Journal of Diabetes Mellitus
Vol.1 No.2(2011), Article ID:5148,9 pages DOI:10.4236/jdm.2011.12004
Relationship between plasma adipokines, inflammation, insulin resistance and subclinical atherosclerosis in newly diagnosed type 2 diabetes
![]()
1Clinical Hospital Colentina – Department of Diabetes, Nutrition and Metabolic Diseases, Bucharest; razvanv@mailbox.ro
2National Institute of Diabetes, Nutrition and Metabolic Diseases “N.Paulescu”, Bucharest
Received 24 February 2011; revised 30 April 2011; accepted 7 May 2011
Keywords: Newly Diagnosed; Type 2 Diabetes; Atheroscleorsis; Common Carotid Artery; Intima-Media Thickness
ABSTRACT
Background: The aim of this study was to investigate the occurrence of subclinical atherosclerosis, evaluated by carotid artery intimamedia thickness (CIMT) in subjects with newly diagnosed type 2 diabetes. Methods: A total of 167 subjects, 50 newly diagnosed type 2 diabetic subjects and 117 non-diabetic subjects were included in the study. Obese and overweight newly diagnosed type 2 diabetic patients were matched for age and BMI with obese and overweight non-diabetic subjects. Only postmenopausal women were selected. The following biomarkers were analyzed: fasting glucose, HbA1c, fasting insulin, fasting proinsulin, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, adiponectin, leptin, hs-CRP, urine albumin/creatinine ratio. 75 g oral glucose tolerance test was performed in all subjects. Ultrasound imaging was used to evaluate IMT of the common carotid artery. Results: CIMT was greater in newly diagnosed type 2 patients compared to non-diabetic subjects. When analyzed by BMI, the difference regarding CIMT between diabetic and non-diabetic subjects was significant only in overweight subjects, in both sexes. In univariate analysis in men with newly diagnosed type 2 diabetes, CIMT was positively correlated with age, SBP, triglycerides, leptin and negatively correlated with HDL-cholesterol and in women CIMT was positively correlated with SBP and leptin. Independent determinants of CIMT in patients with newly diagnosed type 2 diabetes were in men age (β = 0.556, p = 0.0028) and log leptin (β = 0.393, p = 0.049) and in women systolic blood pressure (β = 0.48, p = 0.026). Conclusions: Subclinical atherosclerosis is present in newly diagnosed type 2 diabetic subjects. Body fat accumulation in men and hypertension in postmenopausal women have a primary role in increase carotid intima-media thickness.
1. INTRODUCTION
Diabetes is one of the main risk factors for cardiovascular disease [1]. An increase in the carotid intima-media thickness (CIMT), examined by B-mode ultrasonography, is generally considered an early marker of atherosclerosis [2-6]. Previous studies have shown an association of increased CIMT with cardiovascular risk factors [7,8] and future cerebrovascular and cardiovascular events [9,10]. It has also been demonstrated that CIMT is increased in type 2 diabetic patients [11-14]. In subjects with established diabetes, traditional risk factors such as hypertension, dyslipidemia (high LDL-cholesterol, triglycerides and apoB levels and low HDL-cholesterol levels), obesity as well as lower plasma adiponectin, inflammation markers and insulin resistance are associated with CIMT [15-21]. We noticed large differences between the studies which evaluated the correlation of cardiovascular risk factors with measures of subclinical atherosclerosis, both in diabetic and non-diabetic subjects, mainly because of the wide variations of the populations included. Few studies showed that early atherosclerosis, evaluated by CIMT or arterial stiffness, is present in newly diagnosed type 2 diabetic subjects [22-25]. In the present study we evaluated CIMT in newly diagnosed type 2 diabetic patients, in comparison with nondiabetic subjects, matched for age and sex. In addition we analyzed the influence of traditional cardiovascular risk factors, adipokines (adiponectin and leptin), inflammation markers (fibrinogen and hs-CRP), insulin resistance and albuminuria on CIMT in patients with newly diagnosed type 2 diabetes without cardiovascular disease.
2. METHODS
2.1. Subjects
A total of 167 subjects were included in this study: 50 newly diagnosed type 2 diabetes (25 men and 25 women) and 117 control, non-diabetc (64 men and 53 women). Subjects were recruited at the Department of Diabetes, Nutrition and Metabolic Diseases, Clinical Hospital Colentina, from February 2009 to January 2010. All participants gave written informed consent. Subjects with established diabetes, currently smoking, history of cardiovascular disease, use of drugs for dyslipidemia and hypertension, fasting glucose ≥ 250mg/dl, systolic blood pressure ≥ 170 mmHg, total cholesterol ≥ 300 mg/dl, triglycerides ≥ 300 mg/dl, presence of viral hepatitis, renal disease or thyroid dysfunction were excluded. Women included in the study were post menopause. Subjects were classified by BMI into: lean (18.5 < BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30kg/m2) and obese (BMI ≥ 30 kg/m2). According to BMI newly diagnosed type 2 diabetic subjects were classified into two groups: overweight, 20 subjects (10 men and 10 women) and obese, 30 subjects (15 men and 15 women) and non-diabetic subjects into three groups: lean, 18 subjects (9 men and 9 women), overweight, 43 subjects (24 men and 19 women) and obese, 56 subjects (31 men and 25 women).
2.2. Carotid Artery Ultrasound
CIMT was determined using a B-mode ultrasound scanner (Siemens Sonoline Sienna) and a 7.5 MHz linear probe with subjects in the supine position, by a trained specialist with no knowledge of the subjects clinical characteristics. Longitudinal scan of both the right and left common carotid artery was recorded. Measurement of IMT was made on the near (anterior) and far (posterior) wall of the common carotid artery at 1 cm proximal to the bifurcation, in segments that were free of plaque, plaque being defined as the presence of wall thickening at least 50% greater than the adjacent thickness. For each individual, the CIMT was determined as the average of near and far-wall measurements of both the left and right arteries.
2.3. Laboratory Assay
The samples of blood were taken after 12 hours of overnight fasting. Total adiponectin, leptin, hs-CRP, insulin and proinsulin were measured by ELISA (DRG International, Inc.) on a Dynex analyzer. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and glucose were measured using standard techniques. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of the fasting plasma insulin value (in microunits per liter) and the fasting plasma glucose value (in mg per deciliter) divided by 405. Urine albumin/cretinine ratio (UACR) was calculated from a single spot urine specimen collected in the morning. Microalbuminuria was defined as UACR 30 - 300 mg/g. Percent body fat (%) was measured using a bioelectrical impedance analyzer (Omron BF 500). All participants underwent standard oral glucose tolerance test (OGTT) with 75 g glucose. To diagnose type 2 diabetes we used the following criteria: fasting plasma glucose (FPG) ≥ 126 mg/dl and/or a 2-h plasma glucose in OGTT ≥ 200 mg/dl.
2.4. Statistical Analysis
Statistical analyse was performing using GraphPad Instat version 3.0 and NCSS/2004. Continuous variables were tested for normality of distribution with the use of the Wilk-Shapiro test. Data normally distributed were expressed as mean ± standard deviation (SD) and data skewed distributed were expressed as median (interquartile range). Normally distributed variables were compared by unpaired t-test and not normally distributed variables were compared by Mann-Whitney test. Variables not normally distributed (insulin, proinsulin, proinsulin to insulin ratio, HOMA-IR, adiponectin, leptin, leptin to adiponectin ratio, hs-CRP, UACR) were log transformed before evaluating the correlations. Associations between CIMT and the variables evaluated, in subjects with newly diagnosed type 2 diabetes, were examined using linear regression analysis. P-value < 0.05 was considered statistically significant for all analyses.
3. RESULTS
3.1. Characteristics of Study Subjects
The clinical and biochemical characteristics of the subjects included in this study are presented in Table 1. Obese and overweight newly diagnosed type 2 diabetic patients were matched for age, sex and BMI with obese and overweight non-diabetic subjects. Systolic blood pressure (SBP), triglycerides, fibrinogen, hs-CRP, insu
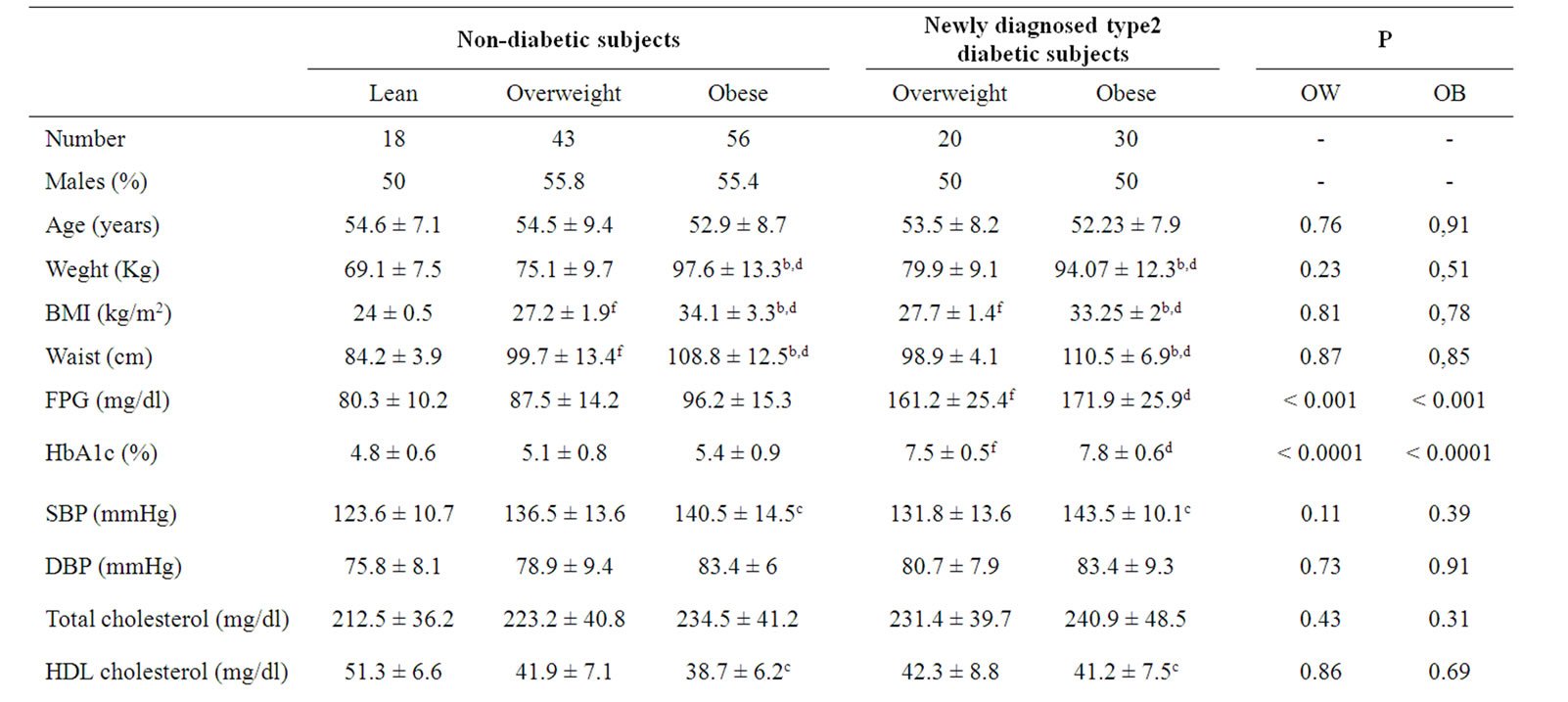

Table 1. Clinical and biochemical characteristics of diabetic patients and control subjects.
lin, proinsulin, leptin and UACR were significantly higher and adiponectin and HDL-cholesterol were significantly lower in obese subjects, both newly diagnosed diabetic and non diabetic, compared to non diabetic, lean subjects. There were no significant differences between obese non diabetic and obese newly diagnosed type 2 diabetic subjects for waist, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, fibrinogen, hs-CRP, adiponectin, leptin, leptin/adiponectin ratio, proinsulin/ insulin ratio and UACR. Similarly, there were no significant differences between overweight non-diabetic and overweight newly diagnosed type 2 diabetic subjects for waist, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, fibrinogen, hs-CRP, adiponectin, leptin, leptin/adiponectin ratio, proinsulin/insulin ratio and UACR. Fasting glucose and HBA1c were higher in newly diagnosed type 2 diabetic, both in obese and overweight, compared to non-diabetic subjects. Fasting insulin, proinsulin and HOMA-R were significantly higher in obese type 2 newly diagnosed diabetic vs.
obese non-diabetic. In non-diabetic subjects BMI, waist and triglycerides were higher in obese vs. overweight. In newly diagnosed type 2 diabetic patients BMI, waist, insulin, HOMA-R and proinsulin were higher in obese vs. overweight. None of the subjects had macroalbuminuria, 7 subjects (5 subjects from the non-diabetic group and 2 subjects from the diabetic group) had microalbuminuria and the rest of the participants had low grade albuminuria.
CIMT was greater in obese and overweight, both in newly diagnosed type 2 diabetic (obese vs. lean, pmen < 0.0001, pwomen < 0.0001; overweight vs. lean, pmen < 0.0001, pwomen < 0.0001) and non-diabetic subjects (obese vs. lean, pmen < 0.0001, pwomen < 0.0001; overweight vs. lean, pmen = 0.0041, pwomen < 0.0001), compared to non-diabetic lean subjects. CIMT was greater in obese subjects, both in newly diagnosed type 2 diabetic and non diabetic subjects, compared to overweight subjects, but the difference was statistically significant only for non-diabetic subjects (non-diabetic, obese vs. overweight, pmen = 0.0005, pwomen = 0.003). There was no significant difference between CIMT in obese, newly diagnosed diabetic patients and CIMT in obese, nondiabetic subjects. CIMT was greater in overweight newly diagnosed diabetic patients compared to overweight, non-diabetic subjects (pmen = 0.008, pwomen = 0.027) (Table 2).
3.2. Univariate Analysis between CIMT and Various Variables in Newly Diagnosed Type 2 Diabetic Subjects
In univariate analysis in men with type 2 diabetes newly diagnosed CIMT was positively correlated with age (CIMT = 0.0122 × age + 0.3033, r2 = 0.35, p = 0.002), systolic blood pressure (CIMT = 0.0044 × SBP + 0.3233, r2 = 0.14, p = 0.046), triglycerides (CIMT = 0.0011 × triglycerides + 0.7018, r2 = 0.2, p = 0.026), leptin (CIMT = 0.2038 × log (leptin) + 0.6764, r2 = 0.19, p = 0.025) and negatively correlated with HDL-cholesterol (CIMT = –0.0082 × HDL-cholesterol + 1.2381, r2 = 0.15, p = 0.044) (Table 3). In univariate analysis in women with type 2 diabetes newly diagnosed CIMT was positively correlated with systolic blood pressure (CIMT = 0.005 × SBP + 0.211, r2 = 0.2, p = 0.021) and leptin (CIMT = 0.1666 × log (leptin) + 0.6053, p = 0.037) (Table 3).
3.3. Multiple Regression Analysis in Subjects with Newly Diagnosed Type 2 Diabetic Subjects
In men, in a model with CIMT as a dependent variable and age, systolic blood pressure, triglycerides, log leptin and HDL-cholesterol as covariates, age (β = 0.556, p = 0.0028) and log leptin (β = 0.393, p = 0.0488) were independent determinant of CIMT (Tabel 4). In a model with age (β = 0.619, p = 0.0002) and log leptin (β = 0.482, p = 0.0021) the coefficient of determination (R2) was 0.578 and the adjusted value was 0.539.
In women, in a model with CIMT as a dependent variable and age, systolic blood pressure, leptin, triglycerides and HDL-cholesterol as covariates, systolic blood pressure (β = 0.48, p = 0.026) was the only independent predictor of CIMT (Table 5).
4. DISCUSSION
In this study we show that patients with newly diagnosed type 2 diabetes have an increase CIMT compared to non-diabetic, lean subjects matched for age. In obese subjects we found no difference in CIMT between newly diagnosed type 2 diabetes and non-diabetic, in both sexes and in overweight subjects CIMT was significantly greater in newly diagnosed type 2 diabetes compared to non-diabetic, in both sexes.
Temelkova et al. [22] showed in 71 newly diagnosed type 2 diabetes, aged 40 - 70 years, 67.61% male with mean HbA1c of 6.33 ± 0.11% and mean BMI of 28.8 ± 0.4 kg/m2 that newly detected type 2 diabetic patients, compared to normal glucose tolerance subjects matched for age and sex, with mean BMI of 27.4 ± 0.4 kg/m2, exhibit a higher degree of early atherosclerosis, evaluated by measurement of the common carotid artery IMT (men, diabetic vs control, 1 ± 0.03 vs.0.88 ± 0.03 mm;
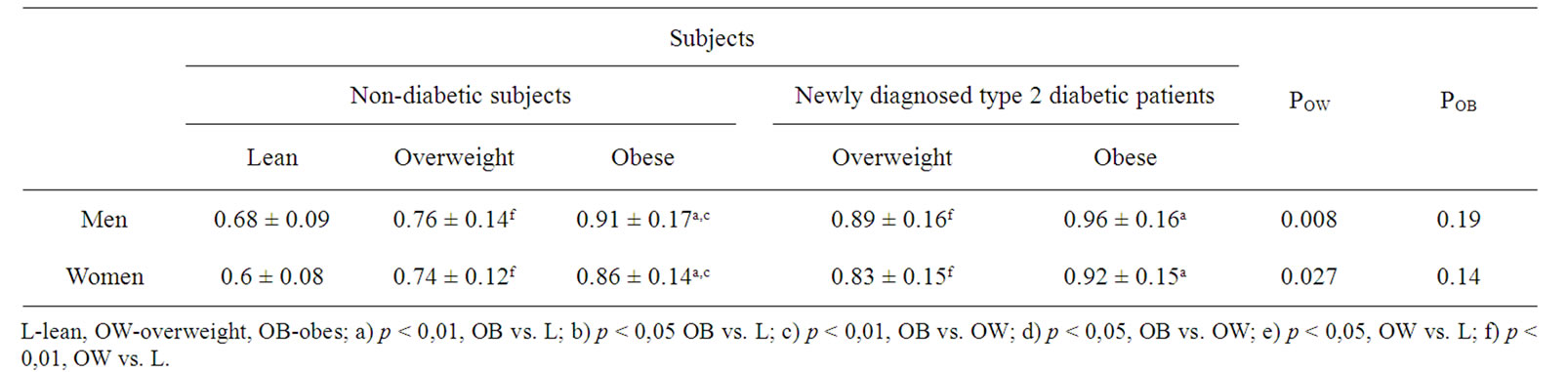
Table 2. Carotid intima-media thickness (CIMT) in newly diagnosed type 2 diabetic patients and non-diabetic subjects.
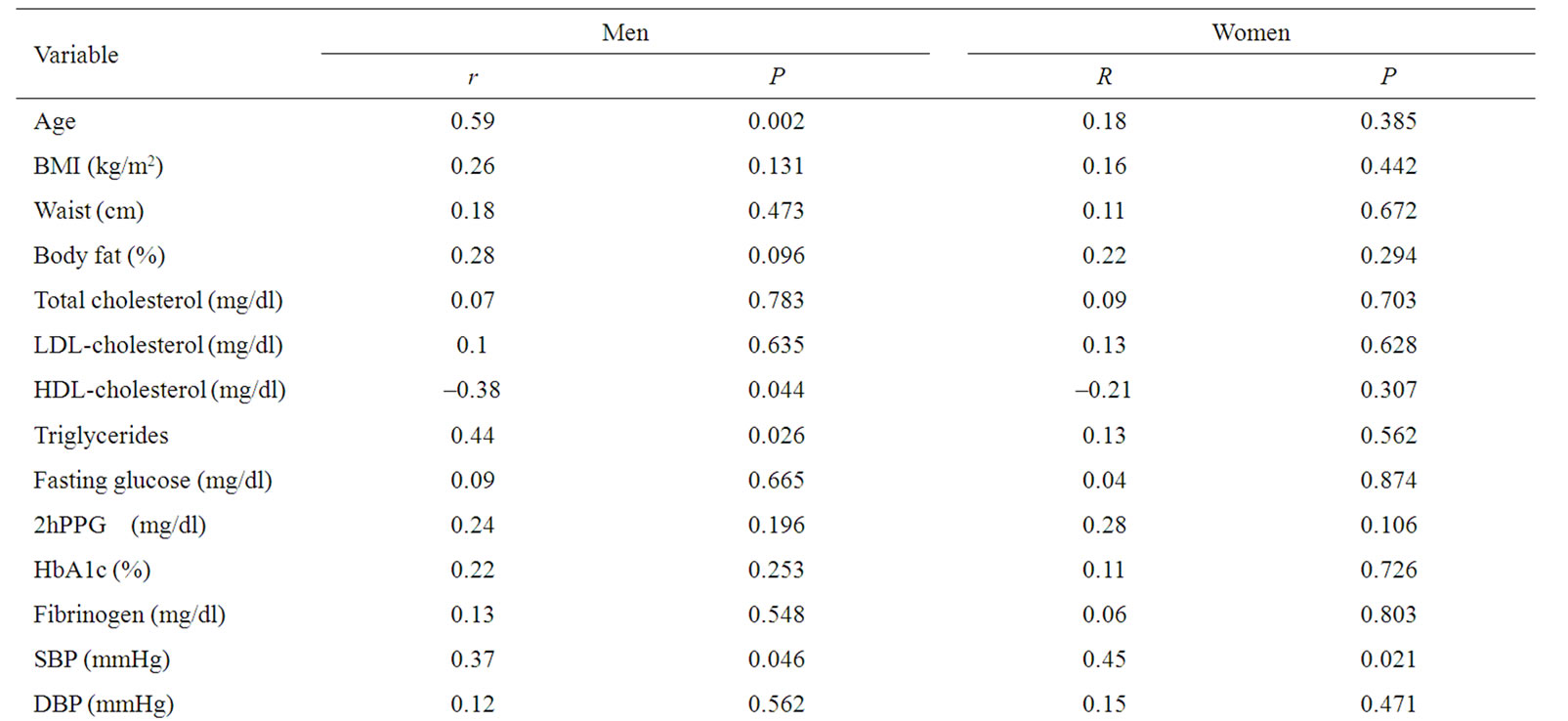
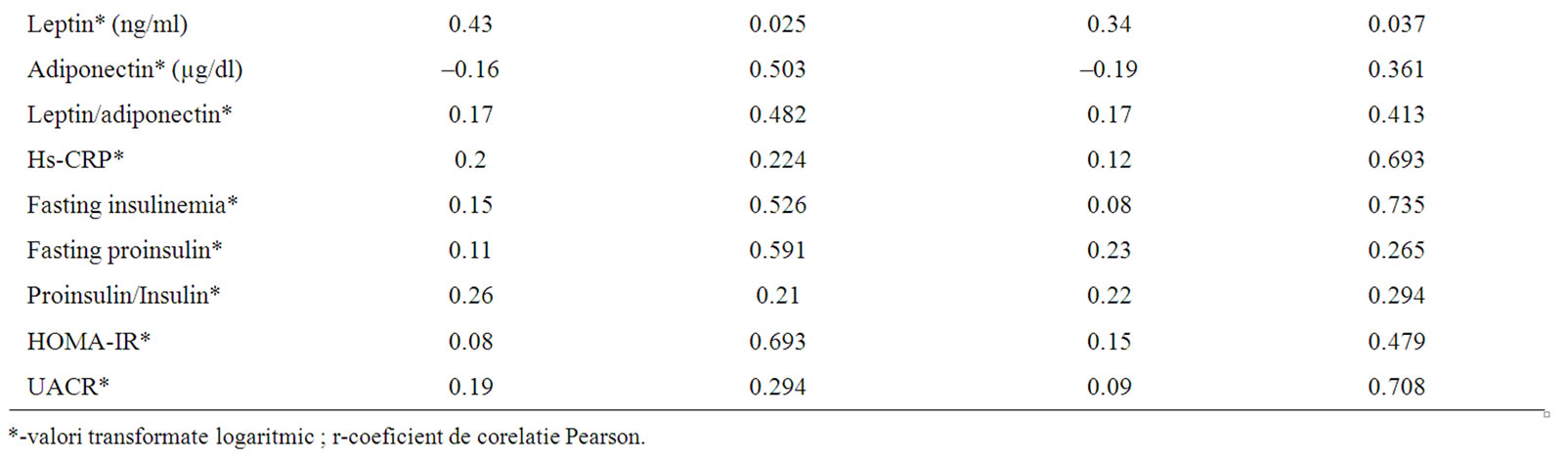
Table 3. Correlation between CIMT and all the other parameters evaluated.
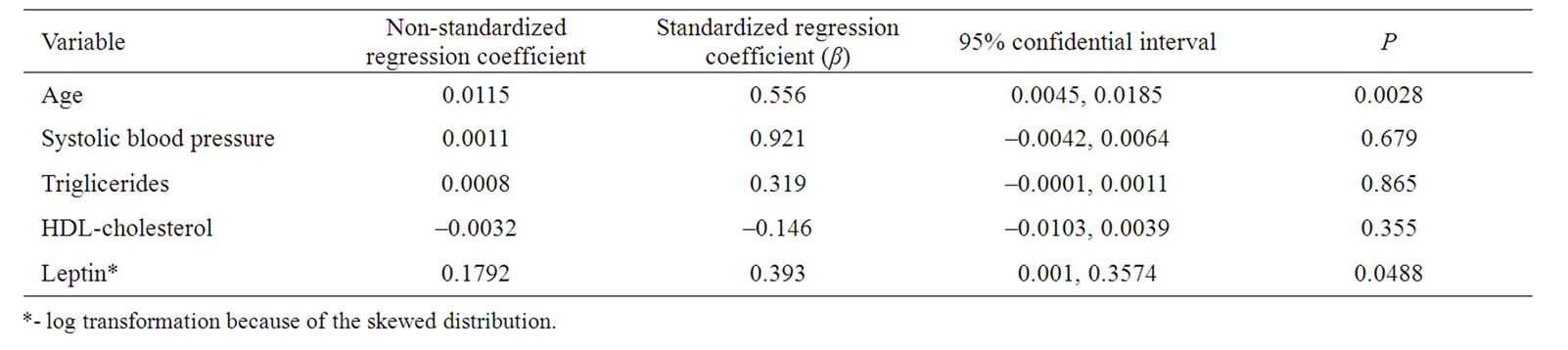
Tabel 4. Multiple regression analysis with CIMT as the dependent variable in men with newly diagnosed type 2 diabetes.
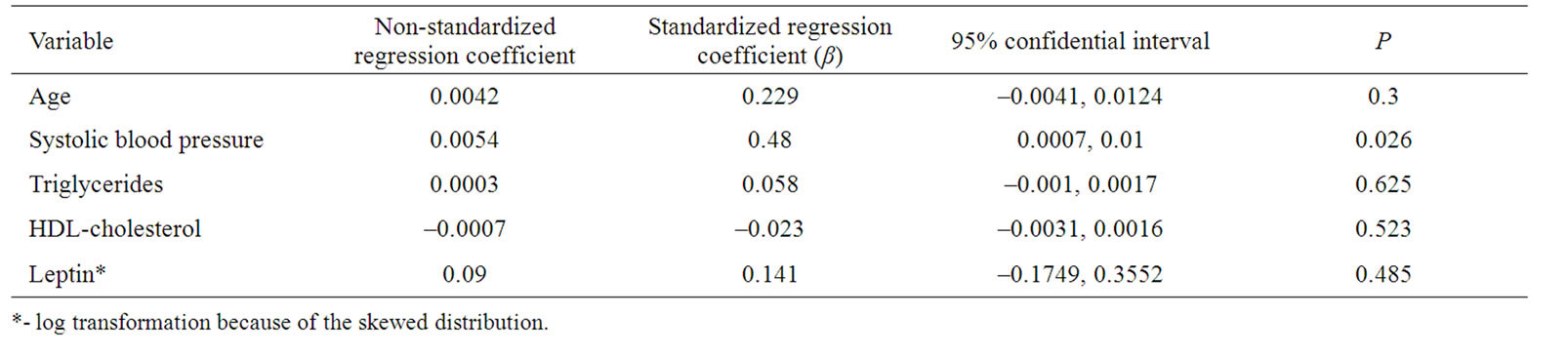
Tabel 5. Multiple regression analysis with CIMT as the dependent variable in women with newly diagnosed type 2 diabetes.
female, diabetic vs. control, 0.94 ± 0.04 vs. 0.79 ± 0.02 mm). Also, the authors showed that in diabetic patients triglycerides and the total-to-HDL cholesterol ratio were significantly correlated to IMT after adjustment for age and sex, but in multivariate analysis no independent determinants of carotid IMT were found. Sigurdardottir et al. [23] showed in 271 men, 61 year old (50 with established diabetes, mean HbA1c of 6.66 ± 1.53% and mean BMI of 29.6 ± 4.7 kg/m2; 24 newly diagnosed diabetes with mean HbA1c of 5.94 ± 1.54% and mean BMI of 29.8 ± 2.9 kg/m2 and 197 healthy control subjects with mean BMI of 25.5 ± 4.3 kg/m2) increased preclinical atherosclerotic changes (evaluated by carotid IMT and plaque size) and increased inflammation (hsCRP) both in men with newly diagnosed and established diabetes compared with healthy control subjects. In this study waist-to-hip ratio, diabetes onset status (newly diagnosed versus established) and triglycerides were independent correlates of carotid IMT in men with diabetes.
We found that in patients with newly diagnosed type 2 diabetes, without cardiovascular diseases and treatment for hypertension or dyslipidemia, there are sex differences in the risk of subclinical atherosclerosis. In men, in univariate analysis age, SBP, triglycerides and leptin correlated positively with CIMT and HDL-cholesterol correlated negatively with CIMT and in multivariate analysis, age and leptin were independent predictors of CIMT and in women, in univariate analysis SBP and leptin correlated positively with CIMT and in multivariate analysis, SBP was an independent predictor of CIMT. Leptin, a hormone produce by white adipose tissue, is primarily involved in the regulation of food intake and energy expenditure. Plasma leptin concentration is increased in obese subjects and reflects increased adiposity and leptin resistance [26-28]. In vitro and in vivo assays demonstrated that leptin has multiple atherogenic effects such as induction of endothelial dysfunction [29,30], stimulation of migration and proliferation of vascular smooth cells [31], production of reactive oxygen species (ROS) [32] stimulation of inflammatory reaction [33], platelet aggregation [34], sympathetic activation [35]. Inhibition of leptin signaling may be a promising strategy to slow the progression of atherosclerosis in hyperleptinemic obese subjects. According to the concept of selective leptin resistance, there is resistance to the effects of leptin on appetite and thermogenic metabolism, whereas its other actions are maintained [36]. There are studies which have shown that serum leptin is lower in subjects with established diabetes mellitus [37,38].
In recent years many studies evaluated the cardiovascular effects of leptin [39-41]. Wallace et al. [42] showed in a large prospective study (the West of Scotland Coronary Prevention Study – WOSCOPS) that leptin is an independent risk factor for coronary heart disease. Reilly et al. [43] found an association between plasma leptin levels and coronary artery calcification (CAC), a measure of coronary atherosclerosis, in type 2 diabetes mellitus patients (age, median – 61.3 year in men and 56.9 year in women, duration of diabetes, mean – 7 year in men and 7.5 year in women and BMI, mean – 31.5 kg/m2 in men and 31.2 kg/m2 in women), after controlling for traditional measures of obesity and plasma CRP. Qasim et al. [44] found in 860 (457 men and 403 women) healthy, nondiabetic, with family histories of premature cardiovascular disease, participants in the SIRCA (Study of Inherited Risk of Coronary Atherosclerosis) a strong association between leptin and CAC. Ciccone et al. [45] showed in 120 healthy subjects (52 men and 68 women), aged 28.1 ± 7 years in men and 28.8 ± 7.8 years in women and with a wide range of BMI (31.2 ± 9.4 kg/m2 in men and 29.4 ± 8.1 kg/m2 in women), that plasma leptin concentrations are independently associated with CIMT. Van den Beld et al. [46] found no association between plasma leptin levels and CIMT in 403 elderly men (aged 73 - 94 years). Bountoris et al. [47] found that plasma leptin levels were decreased in symptomatic patients undergoing carotid endarterectomy. Stork et al. [48] show in 142 non-diabetic postmenopausal women, aged 59.1 ± 4.3 years and with mean BMI 26.4 ± 4.6kg/m2, that low levels of adiponectin are associated with CIMT progression and increased risk of arterial stiffness progression. The authors found no association for leptin.
In the present study we show that SBP, triglycerides, fibrinogen, hs-CRP, insulin, proinsulin, leptin and UACR were significantly higher and adiponectin and HDL-cholesterol were significantly lower in obese subjects, both newly diagnosed diabetic and non-diabetic, compared to non-diabetic, lean subjects. We found no difference in the plasma concentration of total cholesterol, LDL-cholesterol, HDL-cholesterol, fibrinogen, hsCRP, leptin and adiponectin, systolic blood pressure and diastolic blood pressure between the diabetic and nondiabetic subjects, irrespective of BMI (obese or overweight). Plasma triglycerides were higher in both obese and overweight newly diagnosed type 2 diabetes, compared to obese and overweight non-diabetic subjects. Insulin, HOMA-R and proinsulin were higher in obese type 2 diabetic subjects compared to obese non-diabetic subjects. Insulin, proinsulin and HOMA-R were higher in obese type 2 diabetic subjects compared to overweight type 2 diabetic subjects. These data sustained our view[49] that the main pathogenic link of type 2 diabetes with vascular disease is close related with obesity. Hansen and colab.[50] showed in a total of 60 males (20 healthy, non-obese subjects, 20 non-obese and 20 obese (BMI >35 kg/m2) type 2 diabetes patients that elevated plasma leptin, hsCRP, IL-6 and free fatty acid (FFA) are associated with obesity and not necessarily with the type 2 diabetic state.
5. CONCLUSION
Subclinical atherosclerosis is present in patients with newly diagnosed type 2 diabetes. Body fat accumulation in men and hypertension in women have primary roles in development of subclinical atherosclerosis in newly diagnosed type 2 diabetic patients.
REFERENCES
- Kannel, W.B., McGee, D.L. (1979) Diabetes and cardiovascular disease: The framingham study. JAMA, 241, 2035-2038. doi:10.1001/jama.241.19.2035
- Pignoli, P., Tremoli, E., Poli, A., Oreste, P. and Paoletti R. (1986) Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation, 74, 1399-1406.
- Poli, A., Tremoli, E., Colombo, A., Sirtori, M., Pignoli, P. and Paoletti, R. (1988) Ultrasonographic measurement of the common carotid artery wall thickness in hypercholestero-lemic patients. A nnew model for the quantitation and follow-up of preclinical atheroslcerosis in living human subjects. Atherosclerosis, 70, 235-261. doi:10.1016/0021-9150(88)90176-1
- de Groot, E., Hovingh, K., Wiegman, A., Duriez, P., Smit, A.J., Fruchart, J.C., Kastelein, J.J.P. (2004) Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation, 109 (suppl III): 33-38. doi:10.1161/01.CIR.0000131516.65699.ba
- Cupini LM, Pasqualetti P, Diomedi M, Vernieri F, Silvestrini M, Rizzato B, Ferrante F, Bernardi G: Carotid artery intima-media thickness and lacunar versus nonlacunar infarcts. Stroke, 33, 689-694. doi:10.1161/hs0302.103661
- O’Leary, D.H., Bots, M.L. (2010) Imaging of atherosclerosis: Carotid intima-media thickness. European Heart Journal, 31, 1682-1689. doi:10.1093/eurheartj/ehq185
- O’Leary, D.H., Polak, J.F., Kronmal, R.A., Kittner, S.J., Bond, M.G., Wolfson, S.K., Bommer, W., Price, T.R., Gardin, J.M. and Savage, P.J., for the CHS Collaborative Group (1992) Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. Stroke, 23, 1752-1760.
- Salonen, R., Salonen, J.T. (1991) Determinants of carotid intimamedia thickness: A population based ultrasonography study in eastern Finnish men. Journal of Internal Medicine, 229, 225-231. doi:10.1111/j.1365-2796.1991.tb00336.x
- Bots, M.L., Hoes, A.W., Koudstaal, P.J., Hofman, A., Grobbee, D.E. (1997) Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation, 96, 1432-1437.
- Chambless, L.E., Heiss, G., Folsom, A.R., Rosamond, W., Szklo, M., Sharrett, A.R., Clegg, L.X. (1997) Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. American Journal of Epidemiol, 146, 483-494.
- Kawamori, R., Yamasaki, Y., Matsushima, H., Nishizawa, H., Nao, K., Hougaku, H., Maeda, H., Handa, N., Matsumoto, M., Kamada, K. (1992) Prevalence of carotid atherosclerosis in diabetic patients. Diabetes Care, 15, 1290-1294. doi:10.2337/diacare.15.10.1290
- Wagenknecht, L.E., D’Agostino, R. Jr., Savage, P.J., O’Leary, D.H., Saad, M.F. and Haffner, S.M. (1997) Duration of diabetes and carotid wall thickness: the Insulin Resistance Athero-sclerosis Study (IRAS). Stroke, 28, 999-1005.
- Pujia, A., Gnasso, A., Irace, C., Colona, A., Mattioli, P.L. (1994) Common carotid arterial wall thickness in NIDDM subjects. Diabetes Care, 17, 1330-1336. doi:10.2337/diacare.17.11.1330
- Ifrim, S. and Vasilescu, R. (2004) Early detection of atherosclerosis in type 2 diabetic patients by endothelial dysfunction and intima-media thickness. Romanian Journal of Internal Medicine, 42, 343-354.
- Dullaart, R.P.F., de Vries, R., van Tol, A., Sluiter, W.J. (2007) Lower plasma adiponectin is a marker of increased intimamedia thickness associated with type 2 diabetes mellitus and with male gender. European Journal of Endocrinol, 156, 387-394. doi:10.1530/EJE-06-0681
- Niskanen, L., Rauramaa, R., Miettinen, H., Haffner, S.M., Mercuri, M. and Uusitupa, M. (1996) Carotid artery intima-media thickness in elderly people with NIDDM and in non-diabetic subjects. Stroke, 27, 1986-1992.
- Bonora, E., Tessari, R., Micciolo, R., Zenere, M., Targher, G., Padovani, R., Falezza, G., Muggeo, M. (1997) Intimal-medial thickness of the carotid artery in nondiabetic and NIDDM patients. Relationship with insulin resistance. Diabetes Care, 20, 627-631. doi:10.2337/diacare.20.4.627
- Goff, D.C., D’Agostino, R.B., Haffner, S.M., Saad, N.F., Wagenknecht, L.E. (2000) Lipoprotein concentrations and carotid atherosclerosis by diabetes status: results from the Insulin Resistance Atherosclerosis Study. Diabetes Care, 23, 1006-1011. doi:10.2337/diacare.23.7.1006
- Hegazi, R.A.F., Sutton-Tyrrell, K., Evans, R.W., Kuller, L.H., Belle, S., Yamamoto, M., Edmundowicz, D., Kelley, D.E.: Relationship of adiposity to subclinical atherosclerosis in obese patients with type 2 diabetes. Obesity Research; 11, 1597-1605. doi:10.1038/oby.2003.212
- Kang, E.S., Kim, H.J., Kim, Y.M., Lee, S., Cha, B.S., Lim, S.K., Kim, H.J. and Lee, H.C. (2004) Serum high sensitivity C-reactive protein is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Res Clin Pract, 66 (Suppl 1), S115-120. doi:10.1016/j.diabres.2004.05.009
- Tajiri, Y., Mimura, K. and Umeda, F. (2005) Highsensitivity C-reactive protein in Japanese patients with type 2 diabetes. Obesity Research, 13, 1810-1816. doi:10.1038/oby.2005.220
- Temelkova-Kurktschiev, T.S., Koehler, C., Leonhardt, W., Schaper, F., Henkel, E., Siegert, G., Hanefeld, M. (1999) Increased intimal-medial thickness in newly detected type 2 diabetes. Diabetes Care, 22, 333-338. doi:10.2337/diacare.22.2.333
- Sigurdardottir, V., Fagerberg, B. and Hulthe, J. (2004) Preclinical atherosclerosis and inflammation in 61-year-old men with newly diagnosed diabetes and established diabetes. Diabetes Care, 27, 880-884. doi:10.2337/diacare.27.4.880
- Gong, W., Lu, B., Yang, Z., Ye, W., Du, Y., Wang, M., Li, Q., Zhang, W., Pan, Y., Feng, X., Zhou, W., Zhang, Y., Yang, Z., Yang, Y., Zhu, X. and Hu, R. (2009) Early-stage atherosclerosis in newly diagnosed, untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Metab, 35, 458-462. doi:10.1016/j.diabet.2009.05.005
- Ahmed, J., Hammed, B., Das, G., Siddiqui, M.A., Ahmed, I. (2005) Postprandial hypertriglyceridemia and carotid intima-media thickness in north Indian type 2 diabetic subjects. Diabetes Research and Clinical Practice, 69, 142-150. doi:10.1016/j.diabres.2004.11.012
- Beltowski, J. (2006) Leptin and atherosclerosis. Atherosclerosis, 189,47-60. doi:10.1016/j.atherosclerosis.2006.03.003
- Correia, M.L., Rahmouni, K. (2006) Role of leptin in the cardiovascular and endocrine complications of metabolic syndrome. Diabetes, Obesity and Metabolism, 8, 603-610. doi:10.1111/j.1463-1326.2005.00562.x
- Werner, N. and Nickenig, G. (2004) From fat fighter to risk factor: The zigzag trek of leptin. Arterioscler Thromb Vasc Biol, 24, 7-9. doi:10.1161/01.ATV.0000110908.43721.ad
- Chiba, T., Shinozaki, S., Nakazawa, T., Kawakami, A., Ai, M., Kaneko, E., Kitagawa, M., Kondo, K., Chait, A., Shimokado, K. (2008) Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis, 196, 68-75. doi:10.1016/j.atherosclerosis.2007.01.040
- Sundell, J., Huupponen, R., Raitakari, O.T., Nuutila, P., Knuuti, J. (2003) High serum leptin is associated with attenuated coronary vasoreactivity. Obesity Research, 11, 776-782. doi:10.1038/oby.2003.108
- Li, L., Mamputu, J.C., Wiernsperger, N., Renier, G. (2005) Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 54, 2227-2234. doi:10.2337/diabetes.54.7.2227
- Xu, F.P., Chen, M.S., Wang, Y.Z., Yi, Q., Lin, S.B., Chen, A.F. and Luo, J.D. (2004) Leptin induces hypertrophy via endothelin-1-reacttive oxygen species pathway in cultured nenonatal rat cardiomyocytes, 110, 1269-1275.
- Loffreda, S., Yang, S.Q., Lin, H.Z, Karp, C.L., Brengman, M.L., Wang, D.J., Klein, A.S., Bulkley, G.B., Bao, C, Noble, P.W., Lane, M.D. and Diehl, A.M. (1998) Leptin regulates proinflammatory immune responses. The FASEB Journal, 12, 57-65.
- Bodary, P.E., Westrick, R.J., Wickenheiser K.J., Shen, Y., Eitzman, D.T. (2002) Effect of leptin on arterial thrombosis following vascular injury in mice. Journal of the American Medical Association, 287, 1706-1709.
- Hall, J.E., Hildebrandt, D.A. and Kuo, J. (2001) Obesity hypertension: role of leptin and sympathetic nervous system. American Journal of Hypertens, 14, 103S-115S. doi:10.1016/S0895-7061(01)02077-5
- Mark, A.L., Correia, M.L.G., Rahmouni, K. and Haynes, W.G. (2004) Loss of leptin actions in obesity: two concepts with cardiovascular implications. Clinical and experimental hypertension, 26, 629-636. doi:10.1081/CEH-200031948
- Clement, K., Lahlou, N., Ruiz, J., Hager, I., Bougneres, P., Basdevant, A., Guy-Grand, B., Froguel, P. (1997) Association of poorly controlled diabetes with low serum leptin in morbid obesity. International Journal of Obesity, 21, 556-561. doi:10.1016/S1056-8727(02)00167-8
- Sandoval, D.A., Davis, S.N. (2003) Leptin: metabolic control and regulation. Journal of Diabetes and its Complications, 17, 108-113. doi:10.1016/S1056-8727(02)00167-8
- Knudson, J.D., Payne, G.A., Borbouse, L. and Tune, J.D. (2008) Laptin and mechanisms of endothelial dusfunction and cardiovascular disease. Current Hypertens Reports, 10, 434-439. doi:10.1007/s11906-008-0082-2
- Gary, S. (2010) Cardiovascular effects of leptin. Nature Reviews Cardiology, 7, 22-29. doi:10.1038/nrcardio.2009.224
- Koh, K.K., Park, S.M. and Quon, M.J. (2008) Leptin and cardiovascular disease. Response to therapeutic interventions. Ciculation, 117, 3238-3249. doi:10.1161/CIRCULATIONAHA.107.741645
- Wallace, A.M., McMahon, A.D., Packard, C.J., Kelly, A., Shepherd, J., Gaw, A., and Sattar, N. on behalf of the Woscops executive committee, (2001) Plasma leptin and the risk of cardiovascular disease in the West of Scotland Coronary Prevention study (WOSCOPS). Circulation, 104, 3052-3056. doi:10.1161/hc5001.101061
- Reilly, M.P., Iqbal, N, Schutta, M., Wolfe, M.L., Scally, W.M., Localio, A.R., Rader, D.J. and Kimmel, S.E. (2004) Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism, 89, 3872-3878. doi:10.1210/jc.2003-031676
- Qasim, A., Mehta, N.M., Tadesse, M.G., Wolfe, M.L., Rhodes, T., Girman, C. and Reilly, M.P. (2008) Adipokines, insulin resistance and coronary artery calcification. Journal of the American College of Cardiology, 52, 231-236. doi:10.1016/j.jacc.2008.04.016
- Ciccone, M., Vettor, R., Pannacciulli, N., Minenna, A., Bellacicco, M., Rizzon, P., Giorgino, R. and De Pergola, G. (2001) Plasma leptin is independently associated with the intimamedia thickness of the common carotid artery. International Journal of Obesity, 25, 805-810. doi:10.1038/sj.ijo.0801623
- van den Beld, A.W., Bots, M.L., Janssen, J.A., Pols, H.A., Lamberts, S.W. and Grobbee, D.E. Endogenous hormones and carotid atherosclerosis in elderly men. American Journal of Epidemiol, 157, 25-31. doi:10.1093/aje/kwf160
- Bountouris, J., Paraskevas, K.I., Koutousis, M., Tzavara, V., Nikolaou, N., Nomikos, A., Barbatis, C., Andrikopoulos, V., Mikhailidis, D.P., Andrikopoulou, M., Kyriakide, Z.S., Geogopoulos, S., Michail, P.O. and BAstounis, E. (2009) Serum leptin levels in patients undergoing carotid endarectomy: A pilot study. Angiology, 60, 698-704. doi:10.1177/0003319709350133
- Stork, S., Bots, M.L., Angerer, P., von Schacky, C., Grobbee, D.E., Angermann, C.E., Seufert, J. (2007) Low levels of adiponectin predict worsening of arterial morphology and function. Atherosclerosis, 194, e147- e153. doi:10.1016/j.atherosclerosis.2006.11.044
- Ionescu-Tirgoviste, C., Ioacara, S., Guja, C., Sabau, S. et al. (2006) A pathophysiological approach to metabolic syndrome using factor analysis in an adult Romanian population. Archives of Physiology and Biochemistry 112(3), 182-188. doi:10.1080/13813450600976374
- Hansen, D., Dendale, P., Beelen, M., Jonkers, R.A.M., Mullens, A., Corluy, L., Meeusen, R., van Loon, L.J.C. (2010) Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. European Journal of Applied Physiology, 109, 397-404. doi:10.1007/s00421-010-1362-5

