications/fs_management/probiotics2/en [2] Taverniti, V. and Guglielmetti, S. (2011) The Immunomodulatory Properties of Probiotic Microorganisms beyond their Viability (Ghost Probiotics: Proposal of Paraprobiotic Concept). Genes and Nutrition, 6, 261-274. http://dx.doi.org/10.1007/s12263-011-0218-x [3] Saito, T. and Kitazawa, H. (2005) Recent Tendency of Immunogenics Research on Lactic Acid Bacteria. Bulletin of Japan Dairy Technical Association, 55, 34-44. (In Japanese) [4] Kitazawa, H., Tohno, M. Shimosato, T. and Saito, T. (2008) Development of Molecular Immunoassay System for Pro- biotics via Toll-Like Receptors Based on Food Immunology. Animal Science Journal, 79, 11-21. http://dx.doi.org/10.1111/j.1740-0929.2007.00491.x-i1 [5] Kitazawa, H., Villena, J. and Alvarez, S. (2013) Probiotics: Immunobiotics and Immunogenics. CRC Press, Boca Ra- ton. [6] Lebeer, S., Vanderleyden, J. and De Keersmaecker, C.J. (2010) Host Interactions of Probiotic Bacterial Surface Mole- cules: Comparison with Commensals and Pathogens. Nature Reviews Microbiology, 8, 171-184. http://dx.doi.org/10.1038/nrmicro2297 [7] Kleerebezem, M., Hols, P., Bernard, E., Rolain, T., Zhou, M., Siezen, R.J. and Bron, P.A. (2010) The Extracellular Bi-  N. Shigwedha et al. ology of the Lactobacilli. FEMS Microbiology Reviews, 34 , 199-230. http://dx.doi.org/10.1111/j.1574-6976.2009.00208.x [8] Kaji, R., Kiyoshima-Shibata, J., Nagaoka, M., Nanno, M. and Shida, K. (2010) Bacterial Teichoic Acids Reverse Pre- dominant IL-12 Production Induced by Certain Lactobacillus Strains into Predominant IL-10 Production via TLR2 -Dependent ERK Activation in Macrophages. The Journal of Immunology, 184, 3505-3513. http://dx.doi.org/10.4049/jimmunol.0901569 [9] Clancy, R. (2003) Immunobiotics and the Probiotic Evolution. FEMS Immunology and Medical Microbiology, 38, 9-12. http://dx.doi.org/10.1016/S0928-8244(03)00147-0 [10] Underhill, D.M. and Ozinsky, A. (2002) Toll-Like Receptors: Key Mediators of Microbe Detection. Current Opinion in Immunology, 14, 103-110. http:// dx. do i.o rg/1 0. 1016 / S09 52 -7915( 0 1)0 0304 -1 [11] Lebeer, S., Vanderleyden, J. and De Keersmaecker, S.C. (2008) Genes and Molecules of Lactobacilli Supporting Pro- biotic Action. Microbiology and Molecular Biology Reviews, 72 , 728-764. http://dx.doi.org/10.1128/MMBR.00017-08 [12] Hornung, V., Ablasser, A., Charrel-Dennis, M., et al. (2009) AIM2 Recognizes Cytosolic dsDNA and Forms a Cas- pase-1-Activating Inflammasome with ASC. Nature, 458, 514-518. http://dx.doi.org/10.1038/nature07725 [13] Ouwehand, A.C. (2007) Antiallergic Effects of Probiotics. The Journal of Nutrition, 137, 794S-797S. [14] Hemmi, H., Takeuchi, O., Kawai, T., et al. (2000) A Toll-Like Receptor Recognizes Bacterial DNA. Nature, 408, 740- 745. http://dx.doi.org/10.1038/35047123 [15] Kawai, T. and Akira, S. (2011) Toll-Like Receptors and their Crosstalk with other Innate Receptors in Infection and Immunity. Immunity, 34 , 637-650. http://dx.doi.org/10.1016/j.immuni.2011.05.006 [16] Beutler, B.A. (2009) TLRs and Innate Immunity. Blood , 113 , 1399-1407. http://dx.doi.org/10.1182/blood-2008-07-019307 [17] Rhee, S.H. (2011) Basic and Translational Understandings of Microbial Recognition by Toll-Like Receptors in the In- testine. Journal of Neurogastroenterology and Motility, 17, 28-34. http://dx.doi.org/10.5056/jnm.2011.17.1.28 [18] Del-Immune, V. (2014) Del-Immune V®. http:// www.deli mmune.c om/rese arch [19] Tohno, M. and Kitazawa, H. (2013) Molecular Immunoassay Systems for Probiotics via Pattern Recognition Receptors. In: Kitazawa, H., Villena, J. and Alvarez, S., Eds., Probiotics: Immunobiotics and Immunogenics, CRC Press, Boca Raton , 54-88. http://dx.doi.org/10.1201/b15532-5 [20] Humen, M.A., Benyacoub, J., Minnaard, J., et al. (2013) Immunobiotics and Immunity against Parasites. In: Kitazawa, H., Villena, J. and Alvarez, S., Eds., Probiotics: Immunobiotics and Immunogenics, CRC Press, Boca Raton, 194 -214. http://dx.doi.org/10.1201/b15532-9 [21] Stadelmann, B., Merino, M.C., Persson, L. and Svärd, S.G. (2012) Arginine Consumption by the Intestinal Parasite Giardia Intestinalis Reduces Proliferation of Intestinal Epithelial Cells. PloS One, 7, e45325. http://dx.doi.org/10.1371/journal.pone.0045325 [22] Cerutti, A. and Rescigno, M. (2008) The Biology of Intestinal Immunoglobulin A Responses. Immunity, 28, 740-750. http://dx.doi.org/10.1016/j.immuni.2008.05.001 [23] Makino, S., Ikegami, S., Nagai, T. and Yamada, H. (2013) Immunogenics: Extracellular Polysaccharides Reduce the Risk of Infection. In: Kitazawa, H., Villena, J. and Alvarez, S., Eds., Probiotics: Immunobiotics and Immunogenics, CRC Press, Bo c a Rato n, 37 6-397. http://dx.doi.org/10.1201/b15532-16 [24] Yoda, K., Miyazawa, K., Harata, G. and He, F. (2013) Immunobiotics and Antiviral Immunity. In: Kitazawa, H., Vil- lena, J. and Alvarez, S., Eds., Probiotics: Immunobiotics and Immunogenics, CRC Press, Boca Raton, 169 -193. http://dx.doi.org/10.1201/b15532-8 [25] Chr isten sen, H.R. and Frøkiær, H. (2007) Immunomodulating Effects of Lactic Acid Bacteria. In: Shetty, K., Paliyath, G., Pometto, A.L. and Levin, R.E., Eds., Functional Foods and Biotechnology, CRC Press, New York, 435-471. [26] Matsumoto, M., Tani, H., Ono, H., Ohishi, H. and Benno, Y. (2002) Adhesive Property of Bifidobacterium Lactis LKM512 and Predominant Bacteria of Intestinal Microflora to Human Intestinal Mucin. Current Microbiology, 44, 212-215. http://dx.doi.org/10.1007/s00284-001-0087-4 [27] Chen, X.Y., Xu, J.J., Shuai, J.B., Chen, J.S., Zhang, Z.F. and Fang, W.H. (2007) The S-Layer Proteins of Lactobacillus Crispatus Strain ZJ001 Is Responsible for Competitive Exclusion against Escherichia coli O157:H7 and Salmon el la Typhimurium. International Journal of Food Microbiology, 115 , 307-312. http://dx.doi.org/10.1016/j.ijfoodmicro.2006.11.007 [28] Xue, C., Zhang, L., Li, H., et al. (2013) Functionality of the S-Layer Proteins from Lactobacillus in the Competitive against Enteropathogens Infection. European Food Research and Technology, 236, 249-255. http://dx.doi.org/10.1007/s00217-012-1871-z [29] Lee, Y.K., Puong, K.Y., Ouwehand, A.C. and Salminen, S. (2003) Displacement of Bacterial Pathogens from Mucus  N. Shigwedha et al. and Caco-2 Cell Surface by Lactobacilli. Journal of Medical Microbiology, 52, 925-930. http://dx.doi.org/10.10 99/ jmm.0 . 050 09 -0 [30] Klein, G., Schanstra, J.P., Hoffmann, J., Mischak, H., Siwy, J. and Zimmermann, K. (2013) Proteomics as a Quality Control Tool of Pharmaceutical Probiotic Bacterial Lysate Products. PloS One, 8, e66682. http://dx.doi.org/10.1371/journal.pone.0066682 [31] Bowdish, D.M., Davidson, D.J., Scott, M.G. and Hancock, R.E. (2005) Immunomodulatory Activities of Small Host Defense Peptides. Antimicrobial Agents and Chemotherapy, 49, 1727-1732. http://dx.doi.org/10.1128/AAC.49.5.1727-1732. 2005 [32] Afacan, N.J., Janot, L.M. and Hancock, R.E.W. (2013) Host Defense Peptides: Immune Modulation and Antimicrobial Activity in Vivo . In: Hiemstra, P.S. and Zaat, S.A.J., Eds., Antimicrobial Peptides and Innate Immunity, Springer, Ba- sel, 321-358. [33] Valdez, J.C., Ramos, A.N., Fernández, D., et al. (2013) Probiotics and their Potential Use in Wound Treatment. In: Ki- tazawa, H., Villena, J. and Alvarez, S., Eds., Probiotics: Immunobiotics and Immunogenics, CRC Press, Boca Raton, 298-335. http://dx.doi.org/10.1201/b15532-13 [34] Liu, W., Zhang, L., Yi, H., et al. (2014) Qualitative Detection of Class IIa Bacteriocinogenic Lactic Acid Bacteria from Traditional Chinese Fermented Food Using a YGNGV-Motif-Based Assay. Journal of Microbiological Methods, 10 0, 121-127. http://dx.doi.org/10.1016/j.mimet.2014.03.006 [35] Shimosato, T. and Kitazawa, H. (2013) Immunogenics: Immunostimulatory Oligodeoxynucleotides from Probiotics. In: Kitazawa, H., Villena, J. and Alvarez, S., Eds., Probiotics: Immunobiotics and Immunogenics, CRC Press, Boca Raton, 336-350. http://dx.doi.org/10.1201/b15532-14 [36] Ramaprakash, H., Shibata, T., Duffy, K.E., et al. (2011) Targeting ST2L Potentiates CpG-Mediated Therapeutic Ef- fects in a Chronic Fungal Asthma Model. The American Journal of Pathology, 179, 104-115. http://dx.doi.org/10.1016/j.ajpath.2011.03.032 [37] Jolly, L., Vincent, S.J.F., Duboc, P. and Neeser, J.R. (2002) Exploiting Exopolysaccharides from Lactic Acid Bacteria. Antonie van Leeuwenhoek, 82, 367-374. http://dx.doi.org/10.1023/A:1020668523541 [38] Nakajima, H., Suzuki, Y. and Hirota, T. (1992) Cholesterol Lowering Activity of Ropy Fermented Milk. Journal of Food Science, 57, 1327-1329. http://dx.doi.org/10.1111/j.1365-2621.1992.tb06848.x [39] Galdeano, C.M., Dogi, A.C. and Perdigón, G. (2013) Difference in the Signals Induced by Commensal or Probiotic Bacteria to the Gut Epithelial and Immune Cells. In: Kitazawa, H., Villena, J. and Alvarez, S., Eds., Probiotics: Immu- nobiotics and Immunogenics, CRC Press, Boca Raton, 36 -53. http://dx.doi.org/10.1201/b15532-4 [40] Wang, S., Zhang, L., Fan, R., et al. (2014) Induction of HT-29 Cells Apoptosis by Lactobacilli Isolated from Fer- mented Products. Research in Microbiology, 165, 202-214. http://dx.doi.org/10.1016/j.resmic.2014.02.004 [41] Tokunaga, T., Yamamoto, H., Shimada, S., et al. (1984) Antitumor Activity of Deoxyribonucleic Acid Fraction from Mycobacterium Bovis BCG. I. Isolation, Physicochemical Characterization, and Antitumor Activity. Journal of the Na- tional Cancer Institute, 72, 955-962. [42] Klinman, D.M. (2004) Immunotherapeutic uses of CpG oligodeoxynucleotides. Nature Reviews Immunology, 4, 249- 259. http://dx.doi.org/10.1038/nri1329 [43] Yanai, H., Chiba, S., Ban, T., et al. (2011) Suppression of Immune Responses by Nonimmunogenic Oligodeoxynuc- leotides with High Affinity for High-Mobility Group Box Proteins (HMGBs). Proceedings of the National Academy of Sciences , 108 , 11542-11547. http://dx.doi.org/10.1073/pnas.1108535108 [44] Nicholls, D.G. and Ward, M.W. (2000) Mitochondrial Membrane Potential and Neuronal Glutamate Excitotoxicity: Mortality and Millivolts. Trends in Neurosciences, 23, 166-174. http://dx.doi.org/10.1016/S0166-2236( 99) 0153 4 -9
|