 Psychology 2014. Vol.5, No.1, 70-77 Published Online Janu ary 201 4 in Sci R es (http://www.scirp.org/journal/psych) http://dx.doi.org/10.4236/psych.2014.51012 Effects of Psychosocial Stress on the Gene Expression of the Clock Genes hPER1 and hPER2 in Humans Elvira A. Abbruzzese1, Thomas Birchler2, Ulrike Ehlert1* 1Department of Clinical Psychology and Psychotherapy, Psychological Institute, University of Zurich, Zurich, Switzerland 2Division of Neuroimmunology, Institute of Experimental Immunology, Department of Pathology, University Hospital of Zurich, Zurich, Switzerland Email: *u.ehlert@psychologie.uzh.ch Received March 29th, 2013; revised November 30th, 2013; accepted December 15th, 2013 Copyright © 2014 Elvira A. Abbruzzese et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the in tellectu al pro perty Elvira A. Abbruzzese et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian. The circadian clock is a self-sustained time-keeping system which controls behavioral, biochemical and physiological rhythmic processes. In mammals, the cogwheels of this clock are the so-called clock genes which control their own expression via several feedback loops. One of these genes is hPER1, a clock gene which disposes of a glucocorticoid-responsive element and might therefore be influenced by glucocorti- coids. In humans, stress is associated with an increase in the glucocorticoid cortisol and is seen as a major factor in the etiology of numerous mental health problems. For this reason, our goal was to investigate the putative cortisol-mediated influence of acute and chronic psychosocial stress on the gene expression of hPER1 as well as hPER2, another related clock gene from the same family. We therefore applied labora- tory psychosocial stress to thirty-one healthy men and measured cortisol as well as mRNA levels of hPER1 and hP ER2. Our main findings suggest that acute psychosocial stress influences the expression of hPER1 and hPER2 dependent on the subjective experience of chronic stress. We therefore conclude that the reactivity to acute stress on the gene expression level of these two genes differs significantly between subjects with high chronic stress compared to subjects with low chronic stress. Keywords: Circadian Rhythm; Clock Genes; Cortisol; Gene Expression; hPER1; hPER2; Psychosocial Stress; Biological Clock Introduction All biological processes recurring daily, also known as cir- cadian-controlled processes, are governed by the so-called cir- cadian clock, a self-sustained time-keeping system controlling rhythmic behavioral, biochemical and physiological processes (Panda et al., 2002; Reppert & Weaver, 2002). In other words, the circadian clock affects the sleep-wake cycle, body t empera- ture, hormone secretion, enzyme activity, renal blood flow, heart rate, and various other physiological activities as well as the regulation of the cell cycle and of apoptosis (Ko & Takahashi, 2006; Albrecht, 2004; Lévi et al., 2007). The neuronal structure of the suprachiasmatic nuclei (SCN) within the hypothalamus acts as central pacemaker in mammals. Its neurons oscillate in a self-sustained fashion and synchronize the expression of clock genes in the peripheral cells (Ralph et al., 1990; Czeisler et al., 1999). The main Zeitgeber for this vital circadian process con- necting the outside world with the pacemaker is light: photic information is transmitted via specific ganglion cells of the retina over the retino-hypothalamic tract to the SCN (Lucas et al., 1999), where the neurons begin to oscillate. Within the single cells, the mechanism of the molecular clockwork is based on transcriptional/translational feedback loops (TTFLs) of s o-called clock genes. These TTFLs exist not only in the SCN but in almost every cell of the body (Lévi & Schibler, 2007). The inner clock is therefore composed of many different and tissue-speci- fic clocks within single cells (Albrecht & Eichele, 2003), which show a great inter-individual variety of expression patterns in humans (Teboul et al., 2005). The synchronization between the central clock (SCN) and the peri phery seems to b e performed by neuronal and/or hormonal signaling (Kriegsfe ld & Silver, 2006; Buijs et al., 2003). Along with th is synchronization, there appear to be more entrainment factors for peripheral oscillators. In- creasing evidence has demonstrated, for example, that the timing of food intake can “adjust” the peripheral clocks and therefore act as a strong Zeitgeber (Damiola et al., 2000; Stokkan et al., 2001; Schibler et al., 2003). Likewise, it has been shown that glucocorticoids (e.g. dexamethasone) are capable of “resetting” the clock in peripheral tissue (Balsalobre et al., 2000). It has been proposed that amongst other pathways glucocorticoids and genes of the Per family (in particular Per1) interact via gluco- corticoid-responsive elements (GRE) (Yamamoto et al., 2005). Also the gene expression of other core clock genes as e.g. Per2, Rev-ERBα, Npas2, could be stimulated in response to the syn- thetic glucocorticoid dexamethasone in primary mesenchymal Corresponding author. OPEN ACCESS 70 E. A. ABBRUZZESE ET AL. stem cells of mice. These clock genes showed significant chan- ges of their transcript levels within 4 hours after dexamethasone exposure. In Per2, there could be 8 different identified Gluco- corticoid binding sites (GBS’s) of which one seems to be con- stantly occupied during Per2’s rhythmic expression and is fur- thermore essential for the regulation of glucocorticoids (So et al., 2009). In addition, the CLOCK protein has been shown to ace- tylate glucocorticoid receptors thereby reducing their DNA- binding capacity to GRE-sites (Nader et al., 2009). One of the best e xplored glucocorticoids in humans is cortisol. Its presence varies according to a circadian rhythm, peaking in the morning immediately after an individual’s awakening and decreasing as the day progresses. The synthesis of cortisol is induced by the activation of the hypothalamus-pituitary-adrenal (HPA) axis. The corticotropin-releasing hormone (CRH) is released from the paraventricular nucleus (PVN) of the hypo- thalamus, inducing the synthesis of adrenocorticotropin (ACTH). This in turn stimulates the adrenal cortex, resulting in the syn- thesis of cortisol (Kirschbaum et al., 2007). There is abundant evidence that the HPA axis is strongly activated in situations of psychosocial stress, and after which there is a considerable increase in cortisol levels (Kudielka et al., 2003). Moreover, increasing evidence shows that cortisol present in the morning is not exclusively induced through an activation of the HPA axis, but also through light-induced activation of the adrenal glands (Scheer & Buijs, 1999; Ishida et al., 2005). This may imply an even stronger relationship between the circadian clock and glucocorticoids than hitherto assumed. Furthermore recent re- search could show a variation of gene expression in the hippo- campus according to a promoter pol ymorphism in the Per3 gene and its association to the stress response in mice: there was a significantly higher Per3 expression in stressed animals com- pared to non stressed animals (Wang et al., 2012). It appears that a disrupted circadian clock is not only clearly associated w ith physical illness, e.g. c ancer (Steven s, 2005; Fu et al., 2002), diabetes (Woon et al., 2007) or sleeping disorders (Viola et al., 2007), but is also involved in several mental health problems such as affective disorders (Roybal et al., 2007; Benedetti et al., 2008) or schizophrenia (Peng et al., 2007). Due to the fact that stress is considered as a factor strongly involved in the etiology of various mental health problems (Heinrichs et al., 2005; Ehlert et al., 2005; Gaab et al., 2005) and that the disruption of sleep-wake cycles in the form of sleeping disorders is often shown as a symptom in psychiatric disturbances (Costa e Silva, 2006), we were interested in focusing on the relationship between stress, cortisol and the gene expression of hPER1 and hPER2 as markers for the circadian clock. Therefore, the purpose of this study was to investigate whe- ther the increase of cortisol after psychosocial stress is associ- ated with the gene expression of hPER1 as well as hPER2. Additionally, we explored the association between chronic psy- chosocial stress and hP ER1 as well as hPE R 2. Considering a bio-psycho-social understanding of a continuous model of health and disease, the association between these factors could be of particular clinical importance. Materials and Methods Ethics: This study was conducted according to the declara- tion of Helsinki (adopted by the 55th General Assembly of the World Medical Association, Tokyo, 2004). The study protocol was approved by the ethics committee of the canton of Zurich, Department of Internal Medicine of the University Hospital of Zurich, and all participants provided written informed consent regarding participation in the study. Subjects and Recruitment Criteria: Data were collected from 31 healthy men aged 20 to 30. This range was deliberately chosen due to the fact that sleeping habits might change with age (Yamazaki et al., 2002). The investigation took place on two consecutive days in the laboratories of the Department of Clinical Psychology and Psychotherapy of the University of Zurich. Exclusion criteria were jetlag, medication, sleeping disorders, intake of psychotropic substances, psychological or physical illness, hospitalization, smoking and shift work within four months prior to the data acquisition. The study participants were instructed to adhere to a strict schedule for one week be- fore they participated in the investigation: they were asked to sleep and eat on a regular basis according to their own, inherent rhythm. Procedure, Sampling Intervals and Stress/Control Condi- tion: The study participants were instructed not to exercise and to avoid stressful psychological experiences if possible during the two study days. They were asked to arrive at the labora- tory at 1930h. On one of the two evenings, the participants were required to undergo a psychosocial stress test (stress con- dition); on the other evening, they had no intervention at all (control condition). While the sequence of the conditions was randomly assigned, the sampling intervals for cortisol and hPER1 as well as hPER2 were identical on both evenings. For cortisol: 2000h (baseline), 2030h (sample immediately after psychosocial stress in the stress condition), 2040h, 2050h, 2100h, 2115h, 2130 h and 2200h. For hPER1 and hPER2: 2000h (baseline), 2030h (sample immediately after psycho- social stress in the stress condition), 2100h, 2130h, 2200h and 2210h (exactly 2 hours aft er the onset of psychosocial stress in the stress condition). Sampling Methods and Analysis of mRNA and Cortisol: mRNA levels of hPER1 as we ll as hPER2 were assesse d in oral mucosa (Bjarnason et al., 2001) as described previously by Cajochen and colleagues (Cajochen et al., 2006) and analyzed in our biochemical laboratory of the Psychological Institute of the University of Zurich. In brief, each sample was ta ken wi th a pipette tip (epDualfilter T.I.P.S. 50 - 1000, Eppendorf, Ham- burg, Germany), immediately pipetted into a mixture of 0.7 µl of β-Mercaptoethanol and 100 µl Lysis buffer (Absolutely RNA Nanoprep Kit, Stratagene) and then frozen at −80˚C. RNA extraction was performed using the Absolutely RNA Nanoprep Kit (Stratagene), followed by reverse transcription with Superscript III First Strand Synthesis Super Mix for qRT- PCR (Invitrogen). The quantitative real-time PCR was conduct- ed using TaqMan Universal PCR Mastermix (Applied Biosys- tem) on an ABI 7700 Sequence Detection System (Applied Biosystems). The following primer and probes were used: Hs00242988 for hPER1, HS00256143_m1 for hPER2 and 4352934E for the endogenous control hGAPDH (all Applied Biosystems). The amount of target, normalized to the endoge- nous reference (GAPDH) and relative to a calibrator, was cal- culated by 2–ΔΔCT. Cortisol samples were collected using Salivettes (Sarstedt, Sevelen, Switzerland) and subsequently frozen at −20˚C. Sal iva samples were assayed with Luminescence Immunoassay (IBL) in the Laboratory of Biopsychology of the Technical University of Dresden, Germany. OPEN ACCESS 71 E. A. ABBRUZZESE ET AL. Assessment of Psychometric Data: Assessment of chronic stress within the three months prior to measurement was con- ducted using the Trier Inventory of Chronic Stress (TICS). This is a well-validated and reliable instrument used to retrospec- tively collect psychometric data relating to the subjective ex- perience of stress (Schulz & Schlotz, 1999). This questionnaire measures six aspects of chronic stress: Work overload, worries, social stress, lack of social recognition, work discontent and intrusive memories. The answers are given on a five-point rat- ing scal e. Psychosoci al stress test: In the stress condition, the Trier So- cial Stress Test (TSST) was applied. The TSST is a standard- ized laboratory stress protocol which reliably induces psycho- social stress in humans and consists of a speech task and a mental arithmetic task, both of which must be accomplished in front of an audience and a video camera. The subject is in- formed ten minutes before the stress task that he has five min- utes to prepare a short speech in which he should apply for a job. Speeches are made in front of two persons dressed in doc- tors’ overalls, who are instructed to be very serious and un- friendly. After five minutes the speeches are interrupted and subjects are told to count backwards beginning at 2047 with intervals of 17. After each mistake, the subje ct must start again at the beginning (Kirschbaum et al., 1993). Statistics: Statistics were calculated using SPSS 15.0. No participant was excluded from the calculation. However, due to missing data in the measurement of hPER1 and hPER2, there were cases which could not be included (relevant for calcula- tions with linear mixed models: for hPER1 30 subjects, for hPER2 22 subjects were included). The poor analyzability of hPER2 is difficult to explain. We assume that some of the spe- cific primer probes unfortunately did not work well. Psycho- metric and cortisol data were complete. To compare the total a mount of cortisol for both control and stress condition, areas under the curve were calculated (Pruess- ner et al., 2003) using the formula: AUCt = ((m1 + m2)/2 × t1-2) + ((m2 + m3)/2 × t2-3) + ((m3 + m4)/2 × t3-4) + ... + ((mx + my)/2 × tx-y). While mx stands for the height of cortisol at a specific measurement time point, tx-y describes the lengths in minutes between two measurement time points. The total amount of increase/decrease of cortisol was determined using the formula: AUCi = AUCt – m1 × ttotal. In order to compare the amount of cortisol that increased between subjects, the area under the curve is calculated in relation to the first measurement time point; therefore, the product of the first measured cortisol value and the overall measurement period (in minutes: ttotal) is sub- tracted from the total amount of cortisol. Percentage change was calculated using the formula: change in % from m1 to m2 = ((m2 – m1)/m1) × 100. Examinations considering repeated measurements were cal- culated using linear mixed models (LMM). Due to missing data in the measurement of hPER1 and hPER2, this procedure was more appropriate than the general linear model for repeated measures (ANOVA) since it also includes incomplete cases in the analysis. Moreover, general linear models assume inde- pendence of repeated measurements, while linear mixed models are appropriate for dependent and therefore nested data. For these reasons we chose to calculate the data using this hierar- chical linear model procedure (see e.g. Peugh & Enders, 2005). Correlations were calculated using Pearson’s correlation (two-tailed). Results The focus of this study was to investigate the link between the well-researched glucocorticoid cortisol and the clock genes hPER1 and hPER2. Furthermore, it was our goal to explore the influence of chronic as well as acute psychosocial stress on the expression of hPER1 and hPER2 in one group of subjects, namely 31 healthy men aged 20 to 30. Data concerning both gene expression and cortisol were collected at different time points in the evenings of two consecutive days. We distin- guished between a stress and a control condition and compared the levels of cortisol as well as the levels of hPER1 and hPER2 (always in relation to hGAPDH) and psychometric data. Diurnal Decrease and Stress-Induced Increase of Cortisol in the Evenings Subjects participated under a different condition each eve- ning. On the first evening, subjects were randomly assigned to the control or the stress condition and were then placed in the opposite group on the second evening. While in the control condition, cortisol levels decreased by 20% (mean) between 2000h and 2030h due to the regular circadian decline. In the stress condition a stress-induced cortisol increase of 244% (mean) was observed. The diurnal decrease/stress-induced in- crease (AUCi) and the total amount of cortisol (AUCt) in the evening were calculated using the formula for the area under the curve. This was conducted for both control and stress con- ditions: In the control condition, the cortisol decrease in the evening ranged from −735.25 to −3.98 nmol/l (mean = −234.93; sd = 201.88), while in the stress condition cortisol increase varied from −545.08 to 1812.25 nmol/l (mean = 462.03; sd = 521.00). The total amount of cortisol (AUCt) of the control evening ranged from 75.65 nmol/l to 798.75 nmol/l (mean = 334.45; sd = 200.63), while the total amount of cortisol of the stress evening displayed a range from 206.43 nmol/l to 2089.45 nmol/l (mean = 960.96; sd = 494.81). The stress-induced dif- ference is highly significant (F = 173.422; p = 0.0004) and can be seen as a successful induction of psychosocial stress. Stress Matters: The Effect of Chronic Stress on Cortisol To assess the perception of chronic stress levels, subjects were required to fill in a questionnaire (screening scale of the Trier Inventory of Chronic Stress, TICS) which recorded the level of subjectively experienced chronic stress over the three months immediately preceding data collection. The scores var- ied from 26 to 63 (mean = 46.87, sd = 10.51). The scores were median-split, providing two groups of subjects: one group with low levels of subjectively perceived chronic stress (n = 18) and the other with high levels (n = 13). Subjects who reported higher levels of chronic stress showed significantly lower cortisol levels in the eveni ng (control condi- tion: F = 19.637, p < 0.000; see Figure 1(a); stress condition: F = 11.715, p = 0.001; see Figure 1(b)). Subjects with Chronic Stress Show Higher Levels of hPER1 and hPE R2 The baseline amounts of mRNA of hPER1 and hPER2 were measured at 2000h (baseline) and did not differ between day one and day two. All mRNA measurements were calculated in OPEN ACCESS 72 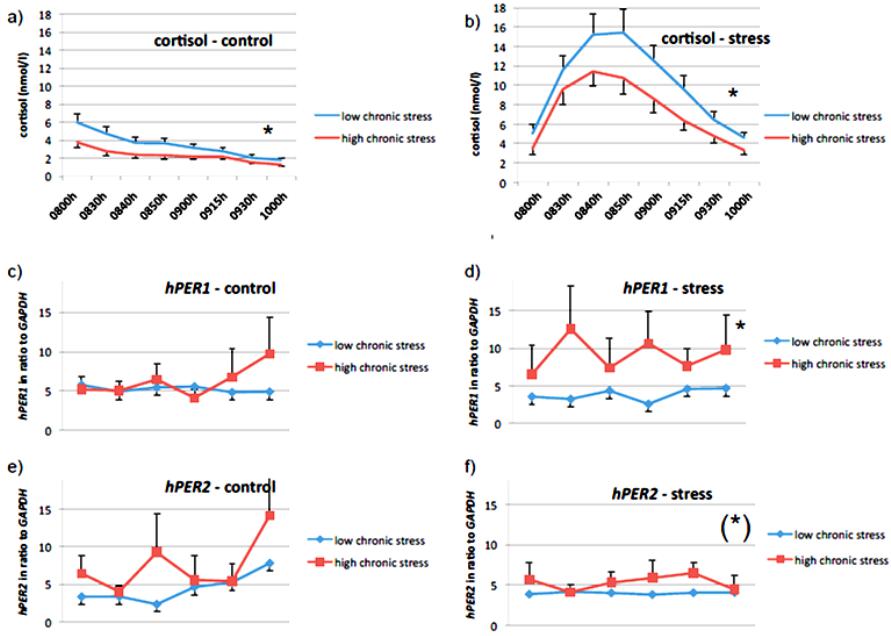 E. A. ABBRUZZESE ET AL. Figure 1. The influence of chronic stress on cortisol levels and gene expression levels of hPER1and hPER2 for both, control a nd s tress condition. ratio to the house-keeping gene hGAPDH. In order to assess the influence of chronic stress levels on the mRNA levels of hPER1 and hPER2, we used the above described psychometric data of the TICS comparing the subjects of the two median-split groups as described above. In contrast to the inverse relation- ship between chronic stress and cortisol, higher chronic stress seems to be associated with higher levels of hPER1. This effect is significant in the stress situation (F = 8.502; p = 0.004; see Figure 1(d)), but not in the control condition (F = 0.502; p = 0.480; see Figure 1(c)). This indicates significantly differing gene expression levels of hPER1 between subjects with high respectively low chronic stress levels immediately after acute psychosocial stress. While subjects with low chronic stress levels show an immediate suppression of mRNA levels of hPER1 with small standard deviations, there are heightened mRNA levels of hPER1 and greater interindividual differences (higher standard deviation) in subjects with high chronic stress levels after acute stress. Similarly, higher hPER2 levels after psychosocial stress seem to be associated with higher levels of chronic stress (F = 3.305; p = 0.075, trend; see Figure 1(f)), whereas this is not the case in the control condition (F = 1.197; 0.287; see Figure 1(e)). Subjects with high levels of perceived chronic stress show lower levels of cortisol in control as well as stress condition, but significantly higher hPER1 levels in the stress condition (p = 0.004). In addition, the same subjects display higher hPER2 levels in the stress condition (p = 0.075). Interestingly also standard deviations of hPER1 and hPER2 seem to be larger in subjects with high levels of perceived chronic stress. The Rise of Cortisol after Acute Psychosocial Stress Seems to Be Associated with the Gene Expression of hPER1 and hPE R2 As reported above, we assessed the simultaneously measured association between the total amount of cortisol and the gene expression levels of hPER1 and hPER2 in the evening between 2000h and 2210h. This relationship was tested separately for the stress and the control conditions, as the total amounts of cortisol were expected to differ between these conditions. In- deed, there was a highly significant difference between the two conditions in cortisol (see above). Such a marked difference between stress and control condition was not found either for hPER1 (F = 0.307; p = 0.580) or for hPER2 (F = 1.864; p = 0.175). However after the induction of psychosocial stress the levels of hPER 1 as well as hPER2 measured begin to correlate strongly with their baseline levels (all p < 0.0001 for hPER1; see also Table 1). This effect was not seen in the control condi- tion (all p > 0.050). This finding contrasts with the correlations between cortisol levels: due to the constant decrease in cortisol in the evening, there is a strong association between the cortisol levels measured in the control condition, but not in the stress condition. Based on the above-mentioned sharp increase in cortisol levels after the stress task, none of the subsequent cor- tisol levels correlate with its baseline. It might therefore be deduced that a sudden increase in cortisol (relative change of OPEN ACCESS 73  E. A. ABBRUZZESE ET AL. Table 1. Highly significant correlations between measurement time points of hPER1 as well as hPER2 after stress-induced increase of cortisol. Condition Compared to baseline measurement at 0800 h Cortisol correlation (p) hPER1 correlation (p) hPER2 correlation (p) Control 0830 h 0900 h 0930 h 1000 h 0.973 (<0.000)** 0.912 (<0.000)** 0.730 (<0.000)** 0.796 (<0.000)** 0.163 (0.546) 0.496 (0.060) −0.005 (0.984) 0.339 (0.216) 0.087 (0.889) −0.214 (0.8 63) −0.440 (0.458) 0.980 (0.128) Stress 0830 h 0900 h 0930 h 1000 h 0.379 (0.035)* 0.107 (0.568) 0.217 (0.242) 0.296 (0.143) 0.928 (<0.000)** 0.912 (<0.000)** 0.887 (<0.000)** 0.815 (<0.000)** 0.666 (0.071) (*) 0.725 (0.042)* 0.805 (0.029)* −0.497 (0.503) cortisol) is involved in “resetting” or “flattening” the fluctua- tion between the measurements of hPER1 and, to a lesser ex ten t, also of hPER2. Time points compared to their baseline at 2000h (compared within conditions): hPER1 and hPER2 seem to react to dra- matic changes in levels of cortisol: As soon as cortisol deviates from its circadian decreasing course by means of a stress-in- duced, sudden increase, hPER1 begins to correlate significantly with its baseline indicating a possible “flattening” of its course. The same effect, although to a lesser extent, is observed in hPER2 (statistical analysis was calculated using Pearson’s cor- relation, two-tailed). This effect is not observed in the control condition where cortisol decreases slowly due to its circadian course. Discussion To our knowledge, this study is the first to examine the in- teraction of psychosocial stress and the gene expression of hPER1 and hPER2 in humans. Regarding the impact of chronic stress, our data show that the levels of hPER1 and hPER2 differ in subjects with high chronic stress compared to subjects with low chronic stress. Similar results have been found in the gene expression of Per3 of stressed animals (Wang et al., 2012). Although reports on the interaction of chronic stress and cortisol are still conflicting (Kudielka et al., 2009), in our sample, high levels of chronic stress are unambiguously associated with lower basal cortisol. In spite of the fact that there is still no reliable reference for “healthy”, or in the statistical sense “normal”, gene expression levels for either hPER1 or hPER2, or any other clock gene, we can draw the conclusion from the data of our sample that sub- jects with high chronic stress levels show blunted basal cortisol in the evening. At the same time and in accordance with find- ings reported above, the expression of hPER1 and also hPER2 is heightened in subjects with higher chronic stress compared to subjects with low reported chronic stress. This interaction is even more pronounced after acute psychosocial stress and might suggest that chronically stressed humans react differently and more instable respectively regarding the influence on the circadian clock. However, we did not find the expected increase of hPER1 and hPER2 after the increase of cortisol due to psy- chosocial stress as expected according to data from animal studies (e.g. Balsalobre et al., 2000). While subjects with low chronic stress show a more coherent “flattening” of the mRNA levels of hPER1 and hPER2, subjects with high chronic stress experience show a far greater standard deviation of the measured time points after the acute psychoso- cial stress, which might indicate a less deterministic and there- fore more inefficient physiological reactivity to acute stress. Recent findings that postulate an increased vulnerability to circadian rhythm disruption after experienced stress in the presence of CLOCK and PER3 polymorphisms in women, support our data (Anty pa e t al. , 2012).Our findings report about the change within the first two hours after psychosocial stress and therefore might describe a very first reaction to psychoso- cial stress at the cost of not fully monitoring the amplitude and frequency of the circadian oscillation of hPER1 and hPER2, which is a short-coming of our study. On the other side, the fact that we “put the spyglass” on the 120 minutes immediately after a stressor occurred, might give new insights and hypotheses about important processes of the first minutes of a stress-reac- tion. Nevertheless, 120 minutes might be too short to detect large effects since the peak of transcribed mRNA accumulation depends on the half-life of the mRNA (and many post-tran - scriptional processes are not known yet or is still unknown how they influence the half life of mRNA), which on average is about two hours after the inducing signal. Usually, the influ- ence of glucocorticoids on the clock gene expression was measured in large intervals of several hours, which better pro- vides for the circadian oscillation of clock genes, but might miss the very first changes of sensitively interacting systems. Balsalobre et al. (2000) postulated that glucocorticoids might reset the clocks in the periphery and therefore play an important role for the synchronization between the SCN and the periphery, but they cannot be the only signals to entrain the periphery. Recent results could show that acute systemic inflammatory processes, which involve the activation of pro-inflammatory cytokines, illustrated a suppressive effect on the gene expres- sion of several clock genes amongst others Per1 and Per2: Okada and colleagues could show that rats which received an intravenous injection of lipopolysaccharide demonstrated nota- ble increases of TNFα, IL-6 and also corticosterone, while the gene expression of Per1 and Per2 in the SCN were slightly suppressed during the day of injection. The same was seen in the cells of the liver with a latency of 4 to 6 hours. Furthermore, a suppressed locomotor activity could be observed, which is also known as “sickness-behavior”. Cavadini et al. (2007) also reported a significant reduction in locomotor activity, pro- longed rest time, and impaired gene expression of clock-related genes after the infusion of TNFα. Data from our research group report that immediately after a psychosocial stressor (TSST) cortisol is elevated, while cytokines are significantly decreased. Moreover, within the first 60 minutes post-stress, LPS-stimula- ted TNFαand IL-6 were predicted by the subjective “control expectancy” as well as the experienced “chall en ge” (Wirtz et al., OPEN ACCESS 74 E. A. ABBRUZZESE ET AL. 2007). Haimovich et al. (2010) found suppressed gene expres- sion of hPER1 and hPE R2 in human peripheral blood leuco- cytes after the administration of endotoxin (LPS) with nadir values 13 to 17 hours after infusion, while levels of TNFα, IL-6 as well as cortisol were elevated during the period of acute systemic inflammation. Gaab et al. (2005) could show that patients with chronic fatigue syndrome did neither differ from controls in the levels of LPS-induced cytokines nor in the stress-induced cortisol-peak after the TSST, but they showed significantly attenuated levels of pro-inflammatory cytokines after the stress task. Amongst others, the authors discuss the possibility of an enhanced sensitivity to inhibitory effects of cortisol. If subjects who report more chronic stress showed higher immunosuppressive effects after a stress-induced corti- sol peak, this would also mean that the cytokine-induced sup- pression or resetting of the gene expression of hPER1 and hPER2 (as shown: Okada et al., 2006; Haimovich et al., 2010) would not be so efficient. This association might be explanatory for our findings and might explain why physical, but also stress-induced mental illness affects the experience of fatigue and/ or disturbed sleep patterns on the long term. In any case, a sudden increase of cortisol seems to affect the mRNA levels of both hPER1 and hPER2 in the periphery or at least in ora l mucosa, albeit the effect is not as pronounced as in animal studies which report an increase of clock gene expres- sion (Balsalobre et al., 2000; Takahashi et al., 2001; Fukuoka et al., 2005). First, this might be due to the fact that in animal studies, high dosage rates of glucocorticoids are administered in order to induce a phase shift of clock genes (e.g. so called “dexamethasone-shock”; Balsalobre et al., 2000). Such a high dosage in relation to the animals’ body weight is, of course, not comparable to a relatively short-lived and natural stress-in- duced rise of cortisol. Therefore, it seems difficult to compare data from human studies with animal studies. Second, it would probably be very dangerous for an organism’s homeostasis if a relatively short-lasting acute stressor would have the impact to severely disturb and/ or disrupt the circadian rhythm. Lastly, we did not monitor the development of gene expression over two hours post-stress; therefore, we might have missed a possible later increase or change of the gene expression of hPER1 and hPER2. Nevertheless, we were able to show that acute psycho- social stress in humans has an impact on the gene expression of hPER1 as well as hPER2. Even a natural increase of corti- sol—induced by an acute psychosocial stressor—seems to “flatten” the fluctuation of hPER1 and hPER2. This is evident based on the highly significant correlations between measure- ments of hPER1 after the stress-induced and considerable in- crease of cortisol. The same effect, although to a lesser extent, was observed in hPER2. This effect failed to occur in the con- trol condition, when cortisol levels were following their steady circadian decrease. Hence, it might be concluded that humans, depending on their individual stress reactivity, minimally “reset” their clocks after subjectively experienced stressful situations. This might be an important result given that mental and physi- cal health strongly depends on an organism’s ability to restore physiological homeostasis (e.g. Mc Ewan & Lasley, 2003). In general, it might be misleading to talk about chronic stress in a cross-sectional study since it is very difficult to retrospec- tively investigate the onset of the so-called chronic stress; therefore, the only appropriate design would be a prospective longitudinal study to adequately investigate chronic stress and its correlates because it is about the time-related process of a maladaptive change (as also alluded in the term “chronic”). This may be the reason why there are many conflicting biopsy- chological data with large interindividual differences when it comes to chronic stress (Kudielka et al., 2009). In allusion to McEwan et al. (2003), chronic stress might be seen as an ongo- ing or repeated strain which can lead to an allostatic load. This means that the sensitive equilibrium of highly adaptive and interacting systems of an organism is disturbed and not able to restore homeostasis any more, which might lead to the deregu- lation of one or more of the interacting psycho-physiological sys tems . Concerning our data, this could lead to the conclusion that the interaction between cortisol and the gene expression of hPER1 and hPER2 might be additionally mediated by other pathway s as e.g. t he immune system. Recent findings suggest a transient, but strong suppressive effect of pro-inflammatory cytokines on the gene expression of clock genes (Okada et al., 2006; Haimovich et al., 2010; see above). It seems important to us to emphasize the influence of the time aspect of chronic stress and to focus the process of change over time since the organism’s interacting systems will most probably display dif- ferent allostatic regulation patterns according to the length of stress endurance. Therefore, cross-sectional studies might be limited in their statements about chronic stress and should not be simplified to universally and generally accepted facts, but at the same time might explain findings which are not conform to the postulated hypotheses. Nevertheless, it is interesting and important to see that subjects who reported about high experi- enced chronic stress in our study react differently compared to subjects who report low experienced chronic stress. This might indicate the beginning of the shift from allostasis to an allostatic load that could lead to severe illness in the long term. Cortisol, as part of the endocrine HPA axis pathway, ge ne- rally seems to have a regulatory role in a putative feedback- mechanism between the inner clock and the endocrine system (Dallmann et al., 2006; Dickmeis et al., 2007), and therefore it seems to be conclusive that even sudden stress-induced in- creases of cortisol level directly as well as indirectly (via other systems mediated), induce short-period effects in the gene ex- pression of hPER1 and hPE R2. On the basis of a strong interaction between the circadian clock and the HPA axis, our finding may be a first meaningful step to explain the disruption of sleep-wake cycles in stress- induced disorders. Shortcomings of our study are the relatively small number of subjects and the broad range of mRNA levels of hPER1 and hPER2, which makes interpretation of the statistical analyses difficult. The latter is not a new problem and it has been ad- dressed before (Brown et al., 2005; Teboul et al., 2005). One aim of future research should be to examine more subjects in order to raise statistical power. A hardly solvable problem in human research concerning the biological clock seems to be the question, if peripherally measured clock gene expression is significantly correlated with the central gene expression in the brain. Recent findings suggest that peripheral measurements can approximately indicate an individual inner period and body time (Pagani et al., 2010; Kasukawa et al., 2012). Furthermore, it would be recommendable to take measure ments at more points spread evenly over the day in order to better account for the circadian rhythms of the parameters- measured. Nevertheless our data fortify the importance to fur- ther research the association between stress and the inert bio- logical clock in humans and might indicate a possible mecha- OPEN ACCESS 75  E. A. ABBRUZZESE ET AL. nism how psychosocial stress might influence or even disrupt an individual’s circadian rhythm on the long term. Three important questions remain and should be addressed in future research: 1) Which systems are actively involved in the stress reaction, which also constitute signaling pathways that therefore affect the inner clock? 2) Which are the molecular processes that mediate the interaction between these systems and the inner clock? 3) How do these processes allostatically change over time in the presence of an ongoing strain? One of the crucial goals for future research will be to deter- mine the effect of short-term changes of psychosocial stress and cortisol on the long-term “ticking of the clock”. REFERENCES Albrecht, U., & Eichele, G. (2003). The mammalian circadian clock. Current Opinion in Genetics & Development, 13, 271-277. http://dx.doi.org/10.1016/S0959-437X(03)00055-8 Albrecht, U. (2004). Human molecul ar chronotyping in sight? Genome Biology, 5, article 246. Antya, N., Mandelli, L., Nearchou, F. A., Vaiopoulos, C., Stefanis, C. N., Serretti, A ., & Stefanis, N. C. (2012). The 3111/C Polymorphism Interacts With Stressfu l Life Events to Influence Patterns of Sleep in Females. Chronobiology Int ernational, 29, 891-897. http://dx.doi.org/10.3109/07420528.2012.699380 Balsalobre, A., Brown, S. A., Marcacci, L., Tronch e, F., Kellend o n k , C ., Reichardt, H. M., Schütz, G., & Schibler, U. (2000). Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Sci- ence, 289, 2344-2347. http://dx.doi.org/10.1126/science.289.5488.2344 Benedetti, F., Dallaspezia, S., Colombo, C., Pirovano, A., Marino, E., & Smeraldi, E. (200 8 ). A leng th p o lymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neuroscience Letters, 445, 184-187. http://dx.doi.org/10.1016/j.neulet.2008.09.002 Bjarnason, G. A., Jordan, R. C. K., Wood, P. A., Li, Q., Lincoln, D. W., Sothern, R. B., Hrushesky, W. J. M., & Ben-David, Y. (2001). Cir- cadian expression of clock genes in human oral mucosa and skin. American Journal of Clinical Pathology, 158, 1793-1801. Brown, S. A., Fl eu ry-Olela, F ., Nagoshi, E., Hauser , C ., Ju g e, C ., Meier, C. A., Chicheportiche, R., Dayer, J . M., Albrecht, U ., & Schibler, U. (2005). The period length of fibroblast circadian gene expression varies widely among human individuals. PLOS Biol., 3, 1813-1818. http://dx.doi.org/10.1371/journal.pbio.0030338 Buijs, R. M., van Eden, C. G., Concharuk, V. D., & Kalsbeek, A. (2003). The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. Journal of Endocri- nology, 177, 17-26. http://dx.doi.org/10.1677/joe.0.1770017 Cajochen, C., Jud, C., Münch, M., Kobialka, S., Wirz-Justice, A., & Albrecht, U. (2006). Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. European Journal of Neuroscience, 23, 1082-1086. http://dx.doi.org/10.1111/j.1460-9568.2006.04613.x Cavadini, G., Petrzilka, S., Kohler, P., Jud, C., Tobler, I., Birchler, T., & Fontana, A. (2007). TNF-alph a suppresses the expression of clock genes by interfering with E-box-mediated transcrip tion. Proceedings of the National Academy of Sciences, 104, 12843-12848. http://dx.doi.org/10.1073/pnas.0701466104 Costa e Silva, J. A. (2006). Sleep disorders in psychiatry. Metabolism, 55, 40-44. http://dx.doi.org/10.1016/j.metabol.2006.07.012 Czeisler, C. A., Duff y, J . F., Shan ahan, T. L., Brown, E. N ., Mitchell, J. F., Rimmer, D . W., Ronda, J. M., Silva, E. W., A llan, J. S., E mens , J . S., Dijk, D. J., & Kronauer, R. E. (1999). Stability, precision, and near-24-hour period of the hum an circadian pacemaker. Science, 284, 2177-2181. http://dx.doi.org/10.1126/science.284.5423.2177 Dallmann, R., Touma, C., Palme, R., Albrecht, U., & Steinlechner, S. (2006). Impaired daily glucocorticoid rhythm in Per1Brd mice. Journal of Comparative Physiology A, 192, 769-775. http://dx.doi.org/10.1007/s00359-006-0114-9 Damiola, F., Le M inh, N., Preitner, N., Ko rnmann, B., Fleury-Olela, F., & Schibler, U. (2000). Restricted feeding uncouples circadian oscil- lators in peripheral tissues from the central pacemaker in the su- prachiasmatic nucleus. Genes & Development, 14, 2950-2961. http://dx.doi.org/10.1101/gad.183500 Dickmeis, T., Lahiri, K., Nica, G., Vallone, D., Santoriello, C., Neu- mann, C. J., Hammerschmidt, M., & Foulkes, N. S. (2007). Gluco- corticoid Play a Key Role in Circadian Cell Cycle Rhythms. PLOS Biol., 5, 854-864. http://dx.doi.org/10.1371/journal.pbio.0050078 Ehlert, U., Nater, U. M., & Böhmelt, A. (2005). High and low unstimu- lated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. Journal of Psychosomatic Re- search, 59, 7-10. http://dx.doi.org/10.1016/j.jpsychores.2005.03.005 Fu, L., Pelicano, H., Liu, J., Huang, P., & Lee, C. C. (2002). The cir- cadian gene Period2 plays an important role in tumor suppression and DNA damage responses in vivo. Cell, 111, 41-50. http://dx.doi.org/10.1016/S0092-8674(02)00961-3 Fukuoka, Y., Burio ka, N., Takat a, M., Oh do, S., Miyata, M., Endo , M., & Shimizu, E. (2005). Glucocorticoid administration increases hPER1 mRNA Levels in human peripheral blood mononuclear cells in vitro or in vivo. Journal of Biological Rhythms, 20, 550-553. http://dx.doi.org/10.1177/0748730405279866 Gaab, J., Rohl eder, N., H eitz, V ., Eng ert, V., Sch ad, T., Sc hürmeyer, T. H., & Ehlert, U. (2005). Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology, 30, 188-198. http://dx.doi.org/10.1016/j.psyneuen.2004.06.008 Godbout, J. F., & Glaser, R. (2006). Stress-induced immune dysre- gulation: Implications for wound healing, infectious disease and cancer. Journal of NeuroImm une Pharmacology, 1, 421-427. http://dx.doi.org/10.1007/s11481-006-9036-0 Haimovich, B., Calv ano, J., Haimovich, A. D., Calvano, S. E., Coyle, S. M., & Lowry, S. F. (2010). In vivo endotoxin synchronizes and sup- presses clock gene expression in human peripheral blood leuko- cytes. Critical Care Medicine, 38, 751-758. http://dx.doi.org/10.1097/CCM.0b013e3181cd131c Heinrichs, M., Wagner, D., Schoch, W., Sorav ia, L. M., Hellhammer, D. H., & Ehlert, U. (2005). Predicting posttraumatic stress symptoms from pretraumatic risk f actors: A 2-year prospective f ollow-up study in firefighters. American Journal of Psy chiatry, 162, 2276-2286. http://dx.doi.org/10.1176/appi.ajp.162.12.2276 Ishida, A., Mu to h , T., Ueyama, T., Band o , H., Mas u b u ch i, S ., Na k ah ara, D., Tsujimoto, G., & Oka mura, H. (2005). Light activat es the ad r en al gland: Timing of gene expression and glucocorticoid release. Cell Metabolism, 2, 297-307. http://dx.doi.org/10.1016/j.cmet.2005.09.009 Kasukawa, T. , Sugimoto, M., Hid a, A., Minami, Y., Mori, M., Hon ma, S., Honma, K. , Mi s h ima, K., Soga, T., & Uedo , H. R. (2012). Human blood metabolite timetable indicates internal body time. Proceedings of the National Academy of Sciences, 109, 15036-15041. http://dx.doi.org/10.1073/pnas.1207768109 Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The “Trier Social Stress Test”—A tool for inv estigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76-81. http://dx.doi.org/10.1159/000119004 Kirschbaum, C., & Hellhammer, D. H. (2007). Encyclopedia of stress (pp. 405-409). Oxford: Academic Press, http://dx.doi.org/10.1016/B978-012373947-6.00334-2 Kudielka, B. M., Sch ommer, N. C., Hellh a mmer, D. H., & Kirschbaum, C. (2003). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology, 29, 983-992. http://dx.doi.org/10.1016/j.psyneuen.2003.08.009 Ko, C. H., & Takahashi, J. S. (2006). Molecular components of the mammalian circadian clock. Human Molecular Genetics, 15, R271- R277. http://dx.doi.org/10.1093/hmg/ddl207 Kriegsfeld, R. J., & Silver, R. (2006). The regulation of neuroendocrine function: Timing is everything. Hormones and Behavior, 49, 557- 574. http://dx.doi.org/10.1016/j.yhbeh.2005.12.011 Kudielka, B. M., Hellhammer, D. H., & Wüst, S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34, 2-18. OPEN ACCESS 76  E. A. ABBRUZZESE ET AL. http://dx.doi.org/10.1016/j.psyneuen.2008.10.004 Lévi, F., Filipski, E., Iuriski, I., Li, X. M., & Innominato, P. (2007). Cross-talks between circadian timing system and cell division cycle determine cancer biology and therapeutics. Cold Spring Harbor Symposia on Quantitative Biology, 72, 465-475. http://dx.doi.org/10.1101/sqb.2007.72.030 Lévi, F., & Schibler, U. (2007). Circadian Rhythms: Mechanisms and Therapeutic Implications. Annual Review of Pharmacology and Toxicology, 47, 593 -628. http://dx.doi.org/10.1146/annurev.pharmtox.47.120505.105208 Lucas, R. J., Freedman, M. S., Munoz, M., Garcia-Fernandez, J., & Foster, R. G. (1999). Non-rod, non-cone ocular photoreceptors regu- late the mammalian pineal. Science, 284, 505-507. http://dx.doi.org/10.1126/science.284.5413.505 McEwan, B., & L asley, E. N. (2003). Allostatic load: When protection gives way to damage. Advances in Mind-Body Medicine, 19, 28-33. Nader, N., Chrousos, G. P., & Kino, T. (2009). Circadian rhythm tran- scription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acet ylating its hinge region lysine cluster: Potential physiological implications. FASEB Journal, 23, 1572-1583. http://dx.doi.org/10.1096/fj.08-117697 Okada, K., Yano, M., Doki, Y., Azama, T., Iwanaga, H., Miki, H., Nakayama, M., Miyata, H., Takiguchi, S., Fujiwara, Y., Yasuda, T., Ishida, N., & Monden, M. (2006). Injection of LPS causes transient suppression of biological clock genes in rats. Journal of Surgical Research, 145, 5-12. http://dx.doi.org/10.1016/j.jss.2007.01.010 Pagani, L., Semenova, E. A., Moriggi, E., Revell, V. L., Hack, L. M., Lockley, S . W., Arendt, J., Skene, D. J., Meier, F., Izakovic, J., Wirz- Justice, A ., Cajo ch en, C., Sergeeva, O. J., Cheresiz, S. V., Danilenko , K. V., Eckert, A., & Brown, S. A. (2010). The physiological period length of the hu man circadian clock in vivo is d irectly propo rtional to period in human fibroblasts. PLoS ONE, 5, e13376. http://dx.doi.org/10.1371/journal.pone.0013376 Panda, S., Hogensch, J. B., & Kay, S. A. (2002). Circadian rhythms from flies to human. Nature, 417, 329-335. http://dx.doi.org/10.1038/417329a Peng, Z., Ch en, X., & Wei, Z. (2007). Cryptochrome 1 maybe a candi- date gene of schizophrenia. Medical Hypotheses, 69, 849-851. http://dx.doi.org/10.1016/j.mehy.2007.02.003 Peugh, J. L., & Enders, C. K. (2005). Using the SPSS mixed procedure to fit cross-sectional and lo ngitudinal multilevel models. Educational and Psychological Measurement, 65, 717-741. http://dx.doi.org/10.1177/0013164405278558 Pruessner, J . C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas fo r computation o f the area und er the curve represent measures of total hormone concentration versus time-de- pendent change. Psychoneuroendocrinology, 28, 916-931. http://dx.doi.org/10.1016/S0306-4530(02)00108-7 Ralph, M. R., Foster, R. G., Davis, F. C., & Menaker, M. (1990). Transplanted suprachiasmatic nucleus determines circadian period. Science, 247, 975-978. http://dx.doi.org/10.1126/science.2305266 Reppert, S. M., & Weaver, D. R. (2002). Coordination of circadian timing in mammals. Nature, 418, 935-941. http://dx.doi.org/10.1038/nature00965 Roybal , K. , Theob old , D ., Grah am, A., D iN ie ri , J. A., Russo, S . J., K ri sh - nan, V., Chakravarty, S., Peevey, J., Oehrlein, N ., Birnbau m, S., Vi- taterna, M. H., Orsulak, P., Takahashi, J. S., Nestler, E. J., Carlezon Jr., W. A., & McClung, C. (2007). Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Acad emy of Sci- ences of the United States of America, 104, 6406-6411. http://dx.doi.org/10.1073/pnas.0609625104 Scheer, F. A., & Buijs, R. M. (1999). Light affects morning salivary cortisol in humans. The Journal of Clinical Endocrinology and Me- tabolism, 84, 3395-3398. http://dx.doi.org/10.1210/jc.84.9.3395 Schibler, U., Rippberger, J., & Brown, S. A. (2003). Peripheral circa- dian oscillators in mammals: Time and food. Journal of Biological Rhythms, 18, 250-260. http://dx.doi.org/10.1177/0748730403018003007 Schulz, P., & Schlotz, W. (1999). Das trierer inventar zur erfassung von chronischem streß (TICS): Skalenkonstruktion, teststatistische über- prüfung und validierung der Skala Arbeitsüberlastung. Diagnostica, 45, 8-19. http://dx.doi.org/10.1026//0012-1924.45.1.8 So, A. Y. L., Bernal, T. U., Pillsbury, M. L., Yamamoto, K. R., & Feldman, B. J. (2009). Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 106, 17582- 17587. http://dx.doi.org/10.1073/pnas.0909733106 Stevens, R. G. (2005). Circadian disruption and breast cancer, from melatonin to clock genes. Epidemiology, 16, 254-258. http://dx.doi.org/10.1097/01.ede.0000152525.21924.54 Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y., & Menaker, M. (2001). Entrainment of the circadian clock in the liver by feeding. Science, 291, 490-493. http://dx.doi.org/10.1126/science.291.5503.490 Takahashi, S., Yokota, S., Hara, R., Kobayashi, T., Akiyama, M., Mo- riya, T., & Shibata, S. (2001). Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventri- cular nucleus of the mouse. Endocrinology, 142, 4910-4917. http://dx.doi.org/10.1210/en.142.11.4910 Teboul, M., Barrat-Petit, M. A., Li, X. M., Claustrat, B., Formento, J. L., Delaunay, F., Levi, F., & Milano, G. (2005). Atypical patterns of circadian clock gene expression in human peripheral blood mono- nuclear cells. Journal of Molecular Medicine, 83, 693-699. http://dx.doi.org/10.1007/s00109-005-0697-6 Viola, A. U., Archer, S. N., James, L. M., Groeger, J. A., Lo, J. C. Y., Skene, D. J., von Schantz, M., & Dijk, D. J. (2007). PER3 polymor- phism predicts sleep structure and waking performance. Current Bi- ology, 17, 613-618. http://dx.doi.org/10.1016/j.cub.2007.01.073 Wang, X., Mo zhui, K., Li, Z., Mulligan, M. K., Ingel s, J. F., Zhou, X., Hori, R. T., Chen, H., Cook, M. N., Williams, R. W., & Lu, L. (2012). A promoter polymorphism in the Per3 gene is associated with alcohol and stress response. Translational Psychiatry, 2, e73. Wirtz, P. H., von Känel, R., Emini, L., Suter, T., Fontan a, A., & Ehlert, U. (2007). Variations in anticipatory cognitive stress appraisal and differential proinflammaotry cytokine expression in response to acute stress. Brain, Be havior, and Immunit y, 21, 651-659. http://dx.doi.org/10.1016/j.bbi.2007.02.003 Woon, P. Y., Kaisaki, P. J., Bragança, J., Bihoreau , M. T., Levy, J. C., Farrall, M., & Gauguier, D. (2007). Aryl hydrocarbon receptor nuc- lear translo cator-like (BMAL1) is as sociated with sus ceptibility to hy- pertension and type 2 diabetes. Proceedings of the National Academy of Sciences of the United States of America, 104, 14412-14417. http://dx.doi.org/10.1073/pnas.0703247104 Yamamoto, T., Nakahata, Y., Tanaka, M., Yoshida, M., Soma, H., Shinohara, K ., Yasuda , A., Mamine, T., & Takumi, T. (2005). Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a gluco corticoid -respon siv e ele ment. Journal of Biological Chemistry, 280, 42036-42043. http://dx.doi.org/10.1074/jbc.M509600200 Yamazaki, S., Straume, M., Tei, H., Sakaki, Y., Menaker, M., & Block, G. D. (2002). Effects of aging on central and peripheral mammalian clocks. Proceedings of the National Academy of Sciences of the United States of America, 99, 10801-10806. http://dx.doi.org/10.1073/pnas.152318499 OPEN ACCESS 77
|