Z. Güler et al. / Open Journal of Animal Sciences 1 (2011) 10-15
Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/ojas/
scores were significant with titratable acidity (0.61, P<
0.001), appearence (–0.59, P < 0.001) and overall
acceptability (–0.57, P < 0.001). Similar results were
obtained by Chamnas et al. (2006) and Harper et al.
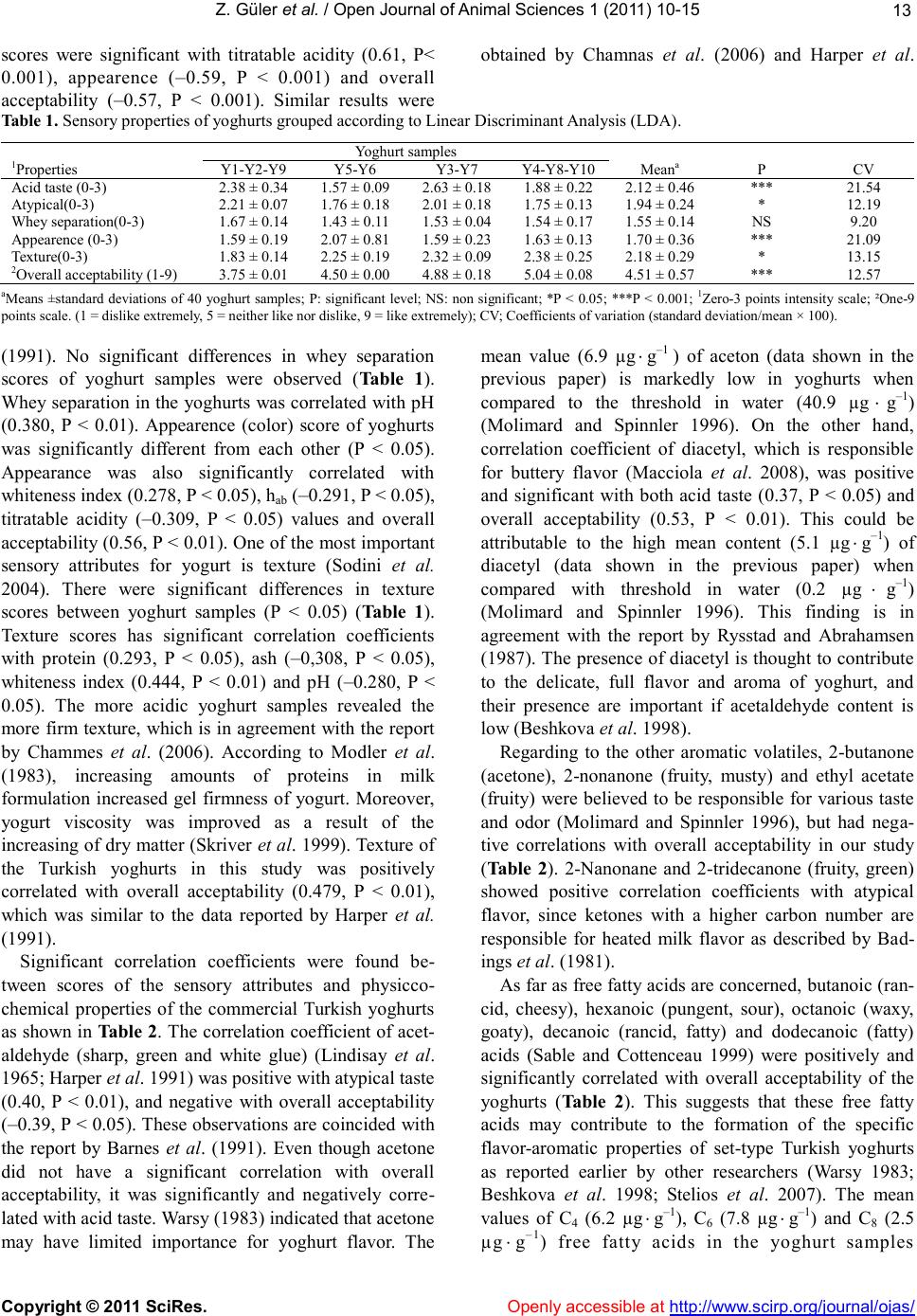
Table 1. Sensory properties of yoghurts grouped according to Linear Discriminant Analysis (LDA).
2Overall acceptability (1-9)
aMeans ±standard deviations of 40 yoghurt samples; P: significant level; NS: non significant; *P < 0.05; ***P < 0.001; 1Zero-3 points intensity scale; ²One-9
points scale. (1 = dislike extremely, 5 = neither like nor dislike, 9 = like extremely); CV; Coefficients of variation (standard deviation/mean × 100).
(1991). No significant differences in whey separation
scores of yoghurt samples were observed (Table 1).
Whey separation in the yoghurts was correlated with pH
(0.380, P < 0.01). Appearence (color) score of yoghurts
was significantly different from each other (P < 0.05).
Appearance was also significantly correlated with
whiteness index (0.278, P < 0.05), hab (–0.291, P < 0.05),
titratable acidity (–0.309, P < 0.05) values and overall
acceptability (0.56, P < 0.01). One of the most important
sensory attributes for yogurt is texture (Sodini et al.
2004). There were significant differences in texture
scores between yoghurt samples (P < 0.05) (Table 1).
Texture scores has significant correlation coefficients
with protein (0.293, P < 0.05), ash (–0,308, P < 0.05),
whiteness index (0.444, P < 0.01) and pH (–0.280, P <
0.05). The more acidic yoghurt samples revealed the
more firm texture, which is in agreement with the report
by Chammes et al. (2006). According to Modler et al.
(1983), increasing amounts of proteins in milk
formulation increased gel firmness of yogurt. Moreover,
yogurt viscosity was improved as a result of the
increasing of dry matter (Skriver et al. 1999). Texture of
the Turkish yoghurts in this study was positively
correlated with overall acceptability (0.479, P < 0.01),
which was similar to the data reported by Harper et al.
(1991).
Significant correlation coefficients were found be-
tween scores of the sensory attributes and physicco-
chemical properties of the commercial Turkish yoghurts
as shown in Table 2. The correlation coefficient of acet-
aldehyde (sharp, green and white glue) (Lindisay et al.
1965; Harper et al. 1991) was positive with atypical taste
(0.40, P < 0.01), and negative with overall acceptability
(–0.39, P < 0.05). These observations are coincided with
the report by Barnes et al. (1991). Even though acetone
did not have a significant correlation with overall
acceptability, it was significantly and negatively corre-
lated with acid taste. Warsy (1983) indicated that acetone
may have limited importance for yoghurt flavor. The
mean value (6.9 µg
g–1 ) of aceton (data shown in the
previous paper) is markedly low in yoghurts when
compared to the threshold in water (40.9 µg
g–1)
(Molimard and Spinnler 1996). On the other hand,
correlation coefficient of diacetyl, which is responsible
for buttery flavor (Macciola et al. 2008), was positive
and significant with both acid taste (0.37, P < 0.05) and
overall acceptability (0.53, P < 0.01). This could be
attributable to the high mean content (5.1 µg
g–1) of
diacetyl (data shown in the previous paper) when
compared with threshold in water (0.2 µg
g–1)
(Molimard and Spinnler 1996). This finding is in
agreement with the report by Rysstad and Abrahamsen
(1987). The presence of diacetyl is thought to contribute
to the delicate, full flavor and aroma of yoghurt, and
their presence are important if acetaldehyde content is
low (Beshkova et al. 1998).
Regarding to the other aromatic volatiles, 2-butanone
(acetone), 2-nonanone (fruity, musty) and ethyl acetate
(fruity) were believed to be responsible for various taste
and odor (Molimard and Spinnler 1996), but had nega-
tive correlations with overall acceptability in our study
(Table 2). 2-Nanonane and 2-tridecanone (fruity, green)
showed positive correlation coefficients with atypical
flavor, since ketones with a higher carbon number are
responsible for heated milk flavor as described by Bad-
ings et al. (1981).
As far as free fatty acids are concerned, butanoic (ran-
cid, cheesy), hexanoic (pungent, sour), octanoic (waxy,
goaty), decanoic (rancid, fatty) and dodecanoic (fatty)
acids (Sable and Cottenceau 1999) were positively and
significantly correlated with overall acceptability of the
yoghurts (Table 2). This suggests that these free fatty
acids may contribute to the formation of the specific
flavor-aromatic properties of set-type Turkish yoghurts
as reported earlier by other researchers (Warsy 1983;
Beshkova et al. 1998; Stelios et al. 2007). The mean
values of C4 (6.2 µg
g–1), C6 (7.8 µg
g–1) and C8 (2.5
µg
g–1) free fatty acids in the yoghurt samples