 Surgical Science, 2011, 2, 52-56 doi:10.4236/ss.2011.22011 Published Online April 2011 (http://www.SciRP.org/journal/ss) Copyright © 2011 SciRes. SS Angiomyolipoma of the Jejunum Mimicking Metastatic Disease in a Patient with Colonic Adenocarcinoma Spyridon Miliaras1, Dimosthenis Miliaras2 1Surgical Department , Papageorgiou Hospital, Medical School, Aristotle University, Thessaloniki, Greece 2Laboratory of Histology and Embryology, Med i c a l School, Aristotle University, Thessaloniki, Greece E-mail: dmiliara@med.auth.gr Received July 27, 2010; revised January 5, 2011; accepted March 9, 2011 Abstract Angiomyolipoma is a benign tumor most commonly arising in the kidney. Very few cases have been re- ported to be located in the small intestine. Here we report the first case located in the jejunum in a patient who was diagnosed with a colonic adenocarcinoma. In the preoperative evaluation this benign lesion was thought it might represent a metastatic nodule. Keywords: Angiomyolipoma, Colon Adenocarcinoma, Jejunum 1. Introduction Angiomyolipoma (AML) is a benign neoplasm com- posed of blood vessels, smooth muscle cells, and mature fat cells [1]. AML most commonly arises in the kidney. Other locations include the liver (most frequent extrare- nal site) [2], the uterus[3], the lungs [4], the skin [5], the subgaleal space [6], the anterior mediastinum [7], the urinary bladder [8], and mucosal membranes such as the lips [9] and the nasal cavity [10]. Very few cases have been reported in the gastrointestinal tract, including nine cases arising from the colon [11-19], and four cases aris- ing from the small bowel [20-23]. Two of the latter cases were located in the duodenum [20,21], and the other two in the ileum [22,23]. In this paper we report a fifth case of AML of the small intestine, this time located at the jejunum. The benign small intestinal tumor was mas- querading a metastatic nodule in the preoperative imag- ing evaluation, since our patient also presented a tumor in the ileocecal region. 2. Case Report An 80 years old male was urgently admitted to our sur- gical department with abdominal pain, vomiting and ab- dominal distension. On clinical examination the patient was afebrile and the abdomen was moderately distended, with mild tenderness on palpation of the right iliac fossa, but no signs of peritonism. Bowel sounds were reduced and digital examination of the rectum was normal. Plain abdominal films confirmed small bowel obstruction and full blood count and biochemistry were unremarkable. Conservative treatment was initiated, including intrave- nous fluids, nasogastric suction and monitoring of the vital organs. After three days of conservative treatment, although there was no clinical deterioration, the obstruc- tive ileus was not resolved and a colonoscopy was per- formed, to further investigate the etiology of obstruction, which revealed an obstructive tumor of the ileocecal valve. A preoperative CT scan of the abdomen per- formed the next day for staging purposes showed re- gional lymphadenopathy of the mesentery of the ileoce- cal region, but no hepatic metastases. However, a soft tissue mass was found in the proximal jejunum with most probable diagnosis to be metastatic implant (Figure 1(a)). In order to resolve the persisting ileus, an exploratory laparotomy was decided and performed through a high suprasubumbilical incision. A large obstructive tumor of the ileocecal valve with regional lymphadenopathy were detected with no signs of hepatic or other metastatic dis- ease. However, a round, well circumscribed tumor of the antimesenteric border of the proximal jejunum was de- tected (Figure 1(b)), which was excised in healthy tis- sues macroscopically through a limited small bowel en- terectomy. A typical right hemicolectomy was then per- formed with ileocolic anastomosis to the transverse colon. Following an uneventful postoperative period, and after gradual mobilization and feeding, the patient was dis- charged home on the seventh postoperative with instruc- tions for regular follow-up and referral to the medical 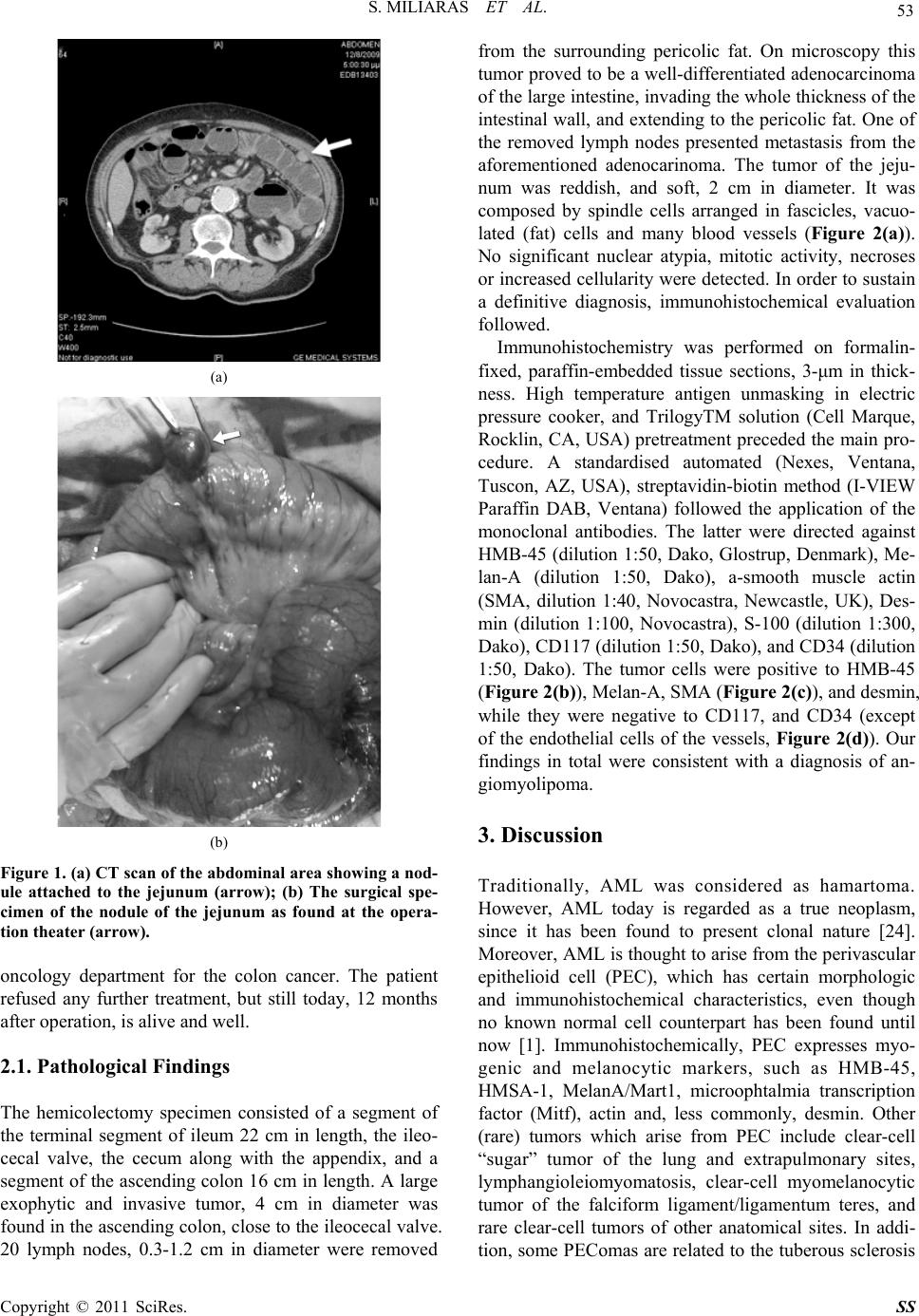 S. MILIARAS ET AL. 53 (a) (b) Figure 1. (a) CT scan of the abdominal area showing a nod- ule attached to the jejunum (arrow); (b) The surgical spe- cimen of the nodule of the jejunum as found at the opera- tion theater (arrow). oncology department for the colon cancer. The patient refused any further treatment, but still today, 12 months after operation, is alive and well. 2.1. Pathological Findings The hemicolectomy specimen consisted of a segment of the terminal segment of ileum 22 cm in length, the ileo- cecal valve, the cecum along with the appendix, and a segment of the ascending colon 16 cm in length. A large exophytic and invasive tumor, 4 cm in diameter was found in the ascending colon, close to the ileocecal valve. 20 lymph nodes, 0.3-1.2 cm in diameter were removed from the surrounding pericolic fat. On microscopy this tumor proved to be a well-differentiated adenocarcinoma of the large intestine, invading the whole thickness of the intestinal wall, and extending to the pericolic fat. One of the removed lymph nodes presented metastasis from the aforementioned adenocarinoma. The tumor of the jeju- num was reddish, and soft, 2 cm in diameter. It was composed by spindle cells arranged in fascicles, vacuo- lated (fat) cells and many blood vessels (Figure 2(a)). No significant nuclear atypia, mitotic activity, necroses or increased cellularity were detected. In order to sustain a definitive diagnosis, immunohistochemical evaluation followed. Immunohistochemistry was performed on formalin- fixed, paraffin-embedded tissue sections, 3-μm in thick- ness. High temperature antigen unmasking in electric pressure cooker, and TrilogyTM solution (Cell Marque, Rocklin, CA, USA) pretreatment preceded the main pro- cedure. A standardised automated (Nexes, Ventana, Tuscon, AZ, USA), streptavidin-biotin method (I-VIEW Paraffin DAB, Ventana) followed the application of the monoclonal antibodies. The latter were directed against HMB-45 (dilution 1:50, Dako, Glostrup, Denmark), Me- lan-A (dilution 1:50, Dako), a-smooth muscle actin (SMA, dilution 1:40, Novocastra, Newcastle, UK), Des- min (dilution 1:100, Novocastra), S-100 (dilution 1:300, Dako), CD117 (dilution 1:50, Dako), and CD34 (dilution 1:50, Dako). The tumor cells were positive to HMB-45 (Figure 2(b)), Melan-A, SMA (Figure 2(c)), and desmin, while they were negative to CD117, and CD34 (except of the endothelial cells of the vessels, Figure 2(d)). Our findings in total were consistent with a diagnosis of an- giomyolipoma. 3. Discussion Traditionally, AML was considered as hamartoma. However, AML today is regarded as a true neoplasm, since it has been found to present clonal nature [24]. Moreover, AML is thought to arise from the perivascular epithelioid cell (PEC), which has certain morphologic and immunohistochemical characteristics, even though no known normal cell counterpart has been found until now [1]. Immunohistochemically, PEC expresses myo- genic and melanocytic markers, such as HMB-45, HMSA-1, MelanA/Mart1, microophtalmia transcription factor (Mitf), actin and, less commonly, desmin. Other (rare) tumors which arise from PEC include clear-cell “sugar” tumor of the lung and extrapulmonary sites, lymphangioleiomyomatosis, clear-cell myomelanocytic tumor of the falciform ligament/ligamentum teres, and rare clear-cell tumors of other anatomical sites. In addi- tion, some PEComas are related to the tuberous sclerosis Copyright © 2011 SciRes. SS  S. MILIARAS ET AL. Copyright © 2011 SciRes. SS 54 (a) (b) (c) (d) Figure 2. (a) The tumor is composed of fascicles of spindle cells, interspersed with groups of vacuolated fat cells and numer- ous blood vessels (H&E, X100); (b) The spindle cells of the lesion are strongly positive to HMB-45 (DAB, Hematoxylin, X100); (c) The spindle cells of the lesion are strongly positive to smooth muscle actin (DAB, Hematoxylin, X100); (d) Only blood ves- sels show positive reaction to CD34 (DAB, Hematoxylin, X100). complex (TSC), an autosomal dominant genetic disease due to losses of TSC1 (9q34) or TSC2 (16p13.3) genes [25,26]. This syndrome is characterized by mental retar- dation, seizures and cellular proliferations (AMLs, sub- ependymal giant cell tumors, cutaneous angiofibromas, cardiac rabdomyomas, lymphangioleiomyomatosis, pulmonary multifocal micronodular hyperplasia). Similar alterations of the TSC genes have been demonstrated in a significant number of PEComas, both occurring within the TSC and in sporadic cases. Sporadic AMLs occur in older patients, in the fourth to sixth decades of life, with a female predominance; they are single, unilateral and larger than those associ- ated with TSC [27]. Classic AML contains more than one cell type; if a particular cell type predominates, AML is consequently named (lipoma-like AML or leio- myoma-like AML). AMLs arising in the GI tract are extremely uncommon and usually present with melena, anemia, diarrhea, abdominal pain, and may even be clini- cally asymptomatic [11-23]. Radiological diagnosis of extrarenal AMLs is difficult because of the rarity of the condition. Even though four other angiomyolipoma cases have been reported to affect small intestine, our case is unique, since to our knowledge is the first to involve jejunum. In addition, in our case the intestinal nodule was thought to represent metastatic disease in the pre-operative evaluation, because our patient was having a colonic tumor as well. So, it represented a critical dif- ferential diagnostic problem in terms of severity, and staging of his primary disease. Moreover, we consider ours to be a genuine AML case, since it is both HMB-45, and Melan-A positive. At least two cases of small intes- tinal AML reported in the literature [8,9] were HMB-45 negative (Table 1), when HM-45 positive immureaction B  S. MILIARAS ET AL. 55 Table 1. Angiomyolipomas of the small intestine reported in the literature: Immunohistochemical reaction to various anti- bodies. Author SMA Desm KIT CD34 Melan-A HMB-45 S-100 Vim De Padua et al. + NA NA NA NA + NA NA Toye and Czarnecki + NA NA NA NA - - NA Lee et al. + + - + NA - NA NA Lin et al. + + + + + + NA + Miliaras and Miliaras + - - - + + - NA SMA = smooth muscle actin, Desm = desmin, Vim = vimentin, NA = not available. is currently considered as a prerequisite for such a diag- nosis [1,14]. In conclusion, intestinal AMLs are very rare, and may cause various symptoms or mimick other condi- tions as metastatic disease in our case. For this reason it is quite important to differentiate such a case from other mesenchymal small intestinal tumors, and especially from gastrointestinal stromal tumor, which is the most frequent tumor in this location and merits specialized targeted immunotherapy. 4. References [1] G. Martignoni, M. Pea, D. Reghellin, G. Zamboni and F. Bonetti, “Pecomas: The Past, the Present and the Future,” Virchows Archives, Vol. 452, No. 2, February 2008, pp. 119-132. doi:10.1007/s00428-007-0509-1 [2] P. J. Xu, Y. Shan, F. H. Yan, Y. Ji, Y. Ding and M. L. Zhou, “Epithelioid Angiomyolipoma of the Liver: Cross-Sectional Imaging Findings of 10 Immunohisto- chemically-Verified Cases,” World Journal of Gastroen- terology, Vol. 15, No. 36, September 2009, pp. 4576-4581. doi:10.3748/wjg.15.4576 [3] A. P. Cil, A. Haberal, S. Hucumenoglu, E. E. Kovalak and M. Gunes, “Angiomyolipoma of the Uterus Associ- ated with Tuberous Sclerosis: Case Report and Review of the Literature,” Gynecologic Oncology, Vol. 94, No. 2, August 2004, pp. 593-596. doi:10.1016/j.ygyno.2004.05.015 [4] D. G. Jr. Guinee, D. S. Thornberry, N. Azumi, R. M. Przygodzki, M. N. Koss and W. D. Travis, “Unique Pul- monary Presentation of an Angiomyolipoma. Analysis of Clinical, Radiographic, and Histopathologic Features,” American Journal of Surgical Pathology, Vol. 19, No. 4, April 1995, pp. 476-480. [5] K. Singh, R. R. Pai, H. Kini and U. A. Kini, “Cutaneous Angiomyolipoma,” Indian Journal of Pathology Micro- biology, Vol. 52, No. 2, April-June 2009, pp. 242-243. doi:10.4103/0377-4929.48932 [6] A. Tamura, O. Ishikawa and Y. Miyachi, “Subgaleal Angiomyolipoma”, Journal of Dermatology, Vol. 21, No. 7, July 1994, pp. 514-517. [7] C. S. Knight, R. J. Cerfolio and T. S. Winokur, “Angio- myolipoma of the Anterior Mediastinum”, Annals of Di- agnostic Pathology, Vol. 12, No. 4, August 2008, pp. 293-295. doi:10.1016/j.anndiagpath.2006.12.007 [8] Y. Huan, R. W. Dillon and P. D. Unger, “Angiomyoli- poma of the Bladder”, Annals of Diagnostic Pathology, Vol. 6, No. 6, December 2002, pp. 378-380. doi:10.1053/adpa.2002.36655 [9] A. A. da Silva, R. Carlos, E. Contreras, O. P. de Almeida, M. A. Lopes and P. A. Vargas, “Angiomyolipoma of the Upper Lip: Case Report and Review of the Literature,” Medicina Oral, Patología Oral y Cirugía Bucal, Vol. 12, No. 2, March 2007, pp. E101-104. [10] K. Watanabe and T. Suzuki T, “Mucocutaneous Angio- myolipoma: A Report of 2 Cases Arising in the Nasal Cavity”, Archives of Pathology and Laboratory Medicine, Vol. 123, No. 9, September 1999, pp. 789-792. [11] K. Oishi, S. Fukuda, H. Sakimoto, T. Eto, M. Takahashi and T. Nishida, “Angiomyolipoma of the Colon: Report of a Case”, Surgery Today, Vol. 39, No. 11, November 2009, pp. 998-1001. doi:10.1007/s00595-009-3973-1 [12] H. Cheng, M. D. Sitrin and A. Rani A, “Angiomyolipoma in the Colon”, Gastrointestinal Endoscopy, Vol. 64, No. 3, September 2006, pp. 443-444. doi:10.1016/j.gie.2006.01.064 [13] A.I. Sharara and A. Tawil A, “Angiomyolipoma of the colon”, Clinical Gastroenterology and Hepatology, vol. 3, no.9, September 2005, p. A35. doi:10.1016/S1542-3565(05)00287-9 [14] J. Pelz, K. Weber, J. Göhl, A. Dimmler and W. Hohen- berger, “Angiomyolipoma of the Colon - Case Report and Review of the Literature”, Zeitschrift für Gastroenterolo- gie, Vol. 41, No. 8, August 2003, pp. 715-718. [15] J. S. Chen, L. J. Kuo, P. Y. Lin and C. R. Changchien, “Angiomyolipoma of the Colon: Report of a Case and Review of the Literature”, Diseases of Colon and Rectum, Vol. 46, No. 4, April 2003, pp. 547-549. doi:10.1007/s10350-004-6598-x [16] H. Maluf and Dieckgraefe B, “Angiomyolipoma of the Large Intestine: Report of a Case”, Modern Pathology, Vol. 12, No.12, December 1999, pp. 1132-1136. [17] C. Maesawa, G. Tamura, H. Sawada, S. Kamioki, Y. Nakajima and R. Satodate, “Angiomyolipoma Arising in the Colon”, American Journal of Gastroenterology, Vol. Copyright © 2011 SciRes. SS  56 S. MILIARAS ET AL. 91, No. 9, September 1996, pp. 1852-1854. [18] R. Verzaro, S. Guadagni, A. Agnifili, I. Ibi, P. Gola, F. Gianfelice, D. Ranalletta, G. Carducci and P. Leocata, “A Case of Extrarenal Angiomyolipoma with Intestinal Lo- calization”, Minerva Chirurgica, Vol. 47, No. 15-16, August 1992, pp. 1317-1319. [19] Y. Hikasa, T. Narabayashi, M. Yamamura, Y. Fukuda, N. Tanida, K. Tamura, T. Ohno, T. Shimoyama and Nishi- gami T, “Angiomyolipoma of the Colon: A New Entity in Colonic Polypoid Lesions”, Gastroenterologia Japonica, Vol. 24, No. 4, August 1989, pp. 407-409. [20] M. De Padua, N. Gupta, S.L. Broor and D. Govil, “Duo- denal Angiomyolipoma: A Case Report,” Indian Journal of Pathology and Microbiology, Vol. 50, No. 3, July 2007, pp. 568-569. [21] L. R. Toye and L. A. Czarnecki, “CT of Duodenal An- giomyolipoma,” American Journal of Roentgenology, Vol. 178, No. 1, January 2002, p. 92. [22] C. H. Lee, J. H. Kim, D. H. Yang, Y. Hwang, M. J. Kang, Y. K. Kim and M. R. Lee, “Ileal Angiomyolipoma Mani- fested by Small Intestinal Intussusception,” World Jour- nal of Gastroenterology, Vol. 15, No. 11, March 2009, pp. 1398-1400. doi:10.3748/wjg.15.1398 [23] C. Y. Lin, H. Y. Chen, S. C. Jwo and S. C. Chan, “Ileal Angiomyolipoma as an Unusual Cause of Small-Intestinal Intussusception,” Journal of Gastroenterology, Vol. 40, No. 2, February 2005, pp. 200-203. doi:10.1007/s00535-004-1527-2 [24] L. Cheng, J. Gu, J. N. Eble, D. G. Bostwick, C. Younger, G. T. MacLennan, F. W. Abdul-Karim, W. A. Geary, M. O. Koch, S. Zhang and T. M. Ulbright, “Molecular Ge- netic Evidence for Different Clonal Origin of Compo- nents of Human Renal Angiomyolipomas,” American Journal of Surgical Pathology, Vol. 25, No. 10, October 2001, pp. 1231-1236. doi:10.1097/00000478-200110000-00002 [25] M. van Slegtenhorst, R. de Hoogt, C. Hermans, M. Nel- list, B. Janssen, S. Verhoef, D. Lindhout, A. van den Ouweland, D. Halley, J. Young, M. Burley, S. Jeremiah, K. Woodward, J. Nahmias, M. Fox, R. Ekong, J. Osborne, J. Wolfe, S. Povey, R. G. Snell, J. P. Cheadle, A. C. Jones, M. Tachataki, D. Ravine, J. R. Sampson, M. P. Reeve, P. Richardson, F. Wilmer, C. Munro, T. L. Hawkins, T. Sepp, J. B. Ali, S. Ward, A. J. Green, J. R. Yates, J. Kwiatkowska, E. P. Henske, M. P. Short, J. H. Haines, S. Jozwiak and D. J. Kwiatkowski, “Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34,” Science, Vol. 277, No. 5327, August 1997, pp. 805-808. doi:10.1126/science.277.5327.805 [26] European Chromosome 16 Tuberous Sclerosis Consor- tium, “Identification and Characterization of the Tuber- ous Sclerosis Gene on Chromosome 16,” Cell, Vol. 75, No. 7, December 1993, pp. 1305-1315. doi:10.1016/0092-8674(93)90618-Z [27] G. Martignoni and M. Amin, “Angiomyolipoma,” In: J. N. Eble, G. Sauter, J. Epstein and I. Sesterhenn (eds), Pa- thology and Genetics of Tumours of the Urinary System and Male Genital Organs Series: WHO Classification of Tumours, IARC Press, Lyon, 2004, pp. 65-67. Copyright © 2011 SciRes. SS
|