Paper Menu >>
Journal Menu >>
 Vol.3, No.3, 227-233 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.33029 Copyright © 2011 SciRes. OPEN ACCESS Induction of testis-ova in nile tilapia (Oreochromis niloticus) exposed to 17-estradiol Piya Kosai1, Wannee Jiraungkoorskul1*, Chaivira Sachamahithinant2, Kanitta Jiraungkoorskul3 1Department of Pathobiology, Faculty of Science, Mahidol University, Bangkok, Thailand; *Corresponding Author: tewjr@mahidol.ac.th 2Mahidol University International College, Mahidol University, Salaya Campus, Nakhonpathom, Thailand 3Department of Occupational Health and Safety, Faculty of Public Health, Mahidol University, Bangkok, Thailand Received 12 November 2010; revised 27 December 2010; accepted 28 December 2010. ABSTRACT The efficacy of Endocrine Disrupting Com- pounds (EDCs), 17β-estradiol was tested on the fish Oreochromis niloticus in order to under- stand the intersex relationship of fish, in which sequential hermaphrodism can consist of a male changing into a female (protandry) or a female changing into a male (protogyny). The fish were equally divided into 3 groups. The first group was the control group; the second and third groups were treated with 10 and 100 g L-1 of 17β-estradiol, respectively, for 30 days. The overall result in this experiment had no signifi- cant effect on the growth parameters. Among the two treated groups, the low concentration group shows results similar to those of the control groups. The high concentration group shows changes to the male reproductive sys- tem with the appearance of the testis-ova pre- sent resulting in an intersex condition of the male gonads. With this experiment, it can be concluded that 17β-estradiol at high concentra- tion reveals positive changes towards the male reproductive system of the fish, Oreochromis niloticus. Keywords: Oreochromis niloticus; Nile Tilapia; Endocrine Disruption; Xenoestrogens; Testis-Ova; 17β-Estradiol 1. INTRODUCTION “Endocrine Disruption” has been defined as exoge- nous chemicals that alter the functions of the endocrine system and consequently cause adverse health effects in the intact organisms [1]. Endocrine Disruptor Screening and Testing Advisory Committee were assigned the task of making recommendations for the development of test- ing and screening programs for endocrine disrupters [2]. Likewise, the Organization for Economic Co-operation and Development also established a special activity for endocrine disrupter testing and assessment [3]. Subse- quently, the World Health Organization tasked the inter- national program on chemical safety with preparing a report describing the global assessment of the scientific literature on endocrine disrupting chemicals [4]. There is increasing concern about male reproductive disorders in humans, and widespread sexual disruption among wildlife. Numerous studies have documented generation rises in testicular cancer, declines in sperm quality and increases in infertility [5]; increases in rates of male infants born with incompletely-formed penises and undescended testicles [6,7]. The ratio of baby boys to baby girls is declining in the U.S. and other industri- alized countries [8]. The causes of these worrying trends are largely unknown, but one possibility is that they might be linked to endocrine disrupting chemicals (EDCs). Among EDCs found in the aquatic environment are the steroid estrogens such as 17β-estradiol, estrone and estriol [9]. In oviparous fish species, the production of vitellogenin, an egg yolk protein precursor, is a critical step for successful reproduction in females. Normally, only mature females produce enough endogenous estro- gen to induce vitellogenesis in fish, but exposure to es- trogenic chemicals in the external environment can trig- ger this response in male and juvenile fish as well. Many wildlife species, especially fish, show signs of feminisa- tion, with male fish producing large quantities of vitel- logenin and developing sexual organs that are interme- diate between male and female features, resulting in so-called intersex fish [10]. In the aquatic environment, EDCs are easily bioavailability to fish through a variety  P. Kosai et al. / Natural Science 3 (2011) 227-233 Copyright © 2011 SciRes. OPEN ACCESS 228 of routes, including aquatic respiration, osmoregulation and maternal transfer of contaminants in lipid reserves of eggs [11]. Dermal contacts with contaminated sediments or ingestion of contaminated food are additional expo- sure routes. EDCs that are apt to cause endocrine modu- lation in vivo have one of three characteristics: they are present in the environment at high concentrations, they are persistent and bioaccumulative, or they are con- stantly entering the environment [12]. EDCs that are resistant to biodegradation and are lipophilic may bio- accumulation in exposed organisms. Such EDCs include organochlorine pesticides, polychlorinated biphenyls and alkylphenols, all of which are found in aquatic environ- ments. Other EDCs i.e., bisphenol A and 17β-estradiol, do not bioaccumulation to any extent but are constantly entering the aquatic environment through operations such as effluent from sewage treatment works and run-off from concentrated animal feeding operations [13]. In the present study, we describe the normal histology of Nile tilapia (Oreochromis niloticus) reproductive or- gan, and illustrate histopathological alterations in this organ associated with endocrine disrupting chemicals in the laboratory studies. 2. MATERIAL AND METHODS 2.1. Experimental Fish This study was performed at the Department of Pathobiology, Faculty of Science, Mahidol University, Bangkok, Thailand. Nile tilapia, O. niloticus, was juve- nile and young adults with 1.22 ± 0.45 g, and 4.25 ± 0.58 g body weight, respectively. Tap water was filtered to eliminate chemical contamination. The physicochemi- cal characteristics of water were measured daily, ac- cording to the experimental procedures described in Standard Methods for the Examination of Water and Wastewater [14]. Under laboratory condition, fish were acclimated for 30 days at 29.0 ± 1.0˚C, pH = 6.6 – 7.0, total hardness = 68 – 80 mg L-1 (as CaCO3), alkalinity = 75 - 80 mg L-1 and conductivity = 190 – 220 µmhos cm-1. A 16:8 hour light-dark cycle was maintained throughout. Chlorine residual and ammonia were below detection limits. Fish were fed twice a day with 37%-protein commercial fish food (Charoen Pokphand Group, Bangkok, Thailand). The quantity of food was 2% of the initial body weight per day. The animal care and han- dling in this research was performed following the in- struction of the Mahidol University-Institutional Animal Care and Use Committee (MU-IACUC). Therefore, this research was followed the mammal animal care and use i.e., 1) Use, care and transportation of fish for toxicopa- thological testing was complied with all applicable ani- mal welfare laws. 2) Number of fish was kept to the minimum requirement for achieve scientifically valid results. 3) All protocols were taken to avoid the discom- fort, distress or pain in the fish. 4) The appropriate dos- age of the anesthesia was 200 mg L-1 ethyl-3-amino- benzoate methanesulfonate salt (MS222, Sigma) and the euthanasia was overdose of this chemical. 2.2. Experimental Design The experiments were carried out in three glass aquaria containing of the same physicochemical charac- teristics of water, with continuous aeration. Fish (n = 30) were randomly assigned to the three groups: Group 1 was the control group; Group 2 and 3 were the treated groups with 10 and 100 g L-1 of 17β-estradiol, respec- tively. After 30 days exposure, fish from each aquarium were anesthetized. Dissection of fish was performed after completing the external examination. The repro- ductive organ was removed and prepared for histopa- thological studies. 2.3. Specimen Preparation for Light Microscopic Studies The procedures for light microscopy were modified Humason’s method [15]. Briefly, small pieces of tissues were fixed in the 10% buffered formaldehyde for 24 h, dehydrate through a graded series of ethanol and clear with xylene solutions. They were embedded in a block using melted paraffin at the embedding station. The par- affin blocks were sectioned at 4 m thickness using a rotary microtome, and stained with hematoxylin and eosin. The tissue glass slides were examined for abnor- malities by a Nikon E600 light microscope and photo- graphed by a Nikon DXM 1200 digital camera (Tokyo, Japan). 3. RESULTS Female gonads were classified of being in one of six maturation stages according to a classification scale proposed by Gupta [16]. Stage I corresponded to imma- ture ovaries, with youngest oocytes diameter 0.05 - 0.15 mm. Stage II showed maturing oocytes, characteristi- cally containing small vacuoles in the cytoplasm diame- ter 0.15 - 0.3 mm. Stage III was characterized by ad- vanced maturing oocytes diameter 0.3 - 0.7 mm. Then, as the ovaries mature, from Stage III onward a number of ova undergo a process of resorption. At this stage the whole follicle loosed its shape and was described as an atretic follicle. Stage IV represented mature oocytes di- ameter 0.7 - 0.8 mm, filled with chromophilic yolk. At Stage V, the mature ova increased in size with yolk ac- cumulation, and at this stage they were ripe. At Stage VI, the ovary did not differ greatly from the previous stage, 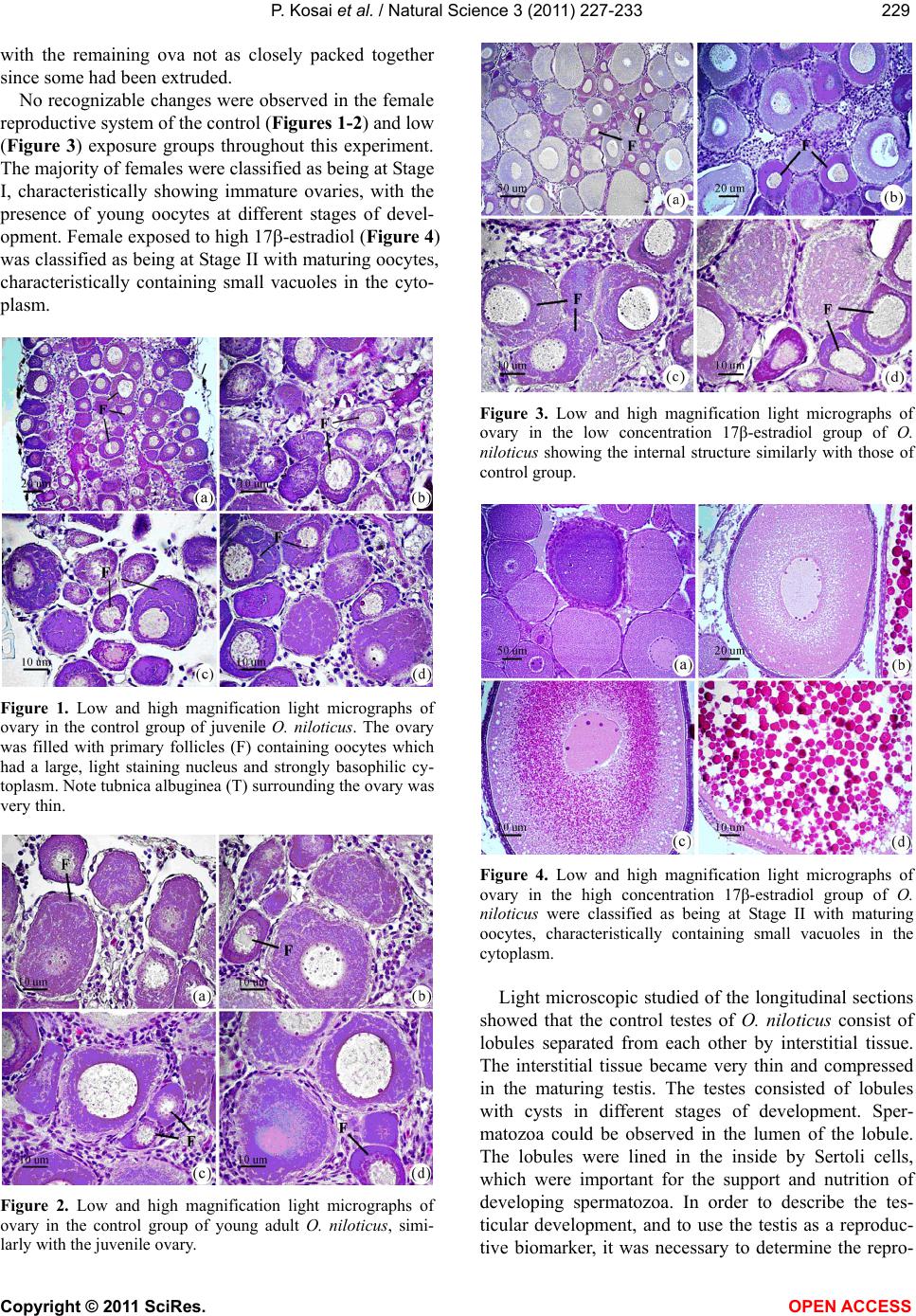 P. Kosai et al. / Natural Science 3 (2011) 227-233 Copyright © 2011 SciRes. OPEN ACCESS 229229 with the remaining ova not as closely packed together since some had been extruded. No recognizable changes were observed in the female reproductive system of the control (Figures 1-2) and low (Figure 3) exposure groups throughout this experiment. The majority of females were classified as being at Stage I, characteristically showing immature ovaries, with the presence of young oocytes at different stages of devel- opment. Female exposed to high 17-estradiol (Figure 4) was classified as being at Stage II with maturing oocytes, characteristically containing small vacuoles in the cyto- plasm. Figure 1. Low and high magnification light micrographs of ovary in the control group of juvenile O. niloticus. The ovary was filled with primary follicles (F) containing oocytes which had a large, light staining nucleus and strongly basophilic cy- toplasm. Note tubnica albuginea (T) surrounding the ovary was very thin. Figure 2. Low and high magnification light micrographs of ovary in the control group of young adult O. niloticus, simi- larly with the juvenile ovary. Figure 3. Low and high magnification light micrographs of ovary in the low concentration 17β-estradiol group of O. niloticus showing the internal structure similarly with those of control group. Figure 4. Low and high magnification light micrographs of ovary in the high concentration 17β-estradiol group of O. niloticus were classified as being at Stage II with maturing oocytes, characteristically containing small vacuoles in the cytoplasm. Light microscopic studied of the longitudinal sections showed that the control testes of O. niloticus consist of lobules separated from each other by interstitial tissue. The interstitial tissue became very thin and compressed in the maturing testis. The testes consisted of lobules with cysts in different stages of development. Sper- matozoa could be observed in the lumen of the lobule. The lobules were lined in the inside by Sertoli cells, which were important for the support and nutrition of developing spermatozoa. In order to describe the tes- ticular development, and to use the testis as a reproduc- tive biomarker, it was necessary to determine the repro- 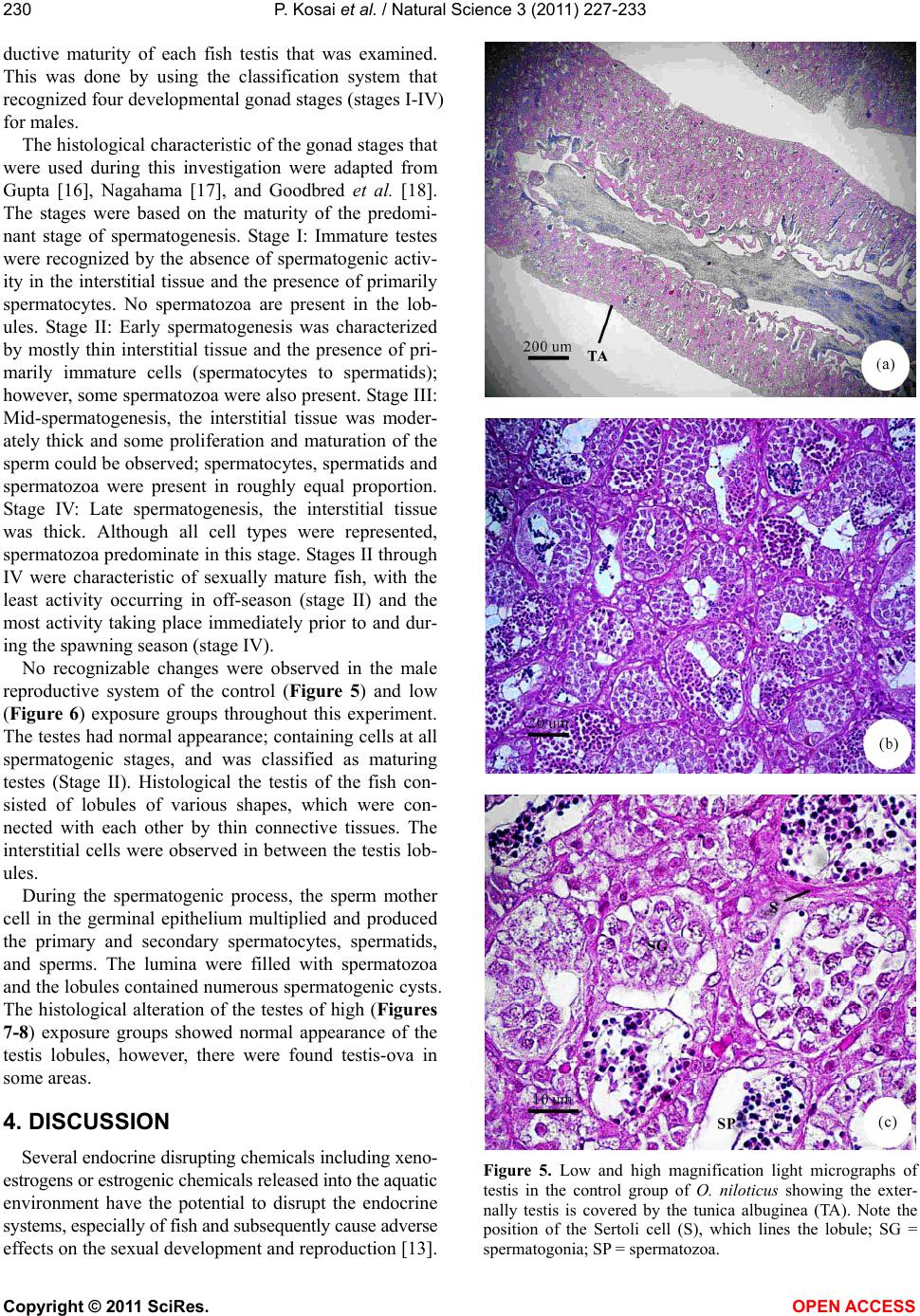 P. Kosai et al. / Natural Science 3 (2011) 227-233 Copyright © 2011 SciRes. OPEN ACCESS 230 ductive maturity of each fish testis that was examined. This was done by using the classification system that recognized four developmental gonad stages (stages I-IV) for males. The histological characteristic of the gonad stages that were used during this investigation were adapted from Gupta [16], Nagahama [17], and Goodbred et al. [18]. The stages were based on the maturity of the predomi- nant stage of spermatogenesis. Stage I: Immature testes were recognized by the absence of spermatogenic activ- ity in the interstitial tissue and the presence of primarily spermatocytes. No spermatozoa are present in the lob- ules. Stage II: Early spermatogenesis was characterized by mostly thin interstitial tissue and the presence of pri- marily immature cells (spermatocytes to spermatids); however, some spermatozoa were also present. Stage III: Mid-spermatogenesis, the interstitial tissue was moder- ately thick and some proliferation and maturation of the sperm could be observed; spermatocytes, spermatids and spermatozoa were present in roughly equal proportion. Stage IV: Late spermatogenesis, the interstitial tissue was thick. Although all cell types were represented, spermatozoa predominate in this stage. Stages II through IV were characteristic of sexually mature fish, with the least activity occurring in off-season (stage II) and the most activity taking place immediately prior to and dur- ing the spawning season (stage IV). No recognizable changes were observed in the male reproductive system of the control (Figure 5) and low (Figure 6) exposure groups throughout this experiment. The testes had normal appearance; containing cells at all spermatogenic stages, and was classified as maturing testes (Stage II). Histological the testis of the fish con- sisted of lobules of various shapes, which were con- nected with each other by thin connective tissues. The interstitial cells were observed in between the testis lob- ules. During the spermatogenic process, the sperm mother cell in the germinal epithelium multiplied and produced the primary and secondary spermatocytes, spermatids, and sperms. The lumina were filled with spermatozoa and the lobules contained numerous spermatogenic cysts. The histological alteration of the testes of high (Figures 7-8) exposure groups showed normal appearance of the testis lobules, however, there were found testis-ova in some areas. 4. DISCUSSION Several endocrine disrupting chemicals including xeno- estrogens or estrogenic chemicals released into the aquatic environment have the potential to disrupt the endocrine systems, especially of fish and subsequently cause adverse effects on the sexual development and reproduction [13]. Figure 5. Low and high magnification light micrographs of testis in the control group of O. niloticus showing the exter- nally testis is covered by the tunica albuginea (TA). Note the position of the Sertoli cell (S), which lines the lobule; SG = spermatogonia; SP = spermatozoa. 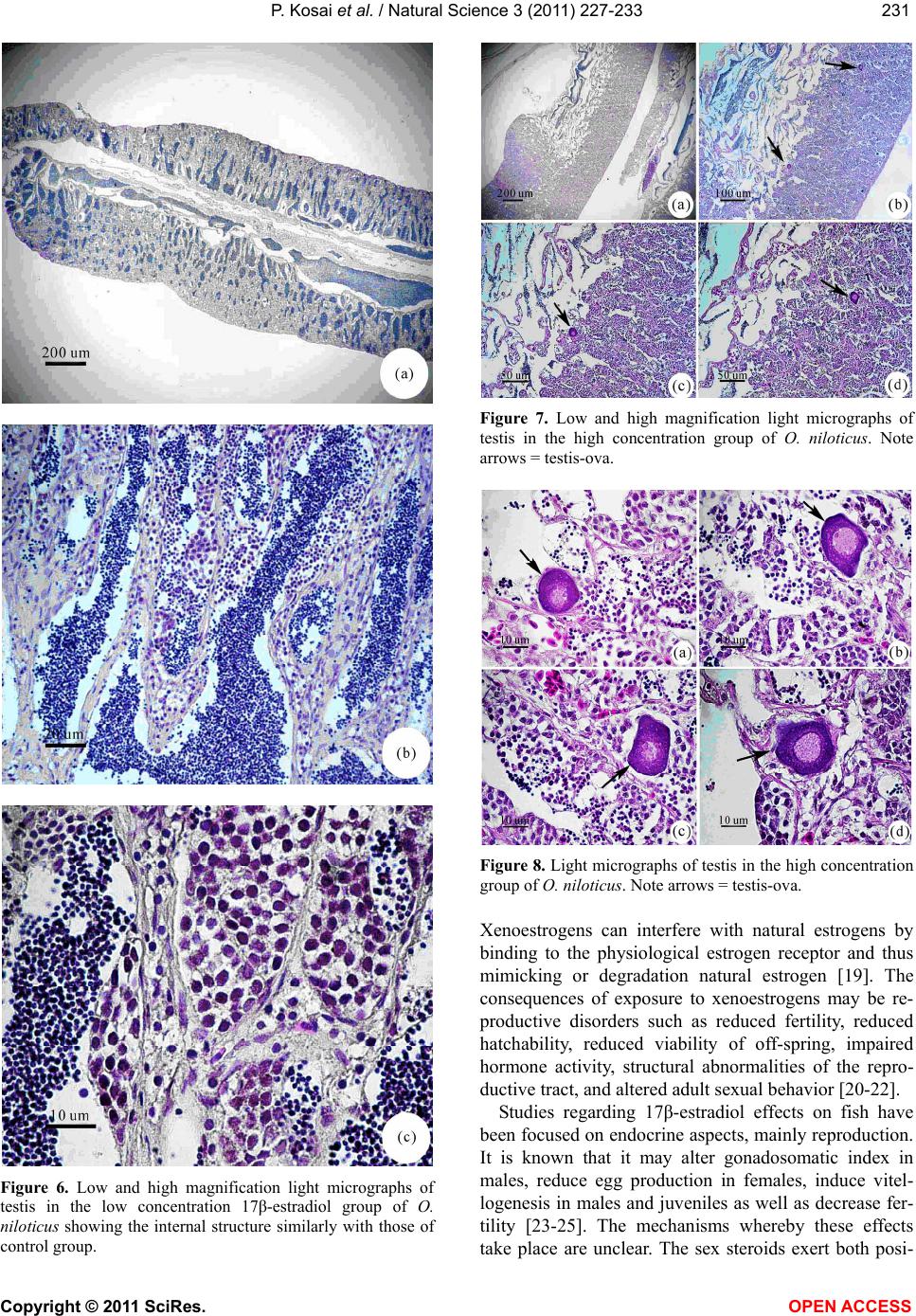 P. Kosai et al. / Natural Science 3 (2011) 227-233 Copyright © 2011 SciRes. OPEN ACCESS 231231 Figure 6. Low and high magnification light micrographs of testis in the low concentration 17β-estradiol group of O. niloticus showing the internal structure similarly with those of control group. Figure 7. Low and high magnification light micrographs of testis in the high concentration group of O. niloticus. Note arrows = testis-ova. Figure 8. Light micrographs of testis in the high concentration group of O. niloticus. Note arrows = testis-ova. Xenoestrogens can interfere with natural estrogens by binding to the physiological estrogen receptor and thus mimicking or degradation natural estrogen [19]. The consequences of exposure to xenoestrogens may be re- productive disorders such as reduced fertility, reduced hatchability, reduced viability of off-spring, impaired hormone activity, structural abnormalities of the repro- ductive tract, and altered adult sexual behavior [20-22]. Studies regarding 17β-estradiol effects on fish have been focused on endocrine aspects, mainly reproduction. It is known that it may alter gonadosomatic index in males, reduce egg production in females, induce vitel- logenesis in males and juveniles as well as decrease fer- tility [23-25]. The mechanisms whereby these effects take place are unclear. The sex steroids exert both posi-  P. Kosai et al. / Natural Science 3 (2011) 227-233 Copyright © 2011 SciRes. OPEN ACCESS 232 tive and negative feedback control on the hypothalamus- pituitary system to regulate the release of gonadotropins. The 17-estradiol has been shown to inhibit gonadotro- pin secretion in fish. This may indicate that 17-estradiol cause its effects on the testes through alterations in go- nadotropin secretion. Mills [26] reported that 17-estradiol treated fish ex- hibited decreased gonadosomatic index (GSI), altered hepatosomatic index, elevated plasma estradiol, reduced plasma testosterone, and high levels of plasma vitel- logenin. Reduced GSI always corresponded with ob- servable histopathological changes indicative of re- gressed gonads. The present study, Nile tilapia was ex- posed to 100 g L-1 of 17β-estradiol for 30 days showed this intersex (testes-ova). These obtained results agree with the previously study, exposure to estrogenic chemicals during the critical periods of sex differentia- tion has induced testis-ova in several fish species [27, 28]. As suggested by Arcand-Hoy and Benson [29], 17β- estradiol given to the male fish can disturb the hypo- thalamus–pituitary-gonadal axis in which several hor- mones, including androgens, normally regulates sexual development, sexual physiology, and sexual behavior. Since androgens, also in male regulate secondary sexual characters and reproductive behavior, a disruption of the androgen synthesis or the processes in which androgens participate can cause severe effects [21]. Gimeno and colleague [10] indicated the role of es- trogens in the induction of intersex gonads in adult gonochoristic species seems similar to that of the normal process of sex reversal in hermaphrodite species. Indeed, the treatment of juvenile male black porgy, Acanthopa- grus schlegeli, a protandrous hermaphrodite marine fish, with 17β-estradiol caused the regression of the testes and the lack of spermiation, as well as the induction of pre- cocious sex change, as shown by the appearance of oo- cytes [30]. In conclusion, the present study shows that water- borne exposure to the xenoestrogens 17β-estradiol cause’s severe effects on the reproductive system of male Nile tilapia. 5. ACKNOWLEDGEMENTS This study was funded by the Thailand Research Fund and the Com- mission on Higher Education: Research Grant for Mid-Career Univer- sity Faculty 2008 (RMU5180001) and in part by Mahidol University International College, and Faculty of Science, Mahidol University. REFERENCES [1] Vos, J.G., Dybing, E., Greim, H.A., Ladefoged, O., Lam- bre, C., Tarazona, J.V., Brandt, I. and Vethaak, A.D. (2000) Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situa- tion. Critical Reviews in Toxicology, 30, 71-133. [2] Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC). (1998) Endocrine disruptor screening and testing advisory committee final report. [3] Organization for Economic Co-operation and Develop- ment (OECD). (1996) Endocrine disruptor testing and assessment. [4] World Health Organization. (2002) Global Assessment of the state-of-the-science of Endocrine Disruptors. WHO/ PCS/EDC/02.2. International Program on Chemical Safety. [5] Adami, H.O., Bergstrom, R., Mohner, M., Zatonski, W., Storm, H., Ekbom, A., Tretli, S., Teppo, L., Ziegler, H. and Rahu, M. (1994) Testicular cancer in nine northern European countries. International Journal of Cancer, 59, 33-38. [6] Toppari, J., Larsen, J.C., Christiansen, P., Giwercman, A., Grandjean, P., Guillette, L.J., Jegou, B., Jensen, T.K., Jouannet, P., Keiding, N., Leffers, H., McLachlan, J.A., Meyer, O., Muller, J., Rajpert-De Meyts, E., Scheike, T., Sharpe, R., Sumpter, J. and Skakkebaek, N.E. (1995) Male reproductive health and environmental chemicals with estrogenic effects. Ministry of Environment and Energy, Copenhagen, Denmark. Miljoprojekt 290, Dan- ish EPA. [7] Hutson, J., Baker, M., Terada, M., Zhou, B. and Paxton, G. (1994) Hormonal control of testicular descent and the cause of cryptorchidism. Reproduction, Fertility and Development, 6, 151-156. [8] Davis, D.L., Gottlieb, M.B. and Stampnitzky, J.R. (1998) Reduced ratio of male to female births in several indus- trial countries: A sentinel health indicator? The Journal of the American Medical Association, 279, 1018-1023. [9] Baronti, C.R., D’Ascenzo, C.G., Di Corcia, A., Gentili, A. and Samperi, R. (2000) Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in receiving river water. Environmental Science and Technology, 34, 5059-5066. [10] Gimeno, S., Komen, H., Gerritsen, A.G.M. and Bowmer, T. (1998) Feminisation {PRIVATE “TYPE = PICT; ALT = next term”} of young males of the common {PRIVATE “TYPE = PICT; ALT = previous term”} carp, {PRIVATE “TYPE = PICT; ALT = next term”} Cyprinus carpio, exposed to 4-tert-pentylphenol during sexual differentia- tion. Aquatic Toxicology, 43, 77-92. [11] van der Kraack, G., Hewitt, M., Lister, A., McMaster, M.E. and Munkittrick, K.R. (2001) Endocrine toxicants and reproductive success in fish. Human and Ecological Risk Assessment, 7, 1017-1025. [12] Tyler, C.R., Jobling, S. and Sumpter, J.P. (1998) Endo- crine disruption in wildlife: A critical review of the evi- dence. Critical Reviews in Toxicology, 28, 319-361. [13] Rasmussen, T.H., Teh, S.J., Bjerregaard, P. and Kors- gaard, B. (2005) Anti-estrogen prevents xenoestrogen- induced testicular pathology of eelpout (Zoarces vivipa- rus). Aquatic Toxicology, 72, 177-194. [14] American Public Health Association (APHA). (2005) Standard methods for the examination of water and wastewater. 21st Edition, Washington, D.C. [15] Humason, G.L. (1972) Animal tissue techniques. 3rd Edition, W.H. Freeman and Company, San Francisco.  P. Kosai et al. / Natural Science 3 (2011) 227-233 Copyright © 2011 SciRes. OPEN ACCESS 233233 [16] Gupta, S. (1975) The development of carp gonads in warm water aquaria. Journal of Fish Biology, 7, 775-782. [17] Nagahama, Y. (1983) The functional morphology of teleost gonads. In: W.S. Hoar, D.F. Randall, and E.M. Donaldson, Eds., Academic Press, Orlando, 223-264. [18] Goodbred, S.L., Gilliom, R.J., Gross, T.S., Denslow, N.P., Bryant, W.L. and Schoeb, T.R. (1997) Reconnaissance of 17β-estradiol, 11-ketotestosterone, vitellogenin, and go- nad histopathology in common carp of United States streams. Geological Survey Open-File Report 96-627, Sacramento (CA), U.S. [19] Muller, A.M.F., Makropoulos, V. and Bolt, H.M. (1995) Toxicological aspects of oestrogen-mimic xenobiotics present in the environment. Toxicology and Ecotoxicol- ogy News, 2, 68-73. [20] van den Belt, K., Wester, P.W., van der Ven, L.T.M., Verheyen, R. and Witters, H. (2002) Effects of ethynylestradiol on the reproductive physiology in ze- brafish (Danio rerio): Time dependency and reversibility. Environmental Toxicology and Chemistry, 21, 767-775. [21] Wibe, A.E., Rosenqvist, G. and Jenssen, B.M. (2002) Disruption of male reproductive behavior in threespine stickleback Gasterosteus aculeatus exposed to 17-es- tradiol. Environmental Research, 90, 136-141. [22] Peters, R.E.M., Courtenay, S.C., Cagampan, S., Hewitt, M.L. and MacLatchy, D.L. (2007) Effects on reproduc- tive potential and endocrine status in the mummichog (Fundulus heteroclitus) after exposure to 17-ethynyl- estradiol in a short-term reproductive bioassay. Aquatic Toxicology, 85, 154-166. [23] Hiramatsu, N., Hara, A., Hiramatsu, K., Fukada, H., We- ber, G., Denslow, N. and Sullivan, C. (2002) Vitel- logenin-derived yolk proteins of white perch, Morone americana: Purification, characterization, and vitel- logenin receptor binding. Biology of Reproduction, 67, 655-667. [24] Angus, R.A., Stankoa, J., Jenkins, R.L. and Watson, R.D. (2005) Effects of 17-ethynylestradiol on sexual devel- opment of male western mosquitofish (Gambusia affinis). Comparative Biochemistry and Physiology Part C: Toxi- cology and Pharmacology, 140, 330-339. [25] Teles, M., Pacheco, M. and Santos, M.A. (2006) Bio- transformation, stress and genotoxic effects of 17β-es- tradiol in juvenile sea bass (Dicentrarchus labrax L.). Environment International, 32, 470-477. [26] Mills, L.J., Gutjahr-Gobell, R.E., Haebler, R.A., Borsay Horowitz, D.J., Jayaraman, S., Pruell, R.J., McKinney, R.A., Gardner, G.R. and Zaroogian, G.E. (2001) Effects of estrogenic (o,p’-DDT; octylphenol) and anti-andro- genic (p,p’-DDE) chemicals on indicators of endocrine status in juvenile male summer flounder (Paralichthys dentatus). Aquatic Toxicology, 52, 157-176. [27] Seki, M., Yokota, H., Maeda, M., Tadokoro, H. and Ko- bayashi, K. (2003) Effects of 4-nonylphenol and 4-tert- octylphenol on sex differentiation and vitellogenin in- duction in medaka (Oryzias latipe). Environmental Toxi- cology and Chemistry, 2, 1507-1516. [28] Flynn, S.R. and Benfey, T.J. (2007) Effects of dietary estradiol-17β in juvenile shortnose sturgeon, Acipenser brevirostrum, Lesueur. Aquaculture, 270, 405-412. [29] Arcand-Hoy, L.D. and Benson, W. (1998) Fish reproduc- tion: An ecologically relevant indicator of endocrine dis- ruption. Environmental Toxicology and Chemistry, 17, 49-57. [30] Chang, C.F., Lau, E.L. and Lin, B.Y. (1995) Stimulation of spermatogenesis or of sex reversal according to the dose of exogenous estradiol-17β in juvenile males of protandrous black porgy, Acanthopagrus schlegeli. Gen- eral and Comparative Endocrinology, 100, 355-367. |

