 Journal of Biosciences and Medicines, 2014, 2, 13-19 Published Online May 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.23003 How to cite this paper: Zhang, L.K., et al. (2014) Effects of Tension Force on Proliferation and Differentiation of Human Pe- riodontal Ligament Cells Induced by Lipopolysaccharides. Journal of Biosciences and Medicines, 2, 13-19. http://dx.doi.org/10.4236/jbm.2014.23003 Effects of Tension Force on Proliferation and Differentiation of Human Periodontal Ligament Cells Induced by Lipopolysaccharides Linkun Zhang1,2*, Chongshan Liao1*, Jiajing Lu1,3, Chengfei Zhang1, Yanqi Yang1# 1Faculty of Dentistry, University of Hong Kong, Hong Kong, China 2Orthodontics, Tianjin Stomatological Hospital of Nankai University, Tian jin, China 3Taizhou Polytechnic College, Taizhou, China Email: #yangyanq@hku.hk Received January 2014 Abstract Human periodontal ligament cells (hPDLCs), with the potential for multi-directional differentia- tion and reproduction, are the target cells of orthodontic tooth movement. The aim of this study was to examine the effect of mechanical tension force and lipopolysaccharides (LPS) on hPDLCs and whether they induce proliferative and differentiated characters in vitro. Tension force was applied to hPDLCs stimulated with and without LPS for 24 hrs. Real-time polymerase chain reac- tion (qPCR) was carried out to analyze the mRNA expression of Cyclin 2 (CCND2), WNT1 inducible signaling pathway protein 1 (WISP1), run t-related transcription factor 2 (RUNX2) and alkaline phosphatase (ALP). Analysis of variance (ANOVA) was used for statistical analysis. Significant dif- ferences were indicated by P < 0.05. The results showed that tension force promoted the mRNA expression of both the proliferation-related genes (CCND2 and WISP1) and differentiation-related genes (RUNX2 and ALP), and that both were enhanced by the simulation of LPS. In addition, the relative expression ratios CCND2/RUNX2 and CCND2/ALP both increased significantly after the application of tension, and this effect was further enhanced by LPS. All results indicated that with the assessed level of mechanical force loading, tension could promote both the proliferation and differentiation of hPDLCs, which could be enhanced by LPS, and that proliferation is promoted to a greater extent than differentiation. These findings may be valuable for understanding the impor- tance of the application of suitable mechanical force in periodontal remodeling, especially in the process of orthodontic tooth movement with inflammation. Keywords Human Periodontal Ligament Cells, Tension Fo rc e, Li p opolysacch arides , Prol iferat ion, Differenti ation *Equal contribution. #Corresponding author.  L. K. Zhang et al. 1. Introduction Orthodontic tooth movement is mediated by bone resorption and deposition on the compression and tension side, mainly due to delicate changes within the periodontal ligament (PDL), which is functionally heterogeneous and contains a subpopulation of cells able to receive mechanical signals of orthodontic force loading and transduce them into biological signals [1] [2]. The regenerative potential of the periodontium is believed to be related to the functions of periodontal ligament cells (PDLCs) [3]. The activation of specific transcription factors is essential for cellular proliferation and commitment to a dif- ferentiation lineage, which are also affected by mechanical force application [2]. As a member of the Cyclin family, Cyclin D2 (CCND2) is a key component for facilitating the G1-to-S-phase transition and subsequently increased cell proliferation [4]. The WNT1 inducible signaling pathway protein 1 (WISP1), a member of the se- creted, cysteine-rich CCN family and a connective tissue growth factor, exerts diverse biological effects such as the proliferation of fibroblasts and smooth muscle cells [5]. In terms of hPDLC differentiation, many experi- ments demonstrated the important role of runt-related transcription factor 2 (Runx2) in regulating osteogenic differentiation, which may be the key to the different signaling pathways involved in mechanotransduction, and can induce the synthesis of alkaline phosphatase (ALP) [6] [7]. ALP, which is produced by PDLCs, can initially respond to force loading with gene expression detected after 24 hrs [8]. ALP activity is involved in the process of calcification in various mineralizing tissues, and is found at much higher levels in PDL than in other connec- tive tissues [9]. Many adults with orthodontic treatment demands have dental problems that involve inflammation such as pe- riodontitis, which aggravates periodontal problems during orthodontic tooth movement if the inflammation is not well controlled [10]. This inflammatory disease leads to gingival connective tissue destruction and irreversi- ble alveolar bone resorption. PDLCs are the target cells of the inflammation. Lipopolysaccharides (LPS) partly comprise the cell wall of periodontal pathogens, and may contribute to alveolar bone loss and connective tissue degradation in periodontal disease [11]. Thus, there is great interest in the tension force involved in the regulation of the expression of hPDLC proli- feration and differentiation in terms of LPS inducement. In this study, we evaluated the mRNA production of CCND2, WISP1, RUNX2 and ALP in hPDLCs induced by tension force and their changes in expression after LPS addition. This information may clarify the importance of suitable mechanical force in periodontal remode- ling, especially in the process of orthodontic tooth movement with inflammation. 2. Materials and Methods 2.1. Cell Culture This study was approved by the Ethics Committee of The University of Hong Kong (NO. UW1 3-120). The hPDLCs were obtained from three healthy individuals aged 13 - 18 years who had undergone premolar extrac- tion for orthodontic treatment. Cell s were obtained from the middle third of the root surfaces of healthy human premolars, as described previously [12]. The primary hPDLCs were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, USA) containing 10% foetal bovine serum (FBS; HyClone, Logan, USA) and antibiotic solution (100 U ml–1 penicillin and 100 U ml–1 streptomycin) at 37˚C in a 5% CO2 incub a- tor. After achieving confluence, cells were detached with 0.25% trypsin and subcultured in fresh DMEM. The hPDLCs were characterized by immunocytochemical staining for vimentin and cytokeratin. hPDLC suspensions (1 × 106 cells ml–1) were plated onto special force-loading plates and cultured to confluence. Before the experi- ments, the cultured cells were serum starved for 12 hrs to be synchronized [13], and the medium was then changed to fresh DMEM containing 1.5% FBS with or without 0.1 μg/ml LPS (InvivoGen, San Diego, USA). All of the experiments were carried out at passages 4 - 7. 2.2. Force Application The cells at the centers of the force-loading plates were loaded with cyclic uniaxial tension (2000 μ of strain, 0.5 Hz) for 24 hrs by a four-point bending system (SXG4201, University of Electronic Science and Technology of China, China) [13]-[15] . The cells in the control group were prepared using the same procedures as the experi- mental groups, except in terms of mechanical loading.  L. K. Zhang et al. 2.3. Real-time Polymerase Chain Reaction Analysis The mRNA levels of CCND2, WISP1, RUNX2, and ALP were determined by system (MyiQ, Bio-Rad, Her- cules, CA, USA). β-actin was analyzed as the housekeeping gene for the internal control. In brief, after the me- chanical loading, the total RNA was extracted from the cells with Trizol reagent (Invitrogen, Carlsbad, USA) immediately and cDNA was reverse transcribed from mRNA using a SuperScript III Reverse Transcriptase (In- vitrogen, Carlsbad, USA) according to the manufacturer’s instructions. Real-time PCR was performed with a Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). The sequences of the primers are listed in Table 1. 2.4. Statistical Analysis All data were expressed as the mean standard deviations (SDs) from three independent experiments. Analysis of variance (ANOVA) was performed with the use of the SPSS 19.0 statistical software package (SAS Institute, Cary, NC, USA). Significant differences were indicated by P < 0.05. 3. Results and Discussion 3.1. Tension Induced CCND2, WISP1, RUNX2 and ALP mRNA Mechanical loading is a fundamental determinant of bone formation and reconstruction. It can be converted into a cellular response involving rapid, kinase-mediated changes in gene expression [1]. Physiological strains re- ported for daily activities in human long bones are of the order of 2000 - 4000 μ strain [16]. Orthodontic tooth movement induced by mechanical stimuli is dependent on the remodeling capacity of the local periodontal li- gament and alveolar bone. In this study, the magnitude (2000 μ strain) was chosen according to stress analysis of the periodontal ligament under various orthodontic loadings [17]. After tension application, there were significant increases in the mRNA expression level of the prolifera- tion-related genes [CCND2 (P < 0.05) and WISP1 (P < 0.05)] and differentiation-related genes [RUNX2 (P < 0.05) and ALP (P < 0.05)] compared with the control group (Figure 1). This indicates that given the assessed level of mechanical force loading, tension might have promoted the proliferation and differentiation of the hPDLCs . The effect of mechanical loading on the proliferation of hPDLCs is controversial. Some studies have shown that an appropriate mechanical force could induce hPDLC growth. Researchers reported cell proliferation on both the “tension” and “pressure” sides of the PDL by measuring 3 H-thymidine incorporation [18] [19]. Anoth- er study reported that a continuous force produced a three-stage proliferative response over 20 hrs [20]. Other researchers found no significant difference in cell proliferation between cells subjected to the tension force and those of control groups [9]. Our findings suggest that tension force could promote the proliferation of hPDLCs. When considering the balance between hPDLC proliferation and tissue-specific differentiation, the cessation of cell proliferation may indicate the onset of osteogenic lineage commitment. The application of mechanical force to the rat model suggested that PDLCs were primarily osteogenic under strained conditions [21]. Our pre- vious study also showed that hPDLCs had osteogenic differentiation potential under mechanical tension loading [22]. Our data were consistent with previous reports stating that mechanical strain induces the expression of the osteogenic transcription factor RUNX2 at mRNA and protein levels [23]. hPDLCs possess high ALP activity, which is an indicator of hPDLC differentiation. However, Yamaguchi et al. found 10% and 42% decreases in the ALP activity of PDLCs exposed to low (9%) and high (24%) tension forces, respectively [9]. We speculate Table 1. Primer sequences for CCND2, WISP1, RUNX2, ALP and β-actin. Genes Primer sequences (5'-3') Forward Rev erse WISP1 CCACCGGGGCCTCTACT CCACACCGACCACCTGT RUNX2 ATCCAGCCACCTTCACTTACACC GGGACCATTGGGAACT GAT AGG ALP T AT GTC T GGAACC GCACTGAAC CACTAGCAAGAAGAAGCCTTTGG β-actin CACCCGCGAGTACAACCTTC CCCATACCCACCATCACACC 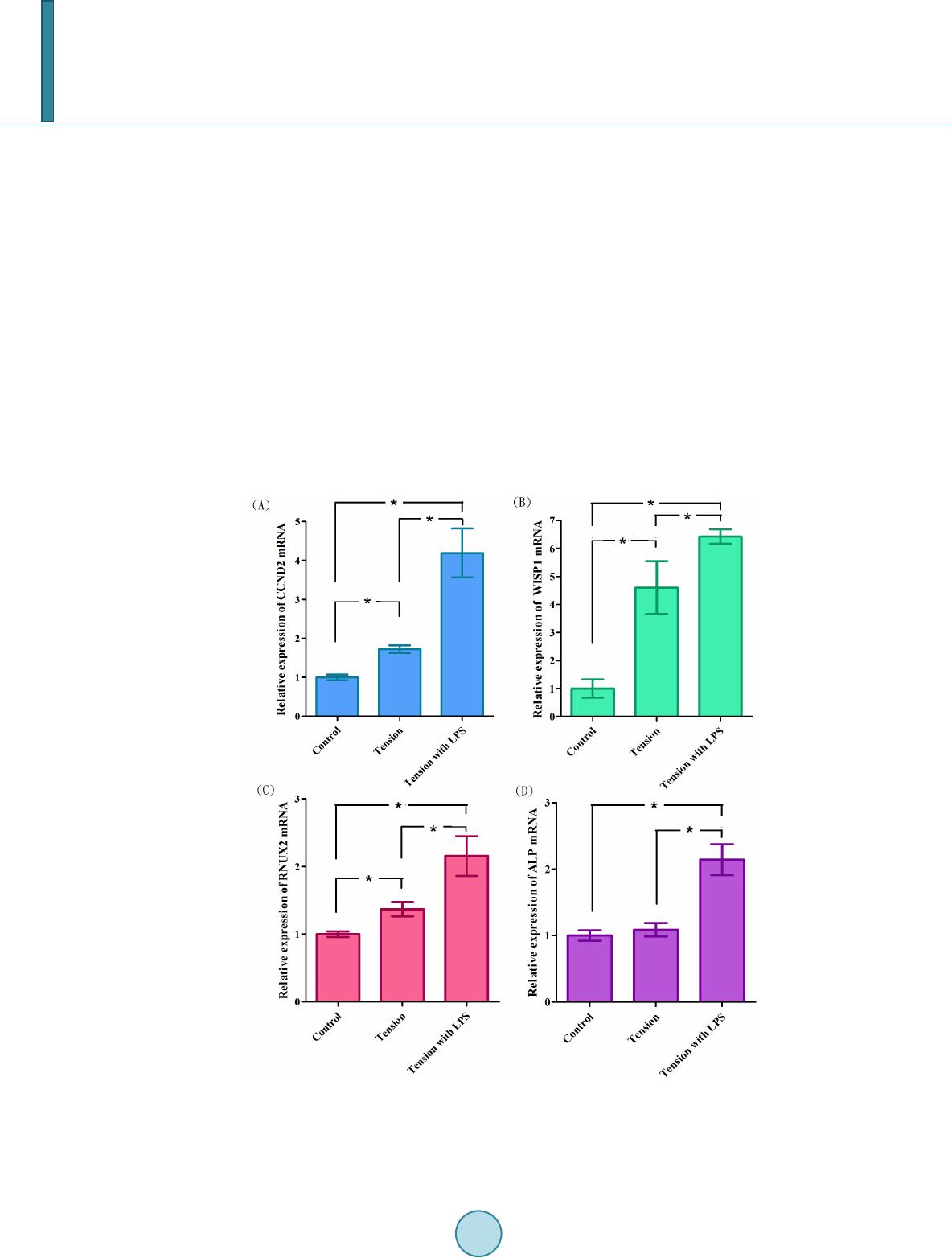 L. K. Zhang et al. that these decreases were dependent on the magnitude of the tension force. 3.2. LPS Enhanced the Effect of Tension The stimulation of LPS at the same force-application time (24 hrs) promoted the mRNA expression of CCND2, WISP1, RUNX2 and ALP to a greater extent than tension alone (Figure 1). LPS is a bacterial cell component that plays multifunctional roles in inflammatory reactions. Cell multiplica- tion is often accompanied by inflammation. Junctional epithelium (JE) cells can enter the proliferating cell cycle when exposed to LPS, and the enhanced proliferating activity in the JE is an important factor in the deepening of the periodontal pocket [24]. One study reported that LPS enhanced the growth of hPDLCs at a concentration of 1 ug/ml [25]. The concept of orthodontic tooth movement as a kind of inflammatory process was revived along with the gradual confirmation of the neurotransmitters, inflammatory mediators, cytokines and P substances in- volved in periodontal remodeling in recent years [2] [26]. As such, the consistency of LPS and tension-force ap- plication for inflammation may lead to the enhanced proliferation of hPDLCs. In terms of differentiation, previous studies showed that LPS from periodontopathic bacterium stimulated os- teoclast formation in mouse bone marrow culture systems and diminished the ALP activity of hPDLCs [27]. This contradicts our data related to the increased osteogenic differentiation factor. We speculate that the differ- ence might have been due to the concentrations of LPS and the addition of tension-force application. Figure 1. Relative mRNA expression of CCND2, WISP1, RUNX2 and ALP. Tension with and without LPS promoted relative mRNA expression of CCND2 (A), WISP1 (B), RUNX2(C) and ALP (D) in hPDLCs, and the effect of the tension was further enhanced by LPS (A-D). (*P < 0.05 was considered statistically significant.)  L. K. Zhang et al. Figure 2. Relative mRNA expression of CCND2/RUNX2 and CCND2/ALP. Tension with and without LPS promoted the ratio of mRNA expression CCND2/RUNX2 (A) and CCND2/ALP (B), and LPS further enhanced the effect of the tension (A, B). (*P < 0.05 was considered statistically signifi- cant.) 3.3. Proliferation was Promoted to a Greater Extent than Differentiation Taking the ratio of the mRNA expression of CCND2 to RUNX2 or ALP into account, CCND2/RUNX2 and CCND2/ALP both increased significantly after tension was applied (P < 0.05). This effect was further enhanced by LPS (P < 0.05) (Figure 2), indicating that proliferation was promoted to a greater extent than differentiation in the hPDLCs by mechanical stimulation with or without LPS. This result was consistent with the observation that LPS is a major virulence factor involved in periodontal diseases that cause inflammatory proliferation [28]. In the future, different tension-force magnitudes should be tested to verify inclinations about hPDLC prolifera- tion and differen tiation. 4. Conclusion Our results indicate that with the assessed level of mechanical force loading (2000 μ), tension can promote both hPDLC proliferation and differentiation, which could be enhanced by LPS. In addition, tension and LPS pro- motes proliferation to a greater extent than differentiation in hPDLCs. These findings may be valuable for un- derstanding the importance of a suitable mechanical force in periodontal remodeling, especially in the process of orthodontic tooth movement with inflammation. However, further studies are needed to elucidate the relation- ship between the tension force and LPS in periodontal remodeling. Acknowledgements The project reported here was supported by small project funding from the University of Hong Kong (201109176111). We thank Mr. Raymond Tong for his technical support. References [1] Pilon, J.J., Kuijpers-Jagtman, A.M. and Maltha, J.C. (1996) Magnitude of Orthodontic Forces and Rate of Bodily Tooth Movement. An Experimental Study. American Journal of Orthodontics and Dentofacial Orthopedics, 110, 16- 23. http://dx.doi.org/10.1016/S0889-5406(96)70082-3 [2] Meikle, M.C. (2006) The Tissue, Cellular, and Molecular Regulation of Orthodontic Tooth Movement: 100 Years after Carl Sandstedt. The European Journal of Orthodontics, 28, 221-240. http://dx.doi.org/10.1093/ejo/cjl001 [3] Le ki c , P. and McCulloch, C.A.G. (1996) Periodontal Ligament Cell Populations: The Central Role of Fibroblasts in Creating a Unique Tissue. The Anatomical Record, 245 , 327-341.  L. K. Zhang et al. http://dx.doi.org/10.1002/(SICI)1097-0185(199606)245:2<327::AID-AR15>3.0.CO;2-R [4] Bartkova, J., Lukas, J., Strauss, M. and Bartek, J. (1998) Cyclin D3: Requirement for G1/S Transition and High Abun- dance in Quiescent Tissues Suggest a Dual Role in Proliferation and Differentiation. Oncogene, 17, 1027-1037. http://dx.doi.org/10.1038/sj.onc.1202016 [5] Reddy, V.S., Valente, A.J., Delafontaine, P. and Chandrasekar, B. (2011) Interleukin-18/ WN T1 -Inducible Signaling Pathway Protein-1 Signaling Mediates Human Saphenous Vein Smooth Muscle Cell Proliferation. Journal of Cellular Physiology, 226, 3303-3315. http://dx.doi.org/10.1002/jcp.22676 [6] Arnsdorf, E.J., Tummala, P., Kwon, R.Y. and Jacobs, C.R. (2009) Mechanically Induced Osteogenic Differentiation— The Role of RhoA, ROCKII and Cytoskeletal Dynamics. Journal of Cell Science, 122 , 546-553. http://dx.doi.org/10.1242/jcs.036293 [7] Lee, K.S., Kim, H.J., Li, Q.L., Chi, X.Z., Ueta, C., Komori, T., et al. (2000) Runx2 Is a Common Target of Tran s- forming Growth Factor β1 and Bone Morphogenetic Protein 2, and Cooperation between Runx2 and Smad5 Induces Osteoblast-S peci fic Gene Expression in the Pluripotent Mesenchymal Precursor Cell Line C2C12. Molecular and Cel- lular Biology, 20, 8783-8792. http://dx.doi.org/10.1128/MCB.20.23.8783-8792.20 00 [8] Pavlin, D. and Gluhak-Heinrich, J. (2001) Effect of Mechanical Loading on Periodontal Cells. Critical Reviews in Oral Biology & Medicine, 12, 414-424. http://dx.doi.org/10.1177/10454411010120050401 [9] Yamaguchi, M., Shimizu, N., Shibata, Y. and Abiko, Y. (1996) Effects of Different Magnitudes of Tension-Force on Alkaline Phosphatase Activity in Periodontal Ligament Cells. Journal of Dental Research, 75, 889-894. http://dx.doi.org/10.1177/00220345960750030501 [10] Rabi e, A.B.M and Yang, Y. (2009) Perio -Ortho Conjoint Treatment of Periodontally Compromised Patients. Chinese Journal of Orthodontics, 16, 181-183. [11] Ueda, N., Koide, M., Ohguchi, M., Ishihara, Y., Noguchi, T., Okahashi, N., et al. (1998) Involvement of Prostaglandin E2 and Interleukin-1α in the Differentiation and Survival of Osteoclasts Induced by Lipopolysaccharide from Actino- bacillus Actinomycetemcomitans Y4. Journal of Periodontal Research, 33, 509-516. http://dx.doi.org/10.1111/j.1600-0765.1998.tb02351.x [12] Howard, P.S., Kucich, U., Taliwal, R. and Korostoff, J.M. (1998) Mechanical Forces Alter Extracellular Matrix Syn- thesis by Human Periodontal Ligament Fibroblasts. Journal of Periodontal Research, 33, 500-508. http://dx.doi.org/10.1111/j.1600-0765.1998.tb02350.x [13] Li, Y., Zhao, Z., Song, J., Feng, Y., Wang, Y., Li, X., et al. (2009) Cyclic Force Upregulates Mechano-Growth Factor and Elevates Cell Proliferation in 3D Cultured Skeletal Myoblasts. Archives of Biochemistry and Biophysics, 490, 171- 176. http://dx.doi.org/10.1016/j.abb.2009.08.016 [14] Fan, X., Zou, R., Zhao, Z., Yang, P., Li, Y. and Song, J. (2009) Tensile Strain Induces Integrin β1 and ILK Expression Higher and Faster in 3D Cultured Rat Skeletal Myoblasts than in 2D Cultures. Tissue and Cell, 41, 266-270. http://dx.doi.org/10.1016/j.tice.2008.12.007 [15] Li, Y., Song, J., Yang, P., Zou, R., Fan, X. and Zhao, Z. (2009) Establishment of a Three-Dimensional Culture and Mechanical Loading System for Skeletal Myoblasts. Cell Biology International, 33, 192-198. http://dx.doi.org/10.1016/j.cellbi.2008.11.002 [16] Burr, D.B., Milgrom, C., Fyhrie, D., Forwood, M., Nyska, M., Finestone, A., et al. (1996) In V ivo Measurement of Human Tibial Strains during Vigorous Activity. Bone, 18, 405-410. http://dx.doi.org/10.1016/8756-3282(96 )00 02 8-2 [17] McGuinness, N.J., Wilson, A.N., Jones, M.L. and Middleton, J. (1991) A Stress Analysis of the Periodontal Ligament under Various Orthodontic Loadings. The European Journal of Orthodontics, 13, 231-242. http://dx.doi.org/10.1093/ejo/13.3.231 [18] Baumrind, S. (1969) A Reconsideration of the Propriety of the “Pressure-Tension” Hypothesis. American Journal of Orthodontics, 55, 12-22. http://dx.doi.org/10.1016/S0002-9416(69)90170-5 [19] Baumrind, S. and Buck, D.L. (1970) Rate Changes in Cell Replication and Protein Synthesis in the Periodontal Liga- ment Incident to Tooth Movement. American Journal of Orthodontics, 57, 109-131. http://dx.doi.org/10.1016/0002-9416(70)90259-9 [20] Smith, R.K. and Roberts, W.E. (1980) Cell Kinetics of the Initial Response to Orthodontically Induced Osteogenesis in Rat Molar Periodontal Ligament. Calcified Tissue International, 30, 51-56. http://dx.doi.org/10.1007/BF02408606 [21] EugeneRoberts, W. and Chase, D.C. (1981) Kinetics of Cell Proliferation and Migration Associated with Orthodonti- cally-Induced Osteogenesis. Journal of Dental Research, 60, 174-181. http://dx.doi.org/10.1177/00220345810600021501 [22] Lia o , C. and Hua, Y. (2013) Effect of Hydrogen Sulphide on the Expression of Osteoprotegerin and Receptor Activator of NF-kB Ligand in Human Periodontal Ligament Cells Induced by Tension-Force Stimulation. Archives of Oral Bi- ology, 58, 1784-1790. http://dx.doi.org/10.1016/j.archoralbio.2013.08.004  L. K. Zhang et al. [23] Fan, X., Rahnert, J.A., Murphy, T.C., Nanes, M.S., Greenfield, E.M. and Rubin, J. (2006) Response to Mechanical Strain in an Immortalized Pre-Osteoblast Cell Is Dependent on ERK1/2. Journal of Cellular Physiology, 207, 454-460. http://dx.doi.org/10.1002/jcp.20581 [24] Takata, T., Miyauchi, M., Ogawa, I., Ito, H., Kobayashi, J. and Nikai, H. (1997) Reactive Change in Proliferative Ac- tivity of the Junctional Epithelium after Topical Application of Lipopolysaccharide. Journal of Periodontology, 68, 531-535. http://dx.doi.org/10.1902/jop.1997.68.6.531 [25] Bodet, C., Andrian, E., Tanabe, S.I. and Grenier, D. (2007) Actinobacillus Actinomycetemcomitans Lipopolysaccharide Regulates Matrix Metalloproteinase, Tissue Inhibitors of Matrix Metalloproteinase, and Plasminogen Activator Pro- duction by Human Gingival Fibroblasts: A Potential Role in Connective Tissue Destruction. Journal of cellular physi- ology, 212, 189-194. http://dx.doi.org/10.1002/jcp.21018 [26] Krishnan, V. and Davidovitch, Z.E. (2006) Cellular, Molecul ar, and Tissue-Level Reactions to Orthodontic Force. American Journal of Orthodontics and Dentofacial Orthopedics, 129, 469.e1-469. e32 . http://dx.doi.org/10.1016/j.ajodo.2005.10.007 [27] Ueda, N., Koide, M., Ohguchi, M., Ishihara, Y., Noguchi, T., Okahashi, N. and Nishihara, T. (1998) Involvement of Prostaglandin E2 and Interleukin-1α in the Differentiation and Survival of Osteoclasts Induced by Lipopolysaccharide from Actinobacillus Actinomycetemcomitans Y4. Journal of Periodontal Research, 33 , 509-51 6. http://dx.doi.org/10.1111/j.1600-0765.1998.tb02351.x [28] Yamaji, Y., Kubota, T., Sasaguri, K., Sato, S., Suzuki, Y., Kumada, H. and Umemoto, T. (1995) Inflammatory C yto- kine Gene Expression in Human Periodontal Ligament Fibroblasts Stimulated with Bacterial Lipopolysaccharides. In- fection and Immunity, 63, 3576-3581.
|