Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2011, 2, 27-34 doi:10.4236/ajps.2011.21003 Published Online March 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS 27 Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Javier Gil-Humanes, Antonio Martín, Francisco Barro Institute for Sustainable Agriculture, CSIC. E-14080, Córdoba, Spain. Email: javigil@ias.csic.es Received August 5th, 2010; revised September 21st, 2010; accepted September 28th, 2010. ABSTRACT Brassica carinata, a natural alloploid formed between B. oleracea and B. nigra, is a potential oil crop for th e Mediter- ranean area in which genetic transformation could help to breed. In vitro culture and shoot regeneration are key fac- tors in developing an efficient transformation method in the genus Brassica. However, the studies for in vitro culture and shoot regeneratio n in B. carinata are limited to only a few g enotypes. The aim of this study was to evaluate the in vitro culture respon se and shoot regeneration in a collection of B. carinata accessions to identify promising genotypes with high shoot regeneration for genetic transformation experiments. Cotyledonary explants from 51 genotypes were cultured in vitro and callus formation and swelling as well as the mode of shoot regeneration evaluated. A highly posi- tive response to in vitro culture, i.e. callus formation or swelling, was observed in all the genotypes tested. Tissue blackening occurred only in eleven genotypes. Parameters like callus formation and swelling and number of shoots per explant were highly variable among genotypes. Fourteen genotypes regenerated only via callus formation, whereas only one regenera ted only via swelling. Most genotyp es showed a higher p ercentage of callus formation than swelling. The average number of shoots regenerating per explant among genotypes was the most variable factor measured. Six genotypes regenerated more than 6 shoots per explant via callus phase. These genotyp es have been identified as having a high regeneration potential and can be used in genetic transformation via Agrobacterium. Keywords: Mustard, Tissue Culture, Genotype, Cotyledons, Swelling, Callu s 1. Introduction Rapeseed (Brassica napus L.) is the third most important source of vegetable oil in the world with 31 million cul- tivated hectares in the year 2009 (FAOSTAT, 2011). Modification of the fatty acid composition is currently an important objective of plant breeding of this crop [1] and there is considerable commercial interest in the devel- opment of high erucic acid and/or low glucosinolates lines targeted toward industrial end-use and in the de- velopment of low (or zero) erucic acid, low linoleic and high oleic lines for food industries. Ethiopian mustard, B. carinata A. Braun (BBCC, 2n = 4x = 34), is a natural alloploid from B. oleracea L. (CC, 2n = 2x = 18) and B. nigra (L.) W.D.J. Koch, (BB, 2n = 2x = 16) which has several agronomical important traits such as nondehiscent siliques and a much more devel- oped and aggressive root system than B. napus. It is re- sistant to a wide range of diseases and pests and is toler- ant to many abiotic stresses [2-4], which makes it a suit- able candidate as a winter crop in the Mediterranean countries. Plant transformation systems have been developed for many economically important species of the genus Brassica such as B. napu s [5], B. oleracea [6], B. juncea [7], B. rapa [8], B. nigra [9] and B. carinata [10]. This technology enables us to obtain transgenic plants with modified agronomic traits. Many genetic improvements, such as herbicide tolerance, improved oil quality and production of pharmacological and industrial products, have been achieved by genetic transformation in the Brassica species. For example, B. napus seeds containing high levels of gamma-linoleic acid were obtained by the introduction of δ12-desaturase genes from the fungus Mortierella alpine [11]. In addition, B. napus genotypes were developed for sulfonylurea resistance and bro-  Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 28 moxynil resistance by Blackshaw et al. [12] and Zhong [13] respectively. In B. carinata, Jadhav et al. [14] in- creased the level of erucic acid in the seeds by co-sup- pression and antisense repression of the endogenous FAD2 gene encoding the oleate desaturase FAD2. In vitro regeneration is a key factor in developing an efficient transformation method in plants. In vitro regen- eration in Brassica ssp. is highly genotype-dependent as reported in previous studies for B. napus [15,16], B. juncea [17], B. rapa [18] and B. oleracea [19]. In addi- tion, Dietert et al. [20] compared 6 species of the genus Brassica for callus growth and plant regeneration and reported a high influence of the genotype, with as much inter-cultivar as inter-species differences in the response to the in vitro culture. However, the available informa- tion for the genotype variability for in vitro culture and shoot regeneration in B. carinata is limited to a small number of genotypes. This genotype-dependence of the in vitro culture is a limiting factor for the application of genetic engineering to a wide number of genotypes. For that reason, it is important to identify highly regenerant genotypes that can be used in transformation via Agro- bacterium tumefaciens. This study aims to evaluate the in vitro culture re- sponse, i.e. callus formation and swelling, and the mode of shoot regeneration of a collection of B. carinata ac- cessions to identify promising genotypes with high re- generation potential. 2. Materials and Methods 2.1. Plant Material Fifty-one lines of B. carinata supplied by the Regional Plant Introduction Station (Iowa State University, Iowa, U.S.) (Table 1) were evaluated for in vitro culture and shoot regeneration response. For comparison, two lines were used as controls; a B. carinata DH line (BC71), which showed a good response to microspore culture [21] and a B. oleracea DH line (AG1012) provided by the Department of Crop Genetics (John Innes Centre, Nor- wich, UK) which was selected for its high regeneration and transformation potential [22]. 2.2. In vitro Culture Seeds were surface sterilized with 100% ethanol for 2 min and with a 15% sodium hypochlorite solution con- taining 0.1% Tween-20 for 15 min. Then, seeds were washed 3 times with sterile distilled water and air-dried in flow hood chamber. Seeds were germinated in 15 x 90 mm Petri dishes containing 25 ml of germination me- dium, which consisted of 4.3 g/l MS salts [23] plus 3% sucrose and 0.8% phytoagar at pH 5.8. After autoclaving, Table 1. List of Brassica. carinata genotypes used in the experiment supplied by the Regional Plant Introduction Station (Iowa State University, Iowa, U.S.). Genotypes are ordered by accession number. Origin Code number Accession number Country State Plant name 1 193459 Ethiopia Shewa Unknown 2 193460 Ethiopia Harer NU 51639 3 193467 Ethiopia Shewa NU 51640 4 193759 Ethiopia Shewa Unknown 5 193760 Ethiopia Shewa NU 51642 6 193959 Ethiopia Shewa NU 51643 7 194251 Ethiopia Kefa NU 51645 8 194252 Ethiopia Kefa NU 51646 9 194253 Ethiopia Kefa NU 51647 10 194254 Ethiopia Kefa NU 51648 11 194255 Ethiopia Kefa NU 51649 12 194900 Ethiopia Gonder NU 51651 13 194901 Ethiopia Gonder Unknown 14 194903 Ethiopia Gonder NU 51653 15 194904 Ethiopia Gonder Unknown 16 195552 Ethiopia Welo NU 40525 17 195923 Ethiopia Welo NU 51657 18 196836 Ethiopia Welega Unknown 19 197402 Ethiopia Unknown Unknown 20 197403 Ethiopia Unknown NU 51660 21 199947 Ethiopia Unknown NU 51661 22 199949 Ethiopia Gonder NU 16543 23 199950 Ethiopia Kefa NU 51663 24 209023 Puerto Rico Unknown Unknown 25 226545 Ethiopia Shewa NU 51664 26 231046 Ethiopia Shewa Unknown 27 273636 Ethiopia Harer NU 51666 28 273640 Ethiopia Shewa Gomenzer 29 274283 Ethiopia Unknown NU 51668 30 280230 Ethiopia Unknown NU 51669 31 331377 Ethiopia Unknown Unknown 32 331378 Ethiopia Unknown Unknown 33 360879 Sweden Unknown 68-5702-1 34 360880 Sweden Unknown 68-5702-4 35 360881 Sweden Unknown 68-5702-6 36 360882 Sweden Unknown 68-5702-10 37 360883 Sweden Unknown 68-5702-16 38 360885 Sweden Unknown 68-5702-12 PLT 1 39 360886 Sweden Unknown 68-5702-15 PLT 9 S 40 360887 Sweden Unknown 68-5702-16 PLT 5 S 41 390133 Pakistan Unknown P-1 42 390134 Pakistan Unknown P-58 43 596535 Spain Cordoba BC-815-2 44 596536 Spain Cordoba BC-876-2 45 596537 Spain Cordoba BC-738-5 46 596538 Spain Cordoba BC-834-2 47 596539 Spain Cordoba BC-831-2 48 597822 Sweden Unknown 85-0572-69 49 633075 Tanzania Unknown Swahili 50 633076 Kenya Unknown WIR 4325 53 633080 Zambia Unknown BRA 1028/79 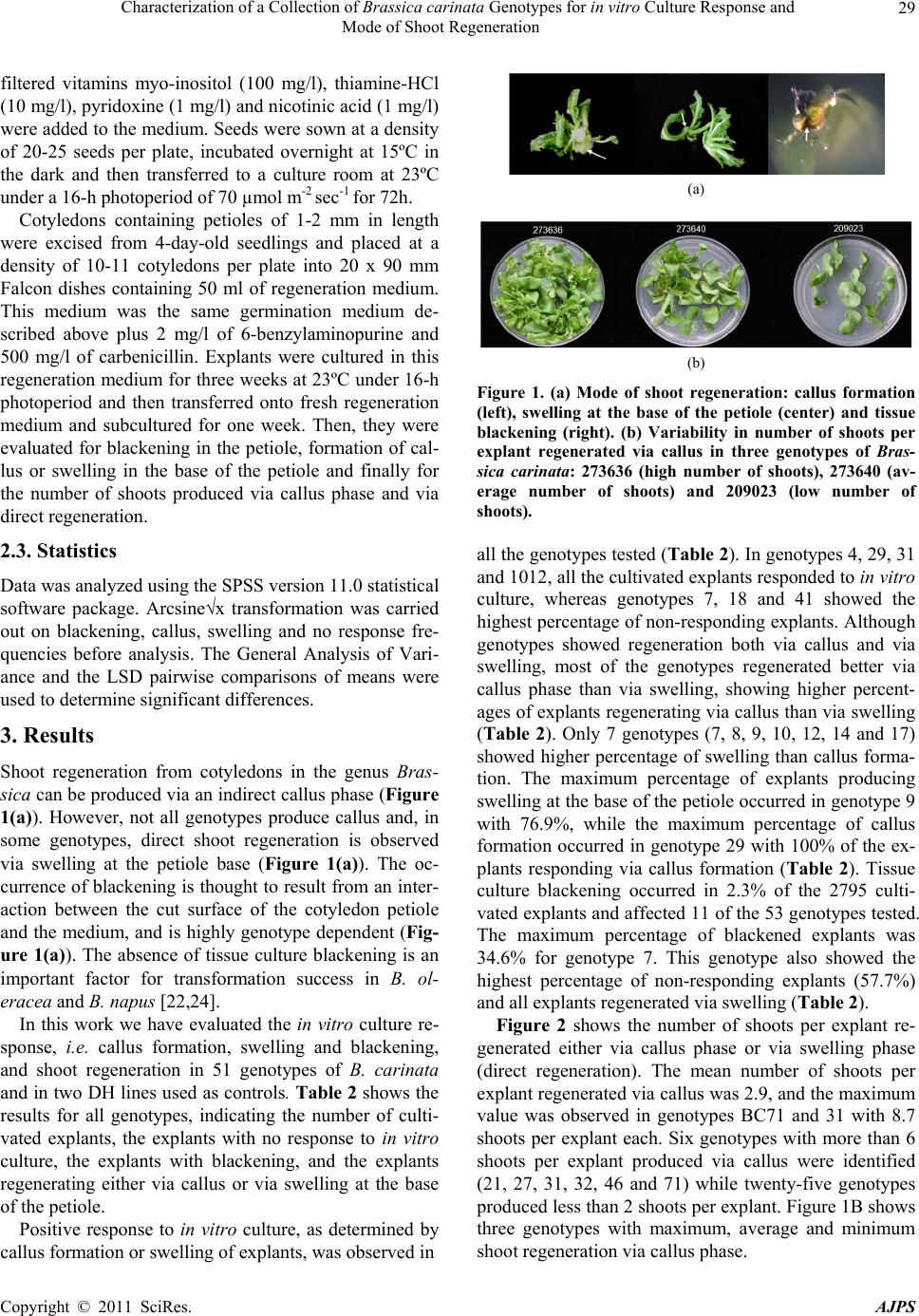 Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 29 filtered vitamins myo-inositol (100 mg/l), thiamine-HCl (10 mg/l), pyridoxine (1 mg/l) and nicotinic acid (1 mg/l) were added to the medium. Seeds were sown at a density of 20-25 seeds per plate, incubated overnight at 15ºC in the dark and then transferred to a culture room at 23ºC under a 16-h photoperiod of 70 µmol m-2 sec-1 for 72h. Cotyledons containing petioles of 1-2 mm in length were excised from 4-day-old seedlings and placed at a density of 10-11 cotyledons per plate into 20 x 90 mm Falcon dishes containing 50 ml of regeneration medium. This medium was the same germination medium de- scribed above plus 2 mg/l of 6-benzylaminopurine and 500 mg/l of carbenicillin. Explants were cultured in this regeneration medium for three weeks at 23ºC under 16-h photoperiod and then transferred onto fresh regeneration medium and subcultured for one week. Then, they were evaluated for blackening in the petiole, formation of cal- lus or swelling in the base of the petiole and finally for the number of shoots produced via callus phase and via direct regeneration. 2.3. Statistics Data was analyzed using the SPSS version 11.0 statistical software package. Arcsine√x transformation was carried out on blackening, callus, swelling and no response fre- quencies before analysis. The General Analysis of Vari- ance and the LSD pairwise comparisons of means were used to determine significant differences. 3. Results Shoot regeneration from cotyledons in the genus Bras- sica can be produced via an indirect callus phase (Figure 1(a)). However, not all genotypes produce callus and, in some genotypes, direct shoot regeneration is observed via swelling at the petiole base (Figure 1(a)). The oc- currence of blackening is thought to result from an inter- action between the cut surface of the cotyledon petiole and the medium, and is highly genotype dependent (Fig- ure 1(a)). The absence of tissue culture blackening is an important factor for transformation success in B. ol- eracea and B. napus [22,24]. In this work we have evaluated the in vitro culture re- sponse, i.e. callus formation, swelling and blackening, and shoot regeneration in 51 genotypes of B. carinata and in two DH lines used as controls. Table 2 shows the results for all genotypes, indicating the number of culti- vated explants, the explants with no response to in vitro culture, the explants with blackening, and the explants regenerating either via callus or via swelling at the base of the petiole. Positive response to in vitro culture, as determined by callus formation or swelling of explants, was observed in (a) (b) Figure 1. (a) Mode of shoot regeneration: callus formation (left), swelling at the base of the petiole (center) and tissue blackening (right). (b) Variability in number of shoots per explant regenerated via callus in three genotypes of Bras- sica carinata: 273636 (high number of shoots), 273640 (av- erage number of shoots) and 209023 (low number of shoots). all the genotypes tested (Table 2). In genotypes 4, 29, 31 and 1012, all the cultivated explants responded to in vitro culture, whereas genotypes 7, 18 and 41 showed the highest percentage of non-responding explants. Although genotypes showed regeneration both via callus and via swelling, most of the genotypes regenerated better via callus phase than via swelling, showing higher percent- ages of explants regenerating via callus than via swelling (Table 2). Only 7 genotypes (7, 8, 9, 10, 12, 14 and 17) showed higher percentage of swelling than callus forma- tion. The maximum percentage of explants producing swelling at the base of the petiole occurred in genotype 9 with 76.9%, while the maximum percentage of callus formation occurred in genotype 29 with 100% of the ex- plants responding via callus formation (Table 2). Tissue culture blackening occurred in 2.3% of the 2795 culti- vated explants and affected 11 of the 53 genotypes tested. The maximum percentage of blackened explants was 34.6% for genotype 7. This genotype also showed the highest percentage of non-responding explants (57.7%) and all explants regenerated via swelling (Table 2). Figure 2 shows the number of shoots per explant re- generated either via callus phase or via swelling phase (direct regeneration). The mean number of shoots per explant regenerated via callus was 2.9, and the maximum value was observed in genotypes BC71 and 31 with 8.7 shoots per explant each. Six genotypes with more than 6 shoots per explant produced via callus were identified (21, 27, 31, 32, 46 and 71) while twenty-five genotypes produced less than 2 shoots per explant. Figure 1B shows three genotypes with maximum, average and minimum shoot regeneration via callus phase.  Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 30 Table 2. In vitro culture response of cotyledonary explants from a collection of Brassica carinata and two DH lines: BC71 (Brassica carinata) and AG1012 (Brassica oleracea). No response Blackening Callus Swelling Code number Accession numberNumber of explantsNo. (%) No. (%) No. (%) No. (%) 1 193459 66 2 3.0 0 0 55 83.3 9 13.6 2 193460 47 5 10.6 0 0 36 76.6 6 12.8 3 193467 63 10 15.9 0 0 35 55.6 18 28.6 4 193759 50 0 0 0 0 39 78.0 11 22.0 5 193760 64 3 4.7 12 18.8 61 95.3 0 0 6 193959 46 3 6.5 0 0 38 82.6 5 10.9 7 194251 26 15 57.7 9 34.6 0 0 11 42.3 8 194252 28 4 14.3 5 17.9 11 39.3 13 46.4 9 194253 39 7 17.9 7 17.9 2 5.1 30 76.9 10 194254 49 10 20.4 0 0 13 26.5 26 53.1 11 194255 40 1 2.5 0 0 37 92.5 2 5.0 12 194900 38 4 10.5 0 0 7 18.4 27 71.1 13 194901 52 3 5.8 0 0 46 88.5 3 5.8 14 194903 41 7 17.1 0 0 13 31.7 21 51.2 15 194904 47 15 31.9 1 2.1 22 46.8 10 21.3 16 195552 57 12 21.1 0 0 42 73.7 3 5.3 17 195923 27 5 18.5 0 0 5 18.5 17 63.0 18 196836 45 25 55.6 0 0 20 44.4 0 0 19 197402 50 3 6.0 0 0 44 88.0 3 6.0 20 197403 39 2 5.1 0 0 36 92.3 1 2.6 21 199947 48 6 12.5 0 0 42 87.5 0 0 22 199949 29 8 27.6 0 0 19 65.5 2 6.9 23 199950 38 1 2.6 1 2.6 29 76.3 8 21.1 24 209023 65 11 16.9 0 0 48 73.8 6 9.2 25 226545 29 3 10.3 4 13.8 24 82.8 2 6.9 26 231046 33 5 15.2 0 0 28 84.8 0 0 27 273636 55 2 3.6 0 0 53 96.4 0 0 28 273640 51 5 9.8 0 0 46 90.2 0 0 29 274283 61 0 0 0 0 61 100.0 0 0 30 280230 60 2 3.3 4 6.7 58 96.7 0 0 31 331377 58 0 0 0 0 53 91.4 5 8.6 32 331378 63 5 7.9 0 0 49 77.8 9 14.3 33 360879 56 7 12.5 0 0 47 83.9 2 3.6 34 360880 59 6 10.2 0 0 49 83.1 4 6.8 35 360881 50 12 24.0 0 0 36 72.0 2 4.0 36 360882 65 6 9.2 0 0 55 84.6 4 6.2 37 360883 58 17 29.3 0 0 41 70.7 0 0 38 360885 54 2 3.7 4 7.4 46 85.2 6 11.1 39 360886 63 12 19.0 0 0 50 79.4 1 1.6 40 360887 66 6 9.1 0 0 60 90.9 0 0 41 390133 65 23 35.4 0 0 42 64.6 0 0 42 390134 66 2 3.0 0 0 52 78.8 12 18.2 43 596535 33 5 15.2 0 0 25 75.8 3 9.1 44 596536 39 3 7.7 9 23.1 36 92.3 0 0 45 596537 48 15 31.3 0 0 33 68.8 0 0 46 596538 66 5 7.6 0 0 59 89.4 2 3.0 47 596539 64 8 12.5 0 0 53 82.8 3 4.7 48 597822 76 6 7.9 0 0 63 82.9 7 9.2 49 633075 53 5 9.4 0 0 42 79.2 6 11.3 50 633076 44 4 9.1 0 0 36 81.8 4 9.1 53 633080 95 15 15.8 0 0 74 77.9 6 6.3 71 BC71 44 5 11.4 0 0 39 88.6 0 0 1012 AG1012 127 0 0 3 2.4 100 78.7 27 21.3  Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 31 Figure 2 Number of shoots per explant regenerated via cal- lus or swelling from cotyledonary explants collected from 51 lines of Brassica carinata. Two DH lines, BC71 (Brassicca carinata) and AG1012 (Brassica oleracea) were included for comparison. The mean number of shoots regenerated via swelling was 1.7, and the genotype producing the maximum number of shoots per explant was also genotype 31 with 10.4 shoots per explant. Only three genotypes (20, 13 and 31) produced more than 6 shoots per explant by di- rect regeneration while thirty-seven genotypes produced less than 2 shoots per explant. In thirty-two genotypes, shoots were produced both by callus and swelling phase, whereas seventeen genotypes regenerated shoots only by callus phase and in genotypes 7, 9 and 17 all the shoots were regenerated by direct re- generation. General analysis of variance (Table 3) revealed that there were highly significant differences among geno- types for the absence of response to the in vitro culture, the mode of response, i.e. percentage of callus or per- centage of swelling, the occurrence of blackening, and the number of shoots produced either via callus or via direct regeneration. 4. Discussion Shoot regeneration in B. carinata can occur directly from explant tissue as well as indirectly from callus that pro- liferate from the cut edge of the explants. In many geno- types, direct shoot regeneration has been described as less genotype dependent [25] and regenerants show more genetic stability [26], whereas callus phase is more asso- ciated with somaclonal variation. However, direct shoot regeneration has limitations in being used for DNA transfer as totipotent cells are less accessible to Agro- bacterium during cocultivation [27]. In fact, dedifferen- tiation of explant cells into callus was necessary for the efficient transformation of B. carinata [10]. Therefore, the identification of genotypes with high regeneration potential that regenerate mostly via callus formation can largely influence the recovery of transgenic plants. Spar- row et al. [22] found three phenotypic markers highly influencing the transformation efficiency in B. oleracea. These are: susceptibility to A. tumefaciens [28], shoot regeneration potential and the mode of shoot regenera Table 3. Analysis of variance for in vitro culture response and shoot regeneration in a collection of Brassica carinata accessions. Values expressed as a percentage were trans- formed using the arcsine√x function for the analysis of va- riance. Parameter MS df F-test No response (%) 0.1298 52 3.48*** Blackening (%) 0.0476 52 3.14*** Callus (%) 0.1865 52 4.61*** Swelling (%) 0.1617 52 4.90*** Shoots per explant from callus 19.742 51 10.00*** Shoots per explant from swelling15.596 38 2.16** Significance probability levels: **1%, ***0.1%. MS; Mean Square, df; degree of freedom.  Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 32 tion. They also found that the absence of blackening tis- sue associated with callus formation is critical for trans- formation success. We have applied these criteria to evaluate a collection of 51 genotypes of B. carinata for in vitro culture and shoot regeneration potential, includ- ing the mode of shoot regeneration. We have found a highly positive response to in vitro culture, i.e. callus formation and swelling, in all the genotypes tested in this study. Other studies have previously confirmed the re- sponsiveness of B. carinata seedling explants to tissue culture [29-31]. For both, callus formation and swelling, there were significant differences among genotypes. In our study, the number of genotypes that proliferate via callus formation was much higher than those via swelling. In addition, the percentage of callus formation was higher than swelling in all the genotypes, except in 7 genotypes (7, 8, 9, 10, 12, 14 and 17) in which the per- centage of swelling formation was higher. Callus formation at the base of the petiole has been demonstrated to be an interesting trait in obtaining a great number of shoots per explant. Sparrow et al. [22] observed that shoot regeneration via the callus phase resulted in well established and morphologically defined shoots that were easy to isolate and propagate. Results reported here showed that shoot production was very variable among genotypes. This result agreed with stud- ies in Brassica spp., which also reported that shoot pro- duction was highly variable among different genotypes, indicating that the regeneration ability is strongly influ- enced by genotype [15-20]. Zhang et al. [18] reported a maximum of 10.7 shoots per explant in a cultivar of Chinese cabbage. In our study the maximum number of shoots per explant was 8.7 and 10.4, regenerated respec- tively from callus and direct regeneration, both by geno- type 31. We have found six genotypes (21, 27, 31, 32, 46 and 71) producing more than 6 shoots per explant via callus formation, although three of them (31, 32 and 46) also produced shoots via swelling. These six genotypes had a percentage of callus formation between 77.8% and 96.4% and no blackening tissue at the base of the petiole was observed. These six genotypes produced an average of 7.1 shoots per explant via callus. This average is 2.4 times higher than the 2.9 shoots of average produced via callus by all genotypes. This high-frequency of regenera- tion from callus might be of great importance in regen- eration of transgenic plants. Mukhopadyay et al. [27] reported that the transformation frequency of B. campes- tris is strongly influenced by the mode of shoot regenera- tion. In addition, Babic et al. [10] reported that efficient regeneration from callus was responsible for the high frequency of transformation in B. carinata. The occurrence of tissue blackening resulted from an interaction between the cut surface of the cotyledon peti- ole and the medium. Although blackening does not pre- vent the formation of shoots, we have observed a nega- tive correlation between the percentage of blackening and the number of shoots per explant. In addition, geno- types with no blackening produce more shoots than those presenting blackening. Therefore, the absence of tissue blackening influences the successful regeneration of shoots and this could be a critical factor to regenerate transgenic plants. In conclusion, in this work we have characterized the tissue culture response and the mode of regeneration of a collection of B. carinata. The mode of shoot regeneration is important in the selection of genotypes with a high regeneration potential and that regenerate mainly from callus. Six genotypes (21, 27, 31, 32, 46 and 71) have been identified as having a high regeneration potential. They produce the highest number of shoots per explant via callus formation and do not present tissue blackening at the base of the petiole. We are currently incorporating these 6 genotypes in transformation experiments to de- termine their susceptibility to A. tu mefac iens. The results of these experiments should allow us to develop a trans- formation program using genotypes highly susceptible to A. tumefaciens and with a high level of regeneration via callus. 5. Acknowledgments The authors acknowledge funding by the Spanish Comi- sión Interministerial de Ciencia y Tecnología (C.I.C.Y.T.), project AGL2004-03361-C02-2 and to the Regional Plant Introduction Station (Iowa State University, Iowa, U.S.) for supplying seeds. Javier Gil-Humanes acknowledges the CSIC for a pre-doctoral I3P fellowship and to Dr. Penelope Sparrow from the Crop Genetics Department of John Innes Center (UK) for the critical reading of the manuscript. Technical assistance by Azahara Vida is also acknowledged. REFERENCES [1] W. Friedt and W. Luhs, “Recent Developments and Per- spectives of Industrial Rapeseed Breeding,” Fett-Lipid, Vol. 100, No. 6, June 1998, pp. 219-226. doi:10.1002/(SICI)1521-4133(199806)100:6<219::AID-L IPI219>3.0.CO;2-Y [2] R. K. Katiyar, G. Saran and G. Giri, “Evaluation of Bras- sica carinata as a New Oilseed Crop in India,” Experi- mental Agriculture, Vol. 22, No. 1, January 1986, pp. 67-70. doi:10.1017/S0014479700014058 [3] A. Getinet, G. Rakow and R. K. Downey, “Agronomic Performance and Seed Quality of Ethiopian Mustard in Saskatchewan,” Canadian Journal of Plant Science, Vol.  Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 33 76, No. 3, July 1996, pp. 387-392. [4] R. S. Malik, “Prospects for Brassica carinata as an Oil- seed Crop in India,” Experimental Agriculture, Vol. 26, No. 1, January 1990, pp. 125-129. doi:10.1017/S0014479700015465 [5] M. M. Moloney, J. M. Walker and K. K. Sharma, “High Efficiency Transformation of Brassica napus Using Agrobacterium Vectors,” Plant Cell Reports, Vol. 8, No. 4, July 1989, pp. 238-242. doi:10.1007/BF00778542 [6] M. Deblock, D. Debrouwer and P. Tenning, “Transfor- mation of Brassica napus and Brassica oleracea Using Agrobacterium tumefaciens and the Expression of the bar and neo Genes in the Transgenic Plants,” Plant Physiol- ogy, Vol. 91, No. 2, October 1989, pp. 694-701. doi:10.1104/pp.91.2.694 [7] D. G. Barfield and E. C. Pua, “Gene Transfer in Plants of Brassica juncea using Agrobacterium tumefaciens Medi- ated Transformation,” Plant Cell Reports, Vol. 10, No. 6-7, September 1991, pp. 308-314. doi:10.1007/BF00193148 [8] S. E. Radke, J. C. Turner and D. Facciotti, “Transforma- tion and Regeneration of Brassica rapa Using Agrobac- terium tumefaciens,” Plant Cell Reports, Vol. 11, No. 10, September 1992, pp. 499-505. doi:10.1007/BF00236265 [9] V. Gupta, G. L. Sita, M. S. Shaila and V. Jagannathan, “Genetic Transformation of Brassica nigra by Agrobac- terium Based Vector and Direct Plasmid Uptake,” Plant Cell Reports, Vol. 12, No. 7-8, May 1993, pp. 418-421. [10] V. Babic, R. S. Datla, G. J. Scoles and W. A. Keller, “Development of an Efficient Agrobacterium-Mediated Transformation System for Brassica carinata,” Plant Cell Reports, Vol. 17, January 1998, pp. 183-188. doi:10.1007/s002990050375 [11] J. W. Liu, S. DeMichele, M. Bergana, E. Bobik, C. Hastilow, L. T. Chuang, P. Mukerji and Y. S. Huang, “Characterization of Oil Exhibiting High Gamma-Lino- lenic Acid from a Genetically Transformed Canola Strain,” Journal of the American Oil Chemists Society, Vol. 78, No. 5, May 2001, pp. 489-493. doi:10.1007/s11746-001-0291-2 [12] R. E. Blackshaw, D. Kanashiro, M. M. Moloney and W. L. Crosby, “Growth, Yield and Quality of Canola Ex- pressing Resistance to Acetolactate Synthase Inhibiting Herbicides,” Canadian Journal of Plant Science, Vol. 74, No. 4, October 1994, pp. 745-751. [13] R. Z. Zhong, F. Zhu, Y. L. Liu, S. G. Li, L. Y. Kang and P. Luo, “Oilseed Rape Transformation and the Establish- ment of a Bromoxynil-Resistant Transgenic Oilseed Rape,” Acta Botanica Sinica, Vol. 39, September 1997, pp. 22-27. [14] A. Jadhav, V. Katavic, E. F. Marillia, E. M. Giblin, D. L. Barton, A. Kumar, C. Sonntag, V. Babic, W. A. Keller and D. C. Taylor, “Increased Levels of Erucic Acid in Brassica carinata by Co-suppression and Antisense Re- pression of the Endogenous FAD2 Gene,” Metabolic En- gineering, Vol. 7, No. 3, May 2005, pp. 215-220. doi:10.1016/j.ymben.2005.02.003 [15] Y. Ono, Y. Takahata and N. Kaizuma, “Effect of Geno- type on Shoot Regeneration from Cotyledonary Explants of Rapeseed (Brassica napus L.),” Plant Cell Reports, Vol. 14, No. 1, November 1994, pp. 13-17. doi:10.1007/BF00233290 [16] S. K. Phogat, P. K. Burma and D. Pental, “High Fre- quency Regeneration of Brassica napus Varieties and Genetic Transformation of Stocks Containing Fertility Restorer Genes for Two Cytoplasmic Male Sterility Sys- tems,” Journal of Plant Biochemistry and Biotechnology, Vol. 9, No. 2, July 2000, pp. 73-79. [17] D. Pental, A. K. Pradhan, Y. S. Sodhi and A. Muk- hopadhyay, “Variation amongst Brassica juncea Culti- vars for Regeneration from Hypocotyl Explants and Op- timization of Conditions for Agrobacterium Mediated Genetic Transformation,” Plant Cell Reports, Vol. 12, No. 7-8, May 1993, pp. 462-467. doi:10.1007/BF00234713 [18] F. L. Zhang, Y. Takahata and J. B. Xu, “Medium and Genotype Factors Influencing Shoot Regeneration from Cotyledonary Explants of Chinese Cabbage (Brassica campestris L. ssp. pekinensis),” Plant Cell Reports, Vol. 17, No. 10, July 1998, pp. 780-786. doi:10.1007/s002990050482 [19] P. A. C. Sparrow, T. M. Townsend, C. L. Morgan, P. J. Dale, A. E. Arthur and J. A. Irwin, “Genetic Analysis of in vitro Shoot Regeneration from Cotyledonary Petioles of Brassica oleracea,” Theoretical and Applied Genetics, Vol. 108, No. 7, May 2004, pp. 1249-1255. doi:10.1007/s00122-003-1539-y [20] M. F. Dietert, S. A. Barron and O. C. Yoder, “Effects of Genotype on in vitro Culture in the Genus Brassica,” Plant Science Letters, Vol. 26, No. 2-3, August 1982, pp. 233-240. doi:10.1016/0304-4211(82)90096-7 [21] F. Barro and A. Martín, “Response of Different Geno- types of Brassica carinata to Microspore Culture,” Plant Breeding, Vol. 118, No. 1, March 1999, pp. 79-81. doi:10.1046/j.1439-0523.1999.118001079.x [22] P. A. C. Sparrow, P. J. Dale and J. A. Irwin, “The Use of Phenotypic Markers to Identify Brassica oleracea Geno- types for Routine High-Throughput Agrobacterium-me- diated transformation,” Plant Cell Reports, Vol. 23, No. 1-2, August 2004, pp. 64-70. doi:10.1007/s00299-004-0818-7 [23] T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cul- tures,” Physiologia Plantarum, Vol. 15, No. 3, July 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x [24] P. A. C. Sparrow, J. W. Snape, P. J. Dale and J. A. Irwin, “The Rapid Identification of B. napus Genotypes, for High-Throughput Transformation, Using Phenotypic Tis- sue Culture Markers,” Acta Horticulture, Vol. 706, April 2006, pp. 239-247. [25] O. Toldi, G. Gyulai, J. Kiss, I. A. Tamas and E. Balazs, “ Antiauxin Enhanced Microshoot Initiation and Plant Regeneration from Epicotyl-Originated Thin-Layer Ex-  Characterization of a Collection of Brassica carinata Genotypes for in vitro Culture Response and Mode of Shoot Regeneration Copyright © 2011 SciRes. AJPS 34 plants of sugarbeet (Beta vulgaris L.),” Plant Cell Re- ports, Vol. 15, No. 11, August 1996, pp. 851-854. doi:10.1007/BF00233155 [26] C. Detrez, R. S. Sangwan and B. S. Sangwannorreel, “Phenotypic and Karyotypic Status of Beta vulgaris Plants Regenerated from Direct Organogenesis in Petiole Culture,” Theoretical and Applied Genetics, Vol. 77, No. 4, April 1989, pp. 462-468. doi:10.1007/BF00274264 [27] A. Mukhopadhyay, N. Arumugam, P. B. A. Nandakumar, A. K. Pradhan, V. Gupta and D. Pental, “Agrobacterium Mediated Genetic Transformation of Oilseed Brassica campestris Transformation Frequency is Strongly Influ- enced by the Mode of Shoot Regeneration,” Plant Cell Reports, Vol. 11, No. 10, September 1992, pp. 506-513. doi:10.1007/BF00236266 [28] P. A. C. Sparrow, T. M. Townsend, A. E. Arthur, P. J. Dale and J. A. Irwin, “Genetic Analysis of Agrobacterium Tumefaciens Susceptibility in Brassica oleracea,” Theo- retical and Applied Genetics, Vol. 108, No. 4, February 2004, pp. 644-650. doi:10.1007/s00122-003-1473-z [29] S. B. Narasimhulu and V. L. Chopra, “Plant Regeneration from Callus Cultures of Brassica carinata A. Br. and Its Implications to Improvement of Oil Seed Brassicas,” Plant Breeding, Vol. 99, No. 1, September 1987, pp. 49-55. doi:10.1111/j.1439-0523.1987.tb01149.x [30] S. B. Narasimhulu and V. L. Chopra, “Species Specific Shoot Regeneration Response of Cotyledonary Explants of Brassic as,” Plant Cell Reports, Vol. 7, No. 2, March 1988, pp. 104-106. doi:10.1007/BF00270115 [31] M. Z. Yang, S. R. Jia and E. C. Pua, “High Frequency of Plant Regeneration from Hypocotyl Explants of Brassica carinata A. Br.,” Plant Cell Tissue and Organ Culture, Vol. 24, No. 2, February 1991, pp. 79-82. doi:10.1007/BF00039734 |

