 Pharmacology & Pharmacy, 2013, 4, 679-683 Published Online December 2013 (http://www.scirp.org/journal/pp) http://dx.doi.org/10.4236/pp.2013.49094 Open Access PP 679 Evolution of Biochemical Effects of Byetta® in Type 2 Diabetics with Cardiovascular Risk Alfonso López Ruiz1, María Ángeles Ibáñez Gil2, Pedro Pujante Alarcón3, Alicia Hernández Torres4, Ana Belen Hernández Cascales3, María Dolores Hellín Gil3 1Pharmaceutical Care Research at the University of Granada, Granada, Spain; 2Health Center Mariano Yago, Yecla, Murcia, Spain; 3Endocrinology and Nutrition Service of the Hospital University Virgen de la Arrixaca, Murcia, Spain; 4Infectious Internal Medicine of the Hospital University Virgen de la Arrixaca, Murcia, Spain. Email: lablilly@gmail.com Received September 8th, 2013; revised October 18th, 2013; accepted October 28th, 2013 Copyright © 2013 Alfonso López Ruiz et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The objective of this study was to examine longitudinally the effects of exenatide on different physical and biochemical markers, evaluated in adult type 2 diabetic patients with cardiovascular risk. Data were recorded from 10 patients who attended the outpatient primary care health center Mariano Iago Yecla, Murcia province, Spain in the period of Decem- ber 2009 to October 2011 and who were treated with Byetta®. Differences were statistically significant (p < 0.05) in HbA1c from the third month of treatment, and trends of decrease in body weight from the third week of treatment. There was a significant and better glycemic control. Overall effect was interpreted as a sensitizer drug of the parameters evaluated. Randomized studies are recommended with a minimum follow-up of 2 years, to see if the results are main- tained over time. Keywords: Diabetes Mellitus Type 2; Exenatide; Cardiovascular Risk 1. Introduction Exenatide (Byetta®, Eli Lilly) is an incretin mimetic, and a synthetic peptide (amide acid peptide of 39 amino acids) which is currently approved in several countries world- wide (marketed since 2006 in the European Union) for use as combination therapy with sulfonylureas and/or metformin in patients with type 2 diabetes mellitus who have not been achieved adequate glycemic control with oral antidiabetic earlier [1,2]. Its therapeutic action is related primarily to reduce both postprandial glucose and fasting, before the consideration of the following four aspects [3-6]: 1) Increased insulin secretion by β cells independently of glucose (reduced insulin release with decreasing blood glucose). 2) Inhibition of glucagon secretion and hepatic glu- coneogenesis as well. 3) Slowing of gastric emptying and, consequently, the transition to the movement of glucose intake. 4) Increased satiety. Exenatide is indicated as an alternative to insulin therapy or other measures of second line therapy in pa- tients with obese type 2 diabetes mellitus in combination with sulfonylurea, metformin or pioglitazone when these options have not achieved adequate glycemic results in a maximum dose [1,7-9]. Exenatide is available as a pre-filled pen of 5 and 10 mg subcutaneous injection administration. We recom- mend starting the treatment administered 5 mg/2 times/ day for 1 month, to increase tolerance. The application is recommended within 60 minutes prior to breakfast and dinner, or two main meals, separated by a minimum of 6 hours, never after a meal. If necessary, to improve gly- cemic control, the dose may be increased to 10 mg/2 times/day, following the above recommendations of ad- ministration [1,2]. The Andalusian Centre of Drug Information (CADIMA) [2] and Campoamor [3], considered high treatment ex- enatide daily and annual cost (considering the two doses) compared with other treatment options and oral insulin (excluding with liraglutide has a higher cost and annual daily exenatide). In 2009, he referred a daily cost of 4.47 €, maximum value followed by vildagliptin (2.25 €), in-  Evolution of Biochemical Effects of Byetta® in Type 2 Diabetics with Cardiovascular Risk 680 sulin detemir ornate feathers (2.09 €), insulin glargine cartridges (2.05 €), insulin glargine ornate feathers (2.05 €), pioglitazone (2.03 €) and sitagliptin (2.00 €). For approved indications, currently controversial in- formation on the effectiveness of exenatide and bio- chemical markers is associated with physical, being in constant research, the main reason for this study. Despite this, there is some consensus that the administration is associated with a significant reduction in glycosylated hemoglobin levels (HbA1c) and body weight [1,2]. It is also very limited availability of scientific informa- tion about its use in obese patients and in combination with other oral agents such as glitazones, as well as on mortality and morbidity and association with cardiovas- cular risk factors and liver. It presents a comparative effectiveness not less than insulin. Its use is associated with a high level of with- drawals from treatment due to adverse effects: 8% com- pared with 3% of placebo and 1% insulin [2]. Among the major adverse effects can be mentioned [1,2,10-14]: Nausea (45% - 51%). Vomiting (12% - 14%). Diarrhea (9% - 17%). Hypoglycemic episodes (28% - 36%) in combination with sulfonylureas. Acute pancreatitis (89 cases in the European Union in the period 2006-2007). These effects depend on the continuity of treatment and combination therapy implemented. The truth is that against the perceived benefits of reducing HbA1c and body weight, low risk of hypoglycemia (except in com- bination with a sulfonylurea), low blood pressure and a potential protective effect of β cells, has the following disadvantages: the administration of injections, frequent gastrointestinal side effects, high costs, little experience in treatment, antibody formation and possible interac- tions with other drugs given delayed gastric emptying. Regarding the cardiovascular risk associated with diabe- tes mellitus, there is a current controversy, the effects of exenatide, finding favorable effects [15-21] or on heart rate and blood pressure [15,22,23]. The aim of this study is to contribute to the evolution- ary analysis of the effects of exenatide on physical and biochemical markers evaluated in the specific case of adult type 2 diabetic patients with cardiovascular risk. 2. Materials and Methods Study Design An experimental study was conducted, longitudinal panel, and quantitative. The scientific data were obtained from medical records of 10 patients with type 2 diabetes mel- litus and cardiovascular risk who attended the outpatient primary care health center Mariano Yago in the town of Yecla, Murcia, Spain for the period December 2009 to October 2011. Physical data were collected: weight, height, shape and body mass index (BMI) and biochemical glycosylated hemoglobin (HbA1c). We considered three evolutionary breakpoints: 3, 6 and 12 months. Not all participants were evaluated in the cuts, but that they were established considering a unit of group analysis. Combined treatment with Byetta® was diverse, en- compassing various alternatives and combinations, de- pending on the needs of each patient and medical profes- sional criteria. It included, among others: Januvia 145®, Prevencor 40®, 20® Coropres, Enalapril 20®, Uniket Re- tard®, add 100®, Glucophage 850® and Actos®. In all cases reported, as appropriate: time of ingestion. In the case of Byetta®, all patients started the first month with Byetta® 5 mg and two months later switched to 10 mg, always 2 times/day, maintaining this dose in subse- quent months. The glycosylatedhemoglogina biochemical parameter was evaluated according to the criteria for optimal con- trol of the Spanish Society of Diabetes (SED), where: HbA1c ≤ 7%. The study was conducted based on various measures of physical and biochemical parameters of 10 adult sub- jects with type 2 diabetes mellitus and cardiovascular risk. The sample was prepared in a non-probabilistic, intentional and accidental, according to glycosylated hemoglobin detected in the patient’s analytic and BMI. Inclusion criteria for the preparation of the sample were: be patient with diagnosed type 2 diabetes mellitus, adult at the time of diagnosis and treatment. We excluded cases that did not record a BMI greater than or equal to 30 and type 1 diabetes was based on the discretion of the physician who treated the patient in due course. Considering the number of subjects, we applied the contrast of the Shapiro-Wilk normality, to inquire about the possibility of parametric analysis tools. All physical and biochemical parameters of analysis, were associated with probabilities >0.05, so that was adopted following a normal statistical distribution. We analyzed the existence of statistically significant differences in both the temporal evolution of parameters (initial-final, or initial-3-6 to 12 months, as applicable) and months of treatment, by a factor univariate ANOVA (time of measurement). In cases of biochemical parameters in which such sig- nificant differences were found, deepened trying dis- criminate analysis results according to certain factors, those presented in the overall profile of the participants (except the initial height and weight, used for calculation of initial BMI, and contour). In this case, we applied a Open Access PP 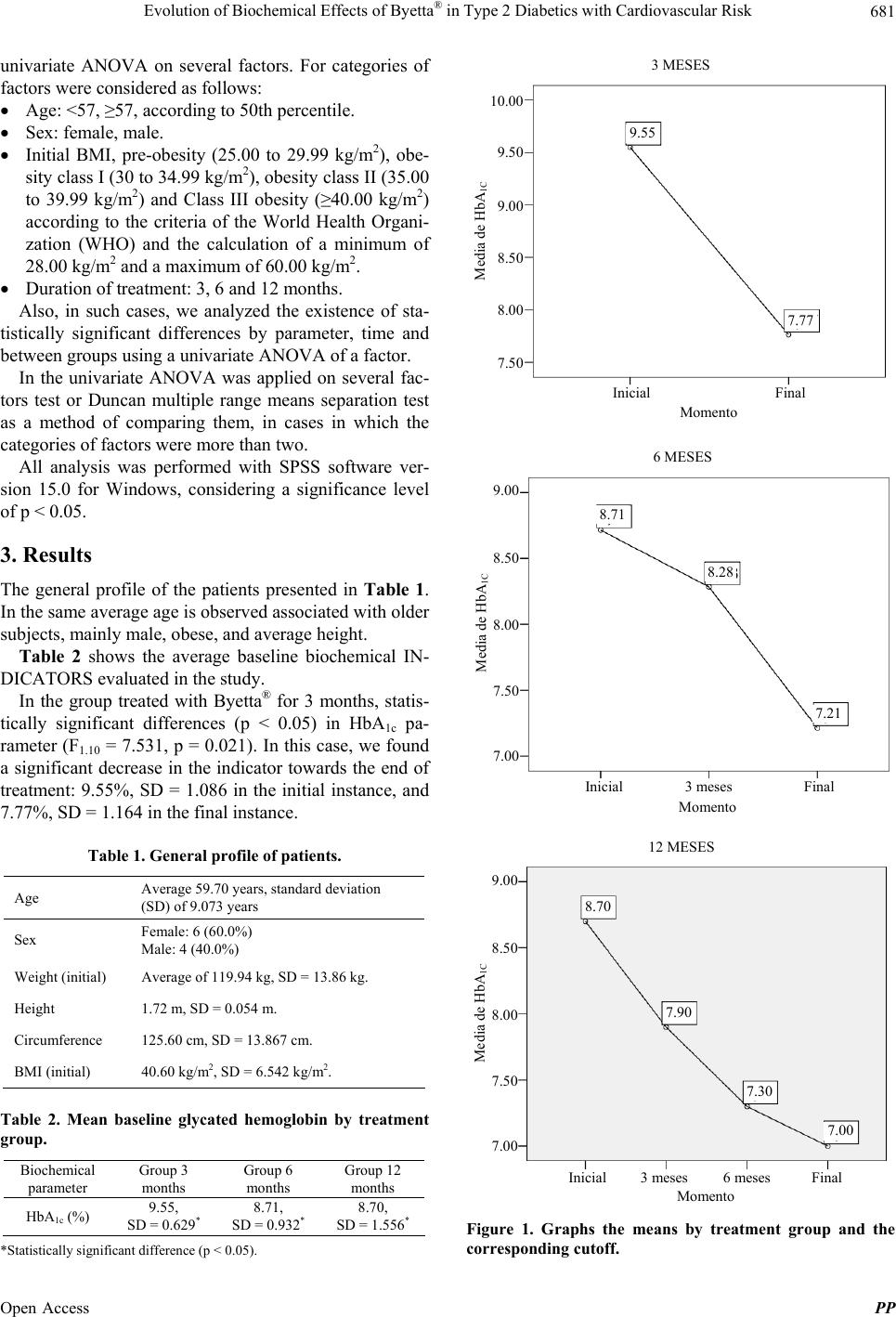 Evolution of Biochemical Effects of Byetta® in Type 2 Diabetics with Cardiovascular Risk 681 univariate ANOVA on several factors. For categories of factors were considered as follows: Age: <57, ≥57, according to 50th percentile. Sex: female, male. Initial BMI, pre-obesity (25.00 to 29.99 kg/m2), obe- sity class I (30 to 34.99 kg/m2), obesity class II (35.00 to 39.99 kg/m2) and Class III obesity (≥40.00 kg/m2) according to the criteria of the World Health Organi- zation (WHO) and the calculation of a minimum of 28.00 kg/m2 and a maximum of 60.00 kg/m2. Duration of treatment: 3, 6 and 12 months. Also, in such cases, we analyzed the existence of sta- tistically significant differences by parameter, time and between groups using a univariate ANOVA of a factor. In the univariate ANOVA was applied on several fac- tors test or Duncan multiple range means separation test as a method of comparing them, in cases in which the categories of factors were more than two. All analysis was performed with SPSS software ver- sion 15.0 for Windows, considering a significance level of p < 0.05. 3. Results The general profile of the patients presented in Table 1. In the same average age is observed associated with older subjects, mainly male, obese, and average height. Table 2 shows the average baseline biochemical IN- DICATORS evaluated in the study. In the group treated with Byetta® for 3 months, statis- tically significant differences (p < 0.05) in HbA1c pa- rameter (F1.10 = 7.531, p = 0.021). In this case, we found a significant decrease in the indicator towards the end of treatment: 9.55%, SD = 1.086 in the initial instance, and 7.77%, SD = 1.164 in the final instance. Table 1. General profile of patients. Age Average 59.70 years, standard deviation (SD) of 9.073 years Sex Female: 6 (60.0%) Male: 4 (40.0%) Weight (initial) Average of 119.94 kg, SD = 13.86 kg. Height 1.72 m, SD = 0.054 m. Circumference 125.60 cm, SD = 13.867 cm. BMI (initial) 40.60 kg/m2, SD = 6.542 kg/m2. Table 2. Mean baseline glycated hemoglobin by treatment group. Biochemical parameter Group 3 months Group 6 months Group 12 months HbA1c (%) 9.55, SD = 0.629* 8.71, SD = 0.932* 8.70, SD = 1.556* *Statistically significant difference (p < 0.05). 3 MESES 10.00 9.50 9.00 8.00 8.50 7.50 7.77 9.55 Inicial Final Momento Media de HbA 1C 6 MESES 9.00 8.00 7.21 8.71 Inicial Final Momento Media de HbA 1C 3 meses 8.50 7.50 7.00 8.28 12 MESES 9.00 8.00 8.70 Inicial Final Momento Media de HbA 1C 3 meses 8.50 7.50 7.00 6 meses 7.90 7.30 7.00 Figure 1. Graphs the means by treatment group and the corresponding cutoff. Open Access PP  Evolution of Biochemical Effects of Byetta® in Type 2 Diabetics with Cardiovascular Risk 682 In the group treated with Byetta® for 6 months, statis- tically significant differences (p < 0.05) in HbA1c pa- rameter (F1.12 = 12.277, p = 0.004), finding a significant decrease towards the end of treatment: 8.71%, SD = 0.932 in the initial instance, and 7.21, SD = 0.644 in the final instance. Related to that, the statistically significant differences (p < 0.05) was found to consider jointly the initial request, 3 and 6 months (F2.17 = 5.839, p = 0.012). In this case, Duncan’s test identified two homogeneous subgroups: early times and 3 months in one (mean 8.71% and 8.28%, respectively) and the other 6 months (mean 7.21%), thus indicating that a significant reduction occurs by 6 months of treatment. Finally, regarding the group treated with Byetta® for 12 months, were found statistically significant differ- ences (p < 0.05), corresponding to average values of 6.70%, SD = 1.556 in the initial instance, 7.90%, DT = 1.272 at 3 months, 7.30%, SD = 0.849 at 6 months and 6.70%, SD = 0.707 at 12 months. At the end of treatment, we found similar effects of Byetta®, effects correlated with the decrease in HbA1c. It is observed that HbA1c is smaller (maximum reduction) in the group treated for 12 months (Figure 1). 4. Discussion Significant effects of treatment with Byetta® in patients with type 2 diabetes mellitus were recorded for the pa- rameter of HbA1c from 3 to 6 months of administering the drug. Even in the latter case, the differences which were statistically significant (p < 0.05) were consistent with intermediate levels. Significant effects occurred for all patients indiscriminately about sex, age and initial BMI. Despite this, and they were presented as baseline bio- chemical parameters inadequate, according to the criteria of optimal control of the SED [10,24], the final results of HbA1c reached the limit of adequacy. Exenatide was associated with a loss of weight com- pared to baseline values in Table 2. Exenatide appears to worsen the cardiovascular status of patients who specifi- cally included in the study by having the risk of that dis- ease. Therefore, the recommendations of this study, in con- clusion, result in the need for randomized studies to evaluate the effects of Byetta®, interpreted as sensitizers, on different physical and biochemical parameters in a greater long-term, proposing to do, at a minimum of 2 years. REFERENCES [1] National Health Service (NHS), “Exenatide,” New Drug Evaluation, Vol. 84, 2007. [2] Andalusian Center for Drug Information (CADIMA), “Exe- natide (INN),” New Therapeutics, 2009, Sheet 2. [3] F. Campoamor, “Exenatide in Type 2 Diabetes Mellitus,” Drug Evaluation Committee, Balearic Islands, 2008. [4] F. Verzegnassi and M. Chinelle, “Exenatide in Type 2 Diabetes,” Lancet, Vol. 376, No. 9746, 2010, pp. 1052- 1053. http://dx.doi.org/10.1016/S0140-6736(10)61485-7 [5] A. Peters, “Incretin-Based Therapies: Review of Current Clinical Trial Data,” American Journal of Medicine, Vol. 123, No. 3, 2010, pp. S28-S37. http://dx.doi.org/10.1016/j.amjmed.2009.12.007 [6] E. Wajcberg and A. Tavari, “Exenatide: Clinical Aspects of the First Incretin Mimetic for the Treatment, of Type 2 Diabetes Mellitus,” Expert Opin Pharmacother, Vol. 10, No. 1, 2009, pp. 135-142. http://dx.doi.org/10.1517/14656560802611832 [7] J. B. Segal, S. M. Dy, E. A. Millman, R. Herbert, E. B. Bass and A. Wu, “Diffusion Into Use of Exenatide for Glucose Control in Diabetes Mellitus: A Retrospective Cohort Study of a New Therapy,” Therapies in Clinical, Vol. 29, No. 8, 2007, pp. 1784-1794. http://dx.doi.org/10.1016/j.clinthera.2007.08.021 [8] J. Philippe, “Role and Indication of GLP-1 Analogues in the Treatment of Type 2 Diabetes,” Revue Médicale Suisse, Vol. 5, No. 206, 2009, pp. 1260-1262, 1264-1265. [9] H. Reuter and E. Erdmann, “Exenatide—An Incretin- Mimetic Agent for the Treatment of Type 2 Diabetes Mellitus,” Deutsche Medizinische Wochenschrift, Vol. 132, No. 11, 2007, pp. 571-574. http://dx.doi.org/10.1055/s-2007-970380 [10] D. M. Nathan, J. B. Buse, M. B. Davidson, R. J. Heine, R. R. Holman, R. Sherwin, et al., “Management of Hyper- glycaemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy: A Con- sensus Statement from the American Diabetes Asso- ciation and the European Association for the Study of Diabetes,” Diabetes Care, Vol. 29, No. 8, 2006, pp. 1963- 1972. http://dx.doi.org/10.2337/dc06-9912 [11] G. Schernthaner, A. H. Barnett, D. J. Betteridge, R. Carmena, A. Ceriello, B. Charbonnel, et al., “Is the ADA/EASD Algorithm for the Management of Type 2 Diabetes (January 2009) Based on Evidence or Opinion? A Critical Analysis,” Diabetologia, Vol. 53, No. 7, 2010, pp. 1258-1269. http://dx.doi.org/10.1007/s00125-010-1702-3 [12] German Diabetes Association, S. Matthaei, R. Bierwirth, A. Fritsche, B. Gallwitz, H. U. Häring, et al., “Medical Treatment of Type Antihyperglycaemic 2 Diabetes Mel- litus: Update of the Evidence-Based Guideline of the German Diabetes Association,” Experimental and Clini- cal Endocrinology & Diabetes, Vol. 117, 2009, pp. 522- 557. [13] C. M. Apovian, R. M. Bergenstal, R. M. Cuddihy, Y. Qu, S. Lenox, M. S. Lewis, et al., “Effects of Exenatide Combined with Lifestyle Modification in Patients with Type 2 Diabetes,” American Journal of Medicine, Vol. 123, No. 5, 2010, pp. 468.e9-468.e17. [14] G. I. Robles and D. Singh-Franco, “A Review of Exenatide as Adjunctive Therapy in Patients with Type 2 Diabetes,” Open Access PP  Evolution of Biochemical Effects of Byetta® in Type 2 Diabetics with Cardiovascular Risk Open Access PP 683 Journal of Drug Design, Development and Therapy, Vol. 3, 2009, pp. 219-240. http://dx.doi.org/10.2147/DDDT.S3321 [15] A. Gill, B. J. Hoogwerf, J. Burger, S. Bruce, L. Mac- conell, P. Yan, et al., “Effect of Exenatide on Heart Rate and Blood Pressure in Subjects with Type 2 Diabetes Mellitus: A Double-Blind, Placebo-Controlled, Rando- mized Pilot Study,” Cardiovascular Diabetology, Vol. 9, 2010, p. 6. [16] T. J. Moretto, D. R. Milton, T. D. Ridge, L. A. Macconell, T. Okerson, A. M. Wolke, et al., “Efficacy and Tolerability of Exenatidemonotherapy over 24 Weeks in Antidiabetic Drug-Naive Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study,” Clinical Therapeutics, Vol. 30, No. 8, 2008, pp. 1448- 1460. http://dx.doi.org/10.1016/j.clinthera.2008.08.006 [17] D. C. Klonoff, J. B. Buse, L. L. Nielsen, X. Guan, C. L. Bowlus, J. H. Holcombe, et al., “Exenatide Effects on Diabetes, Obesity, Cardiovascular and Hepatic Biomarkers Risk Factors in Patients Treated with Type 2 Diabetes at Least for 3 Years,” Current Medical Research and Opinion, Vol. 24, No. 1, 2008, pp. 275-286. [18] D. J. Drucker, J. B. Buse, K. Taylor, D. M. Kendall, M. Trautmann, D. Zhuang, DURATION-1 Study Group, et al., “Exenatide Twice Daily versus Weekly Eleven for the Treatment of Type 2 Diabetes: A Randomized, Open- Label, Non-Inferiority Study,” Lancet, Vol. 372, 2008, pp. 1240-1250. http://dx.doi.org/10.1016/S0140-6736(08)61206-4 [19] T. Okerson, P. Yan, A. Stonehouse, R. Brodows and D. Bhole, “Improved Systolic Blood Pressure Exenatide Compared to Insulin or Placebo in Patients with Type 2 Diabetes,” Diabetologia, Vol. 51, 2008, p. S350. [20] R. Bergenstal, T. Kim, P. Yan, T. Darsow, B. Walsh, T. Okerson, et al., “Eleven Weekly Exenatide Improved Cardiometabolic Risk Factors in Subjects with Type 2 Diabetes during One Year of Treatment,” Diabetes, Vol. 58, 2009, p. A43. [21] R. Bhushan, K. E. Elkind-Hirsch, M. Bhushan, W. J. Butler, K. Duncan and O. Marrioneaux, “Improved Gly- cemic Control and Cardiometabolic Reduction of Risk Factors in Subjects with Type 2 Diabetes and Metabolic Syndrome Treated with Exenatide in a Clinical Practice Setting,” Diabetes Technology & Therapeutics, Vol. 11, No. 6, 2009, pp. 353-359. http://dx.doi.org/10.1089/dia.2008.0090 [22] P. A. Kothare, H. Linnebjerg, Y. Isaka, K. Uenaka, A. Yamamura, K. P. Yeo, et al., “Pharmacokinetics, Pharma- codynamics, Tolerability, and Safety of Exenatide in Japanese Patients with Type 2 Diabetes Mellitus,” Jour- nal of Clinical Pharmacology, Vol. 48, No. 12, 2008, pp. 1389-1399. http://dx.doi.org/10.1177/0091270008323750 [23] H. Linnebjerg, P. Kothare, S. Park, K. Mace and M. Mitchell, “The Effect of Exenatide on Lisinopril Pharma- codynamics and Pharmacokinetics in Patients with Hyper- tension,” International Journal of Clinical Pharmacology and Therapeutics, Vol. 47, No. 11, 2009, pp. 651-658. http://dx.doi.org/10.5414/CPP47651 [24] E. M. Torre, J. L. Tejedor, S. A. Menendez, J. M. Nunez- Cortes, A. A. Garcia, M. P. Sunday, et al., “Recom- mendations for the Pharmacological Treatment of Hyper- glycemia in Type 2 Diabetes,” Avances en Diabetología, Vol. 26, No. 6, 2010, pp. 331-338.
|