 Open Journal of Medicinal Chemistry, 2013, 3, 128-135 Published Online December 2013 (http://www.scirp.org/journal/ojmc) http://dx.doi.org/10.4236/ojmc.2013.34015 Open Access OJMC Synthesis and Evaluation of 2-Amino-4H-Pyran-3-Carbonitrile Derivatives as Antitubercular Agents Chunxia Chen1*, Minghui Lu2*, Zhihui Liu3, Junting Wan2, Zhengchao Tu2, Tianyu Zhang2#, Ming Yan1# 1Institute of Drug Synthesis and Pharmaceutical Process, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, China 2State Key Laboratory of Respiratory Diseases, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Science, Guangzhou, China 3Guangzhou Chest Hospital, Guangzhou, China Email: #zhang_tianyu@gibh.ac.cn, #yanming@mail.sysu.edu.cn Received March 2, 2013; revised April 2, 2013; accepted April 9, 2013 Copyright © 2013 Chunxia Chen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT A series of 2-amino-4H-pyran-3-carbonitrile derivatives were designed and synthesized. Their antitubercular activities were evaluated against autoluminescent M. tuberculosis H37Ra and standard strain M. tuberculosis H37Rv. No obvious antitubercular activities could be observed (MIC > 10 g/mL). The results are in sharp contrast with the previously re- ported data. Keywords: 2-Amino-4H-Pyran-3-Carbonitrile; Synthesis; Antitubercular Activity 1. Introduction Tuberculosis (TB) is a chronic disease caused by My- cobacterium tuberculosis. It continues to be a serious threat for human health [1,2]. Every year about two mil- lion people die of this disease and almost eight million people get tuberculosis. The present antitubercular treat- ment typically requires the combination of at least two first-line drugs (rifampicin, isoniazid, ethambutol and pyrazinaimde) for an extended period (6 - 12 months). The poor compliance to the rigid implementation of the- rapy leads to the emergence of multi-drug-resistant (MDR) and extensively drug-resistant (XDR) strains of Myco- bacterium tuberculosis, which have brought new chal- lenges for clinical treatment [3-6]. In addition, the rising incidence of TB and HIV co-infection makes the treat- ment more difficult. The development of antitubercular agents with new action mechanism is an urgent task [7,8]. In recent ten years, a number of candidates have appeared with prom- ising activities against sensitive and resistant Mycobacte- rium tuberculosis strains. In 2007, Perumal and co- workers reported 2-aminopyranopyridine-3-carbonitriles as a new type of antitubercular agents (Scheme 1) [9]. Several compounds showed excellent antitubercular ac- tivity comparable with isoniazid. Recently Perumal, Sri- ram and co-workers also found that 1,2,4-oxadiazoles de- rived from 2-aminopyranopyridine-3-carbonitriles show- ed enhanced antitubercular activity (Scheme 1) [10]. These results strongly suggest that 2-amino-4H-pyran-3-carbo- nitrile is a new pharmacophore of antitubercular agents. Recently we have developed efficient methods for the synthesis of homochiral 2-amino-4H-pyran-3-carboni- triles [11,12]. We are interested in the effect of chiral center of these compounds on the antitubercular activity. We are also interested in the further improvement of the antitubercular activity of 2-amino-4H-pyran-3-carboni- triles by structural modifications. In this paper, we report the synthesis of racemic and homochiral 2-amino-pyra- nopyridine-3-carbonitriles as well as their structural ana- logs. The antitubercular activity was evaluated in vitro against autoluminescent Mycobacterium tuberculosis H37Ra and standard strain Mycobacterium tuberculosis H37Rv. *These authors contribute equally to this study. #Corresponding authors.  C. X. CHEN ET AL. Open Access OJMC 129 Ar O NH 2 CN Ar N Ar = 4-Cl-C 6 H 4 , MIC0.92 (TB), 1.84 (MDRTB) Ar = 4-F-C 6 H 4 , MIC 0.97 (TB),0.97 (MDRTB) Ar = 4-Me 2 N-C 6 H 4 , MIC0.43 (TB),0.43 (MDRTB) Ar O NH 2 Ar N Ar = 4-Cl-C 6 H 4 -, MIC 0.35(TB) Ar= 2,4-diCl-C 6 H 3 , MIC 0.31 (TB) N N OCl Scheme 1. 2-Amino-4H-pyran-3-carbonitrile and their derivatives with potent antitubercular activity. 2. Results and Discussion 2.1. Chemistry Racemic 2-aminopyranopyridine-3-carbonitriles 2a-2g were prepared from dienones 1a-1g and malononitrile in the presence of piperidine. Generally excellent yields (92% - 99%) were obtained (Scheme 2). To explore the effect of chiral centers in 2a-2g, ho- mochiral (S)-2a, (S)-2d, (R)-2a, and (R)-2d were pre- pared. Excellent yields and enantioselectivities were achiev- ed using chiral thiourea-tertiary amines 3a and 3b as the catalysts (Scheme 3) [11]. 2-Aminopyranopyridine-3-carbonitriles 2h-2n derived from monoenones 1h-1n were also prepared (Scheme 4) [12]. Cyclic enones 1h-1j reacted with malononitrile in the presence of triethylamine. The reaction of acyclic enones 1k-1n was achieved using piperidine as the cata- lyst. Generally products 2h-2n were obtained in good yields. For a further understanding the effect of C (sp3) chiral structure in 2-aminopyranopyridine-3-carbonitriles, the compounds 4a and 4j with achiral pyridine structure were designed. The treatment of 2-amino-pyran 2a and 2j with ammonium acetate provided 4a and 4j in good yields (Scheme 5) [13]. 2.2. Evaluation of Antitubercular Activity The antitubercular activity of racemic 2-amino-4H-pyran- 3-carbonitriles 2a-2e, homochiral 2-amino-4H-pyran-3- carbonitriles (S)-2a, (S)-2d, (R)-2a, and (R)-2d were eva- luated against autoluminescent M. tuberculosis H37Ra [14]. This screen model is fast and cost-efficient for the preliminary evaluation of antitubercular activity. Isoni- azid and rifampicin were used as the positive control and the results are listed in Figure 1. The bacteria growth was conveniently monitored by the bioluminescence in- tensity. Unexpectedly all compounds including 2a and 2d did not showed obvious antitubercualr activity. We further examined the inhibitive activity of the compounds against standard strain M. tuberculosis H37Rv and the results are summarized in Table 1. Perumal and co-workers reported that compound 2a and 2d possess excellent antitubercular activities (MIC 0.97 and 0.92 g/mL against H37Rv respectively) [9]. Our present study led to significantly different results. Racemic 2a, 2d and their homochiral enantiomers did not show obvi- ous antitubercular activities (MIC > 10 g/mL). Other structural analogs 2b, 2c, 2e-2g also appeared to be in- efficient. The compounds 2h-2n and 4a, 4j with further structural diversities still showed disappointed antituber- cular activities. These results brought the question about the reported antitubercular activity of 2-amino-4H-pyran- 3-carbonitriles by Perumal and co-workers. Although a clear conclusion could not be achieved so far, the further examination of the reported data is highly desirable. 3. Conclusion In conclusion, we designed and synthesized a series of 2- amino-4H-pyran-3-carbonitriles and their structural ana- logs. Homochiral 2-amino-4H-pyran-3-carbonitriles were also prepared via the organocatalytic enantioselctive re- action. The antitubercualr activities of these compounds were determined against autoluminescent M. tuberculosis H37Ra and standard strain M. tuberculosis H37Rv, how- ever, no obvious inhibitive activities could be observed. The results are in sharp contrast with the previously re- ported data. Before the further attempt to develop 2- amino-4H-pyran-3-carbonitriles as potential antituber- cular agents, the clarification of the contradictive activity data is required. 4. Experimentals 1H and 13C NMR spectra were recorded on a Bruker Ad- vance 400 MHz spectrometer as solutions in CDCl3. Chemical shifts in 1H NMR spectra are reported in parts per million (ppm, δ) downfield from the internal standard Me4Si (TMS, δ = 0 ppm). Chemical shifts in 13C NMR spectra are reported relative to the central line of the chloroform signal (δ = 77.0 ppm). The following abbre- viations are used to designate chemical shift mutiplicities: s = singlet, d = doublet, m = multiplet. High-resolution mass spectra were obtained with Shimadazu LCMS-IT- TOF mass spectrometer. Infrared (IR) spectra were re- corded on a Bruker Tensor 37 spectrophotometer. Data are represented as follows: frequency of absorption (cm−1), intensity of absorption (s = strong, m = medium, w = weak). The flash column chromatography was car- 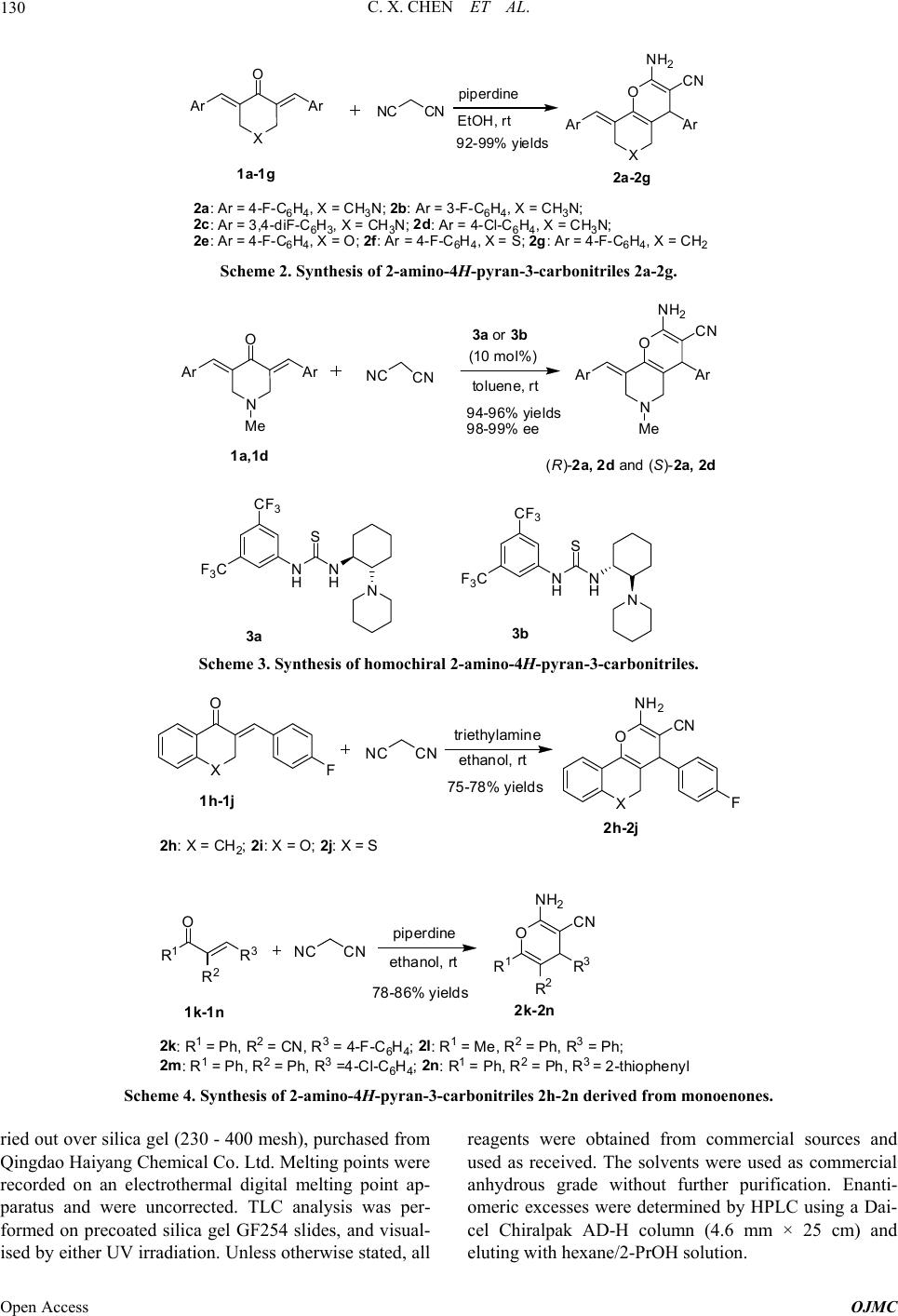 C. X. CHEN ET AL. Open Access OJMC 130 Ar O Ar NCCN piperdine EtOH, rtAr O NH 2 CN Ar 1a-1g 2a-2g XX 92-99% yields 2a:Ar=4-F-C 6 H 4 ,X=CH 3 N; 2b:Ar=3-F-C 6 H 4 ,X=CH 3 N; 2c: Ar = 3,4-diF-C 6 H 3 ,X=CH 3 N; 2d:Ar=4-Cl-C 6 H 4 ,X=CH 3 N; 2e:Ar=4-F-C 6 H 4 ,X=O;2f:Ar=4-F-C 6 H 4 ,X=S;2g:Ar=4-F-C 6 H 4 ,X=CH 2 Scheme 2. Synthesis of 2-amino-4H-pyran-3-carbonitriles 2a-2g. Ar O Ar NC CN 3a or 3b toluene, rt (10 mol%)Ar O NH 2 CN Ar 1a,1d (R)-2a,2dand (S)-2a, 2d CF 3 F 3 C N HN H S N CF 3 F 3 C N HN H S N 3a 3b NN Me Me 94-96% yields 98-99% ee Scheme 3. Synthesis of homochiral 2-amino-4H-pyran-3-carbonitriles. X O FNC CN X O F NH 2 CN triethylamine ethanol, rt 1h-1j 2h-2j 75-78% yields 2h:X=CH 2 ;2i:X= O;2j:X=S O NH 2 CN R 1 R 2 R 3 R 1 O R 2 R 3 NC CN piperdine ethanol, rt 1k-1n 2k-2n 78-86% yields 2k:R 1 =Ph,R 2 =CN,R 3 =4-F-C 6 H 4 ;2l:R 1 =Me,R 2 =Ph,R 3 =Ph; 2m: 1 =Ph, 2 =Ph, 3 =4-Cl- C H ;2n: 1 =Ph, 2 =Ph, 3 =2-thiophen l Scheme 4. Synthesis of 2-amino-4H-pyran-3-carbonitriles 2h-2n derived from monoenones. ried out over silica gel (230 - 400 mesh), purchased from Qingdao Haiyang Chemical Co. Ltd. Melting points were recorded on an electrothermal digital melting point ap- paratus and were uncorrected. TLC analysis was per- formed on precoated silica gel GF254 slides, and visual- ised by either UV irradiation. Unless otherwise stated, all reagents were obtained from commercial sources and used as received. The solvents were used as commercial anhydrous grade without further purification. Enanti- omeric excesses were determined by HPLC using a Dai- cel Chiralpak AD-H column (4.6 mm × 25 cm) and eluting with hexane/2-PrOH solution. 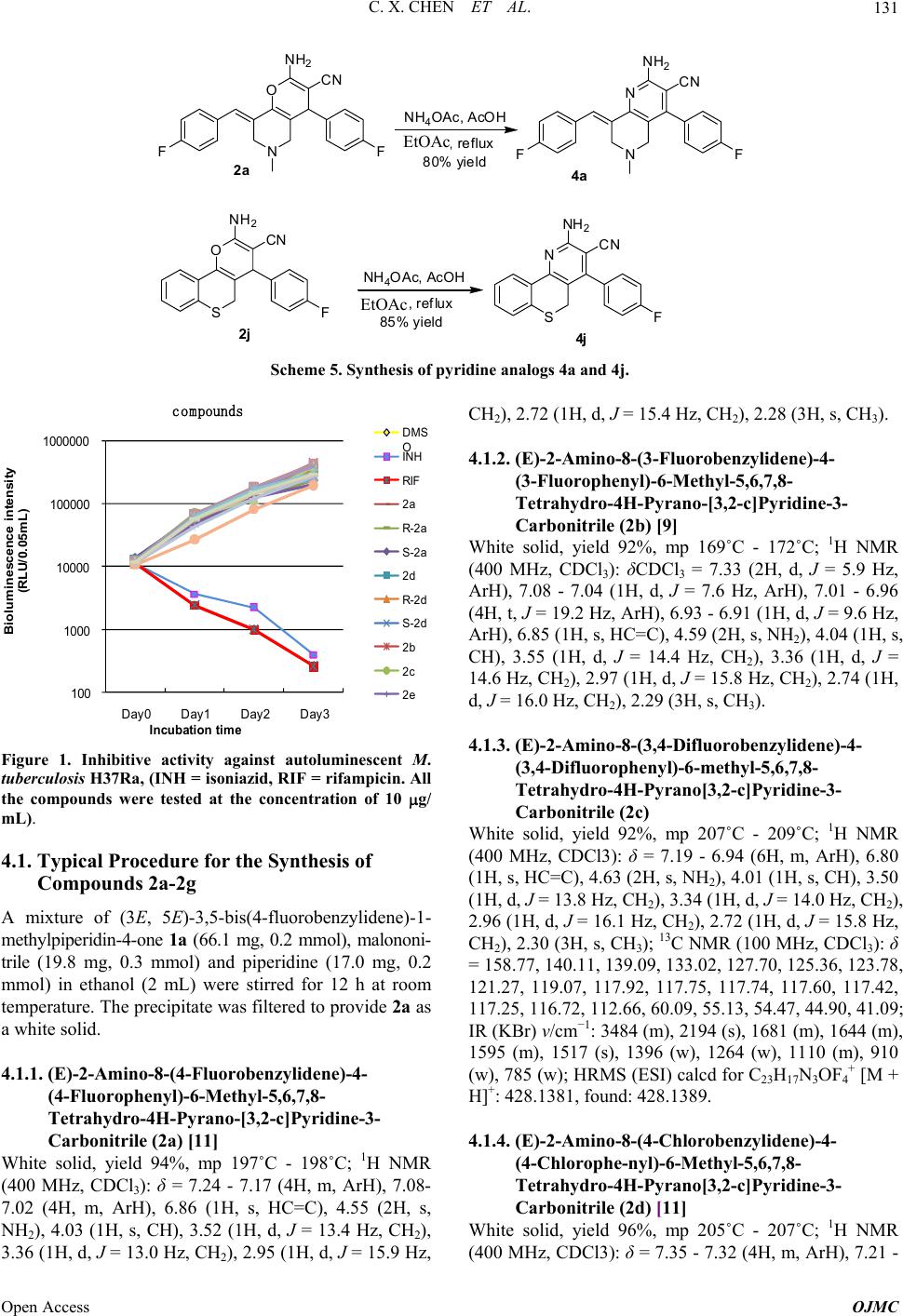 C. X. CHEN ET AL. Open Access OJMC 131 N O NH2CN FF EtOAC, refluxN N NH2CN F F 2a 4a S O NH2CN FS N NH2 CN F 2j 4j 80%yield 85% yield NH4OAc, AcOH EtOAC, reflux NH4OAc, AcOH EtOAc EtOAc Scheme 5. Synthesis of pyridine analogs 4a and 4j. 100 1000 10000 100000 1000000 Day0Day1 Day2 Day3 Biolum inescence intensity (RLU/0.05mL) I n cu b atio n ti me compounds DMS O INH RIF 2a R-2a S-2a 2d -2d S-2d 2b 2c 2e Figure 1. Inhibitive activity against autoluminescent M. tuberculosis H37Ra, (INH = isoniazid, RIF = rifampicin. All the compounds were tested at the concentration of 10 g/ mL). 4.1. Typical Procedure for the Synthesis of Compounds 2a-2g A mixture of (3E, 5E)-3,5-bis(4-fluorobenzylidene)-1- methylpiperidin-4-one 1a (66.1 mg, 0.2 mmol), malononi- trile (19.8 mg, 0.3 mmol) and piperidine (17.0 mg, 0.2 mmol) in ethanol (2 mL) were stirred for 12 h at room temperature. The precipitate was filtered to provide 2a as a white solid. 4.1.1. (E)-2 - A mi no- 8-(4-Fluorobenzylidene)- 4- (4-Fluorophenyl)-6-Methyl-5,6,7,8- Tetrahydro-4H-Pyrano-[3,2-c]Pyridine-3- Carbonitrile (2a) [11] White solid, yield 94%, mp 197˚C - 198˚C; 1H NMR (400 MHz, CDCl3): δ = 7.24 - 7.17 (4H, m, ArH), 7.08- 7.02 (4H, m, ArH), 6.86 (1H, s, HC=C), 4.55 (2H, s, NH2), 4.03 (1H, s, CH), 3.52 (1H, d, J = 13.4 Hz, CH2), 3.36 (1H, d, J = 13.0 Hz, CH2), 2.95 (1H, d, J = 15.9 Hz, CH2), 2.72 (1H, d, J = 15.4 Hz, CH2), 2.28 (3H, s, CH3). 4.1.2. (E)-2 - A mi no- 8-(3-Fluorobenzylidene)- 4- (3-Fluorophenyl)-6-Methyl-5,6,7,8- Tetrahydro-4H-Pyrano-[3,2-c]Pyridine-3- Carbonitrile (2b) [9] White solid, yield 92%, mp 169˚C - 172˚C; 1H NMR (400 MHz, CDCl3): δCDCl3 = 7.33 (2H, d, J = 5.9 Hz, ArH), 7.08 - 7.04 (1H, d, J = 7.6 Hz, ArH), 7.01 - 6.96 (4H, t, J = 19.2 Hz, ArH), 6.93 - 6.91 (1H, d, J = 9.6 Hz, ArH), 6.85 (1H, s, HC=C), 4.59 (2H, s, NH2), 4.04 (1H, s, CH), 3.55 (1H, d, J = 14.4 Hz, CH2), 3.36 (1H, d, J = 14.6 Hz, CH2), 2.97 (1H, d, J = 15.8 Hz, CH2), 2.74 (1H, d, J = 16.0 Hz, CH2), 2.29 (3H, s, CH3). 4.1.3. (E)-2 - Amino-8-( 3,4-Difluorobenzylidene)-4- (3,4-Difluorophenyl)-6-methyl-5,6,7,8- Tetrahydro-4H-Pyrano[3,2-c]Pyridine-3- Carbonitrile (2c) White solid, yield 92%, mp 207˚C - 209˚C; 1H NMR (400 MHz, CDCl3): δ = 7.19 - 6.94 (6H, m, ArH), 6.80 (1H, s, HC=C), 4.63 (2H, s, NH2), 4.01 (1H, s, CH), 3.50 (1H, d, J = 13.8 Hz, CH2), 3.34 (1H, d, J = 14.0 Hz, CH2), 2.96 (1H, d, J = 16.1 Hz, CH2), 2.72 (1H, d, J = 15.8 Hz, CH2), 2.30 (3H, s, CH3); 13C NMR (100 MHz, CDCl3): δ = 158.77, 140.11, 139.09, 133.02, 127.70, 125.36, 123.78, 121.27, 119.07, 117.92, 117.75, 117.74, 117.60, 117.42, 117.25, 116.72, 112.66, 60.09, 55.13, 54.47, 44.90, 41.09; IR (KBr) v/cm−1: 3484 (m), 2194 (s), 1681 (m), 1644 (m), 1595 (m), 1517 (s), 1396 (w), 1264 (w), 1110 (m), 910 (w), 785 (w); HRMS (ESI) calcd for C23H17N3OF4 + [M + H]+: 428.1381, found: 428.1389. 4.1.4. (E)-2 - A mi no- 8-(4-Chloro benzylidene)-4- (4-Chlorophe-nyl)-6-Methyl-5,6,7,8- Tetrahydro-4H-Pyrano[3,2-c]Pyridine-3- Carbonitrile (2d) [11] White solid, yield 96%, mp 205˚C - 207˚C; 1H NMR (400 MHz, CDCl3): δ = 7.35 - 7.32 (4H, m, ArH), 7.21 -  C. X. CHEN ET AL. Open Access OJMC 132 Table 1. Evaluation of antitubercular activity against M. tuberculosis H37Rv. Compounds MIC (g/mL) 2a >10 2b >10 2c >10 2d >10 2e >10 2f >10 2g >10 (R)-2a >10 (R)-2d >10 (S)-2a >10 (S)-2d >10 2h >10 2i >10 2j >10 2k >10 2l >10 2m >10 2n >10 4a >10 4j >10 Isoniazid 0.03 Rifampicin <0.25 7.13 (4H, m, ArH), 6.84 (1H, s, HC=C), 4.57 (2H, s, NH2), 4.02 (1H, s, CH), 3.51 (1H, d, J = 13.8 Hz, CH2), 3.35 (1H, d, J = 13.6 Hz, CH2), 2.94 (1H, d, J = 16.0 Hz, CH2), 2.72 (1H, d, J = 16.0 Hz, CH2), 2.27(3H, s, CH3). 4.1.5. (E)2-Amino-8-(4 -F luorobenzylidene)-4- (4-Fluorophenyl)-4,5,7,8-Tetrahydropyrano [4,3-b]Pyran-3-Carbonitrile (2e) While solid, yield 99%, mp 221˚C - 223˚C; 1H NMR (400 MHz, DMSO-d6): δ = 7.44 - 7.05 (8H, m, ArH), 6.92 (1H, s, HC=C), 6.90 (2H, s, NH2), 4.59 (1H, d, J = 14.0 Hz, CH2), 4.48 (1H, d, J = 13.9 Hz, CH2), 4.17 (1H, d, J = 15.5, CH2), 4.15 (1H, s, CH), 3.72 (1H, d, J = 15.6 Hz, CH2); 13C NMR (100 MHz, DMSO): δ = 162.50, 160.08, 159.70, 139.17, 138.07, 131.72, 131.02, 130.99, 129.42, 129.30, 126.09, 120.38, 120.15, 115.61, 115.55, 115.40, 115.34, 112.96, 65.12, 64.90, 55.77; IR (KBr) v/cm−1: 3472 (m), 3312 (w), 2220 (s), 1685 (m), 1660 (m), 1603 (s), 1508 (s), 1414 (w), 1391 (w), 1264 (w), 1228 (s), 1155 (w), 1098 (m), 883 (w), 838 (w), 780 (w), 744 (m); HRMS (ESI) calcd for C22H16N2O2F2 + [M + Na]+: 401.1072, found: 401.1065. 4.1.6. 2-Amin o-8-(4-Flu orobenzyli dene)- 4- (4-Fluorophenyl)-4,5,7,8-Tetrahydrothiopyrano [4,3-b]Pyran-3-Carbonitrile (2f) White solid, yield 98%, mp 205˚C - 207˚C; 1H NMR (400 MHz, CDCl3): δ = 7.25 - 7.21 (4H, m, ArH), 7.10 - 7.03 (4H, m, ArH), 6.93 (1H, s, HC=C), 4.53 (2H, s, NH2), 4.03 (1H, s, CH), 3.58 (2H, q, J = 9.0 Hz, CH2), 3.04 (2H, q, J = 12.0 Hz, CH2); 13C NMR (100 MHz, DMSO-d6): δ = 159.65, 143.42, 141.39, 135.84, 129.13, 128.74, 128.51, 127.51, 127.39, 127.16, 126.35, 124.39, 120.23, 114.04, 55.97, 43.21, 27.20, 27.15; IR (KBr) v/cm−1: 3452 (m), 3355 (m), 2360 (s), 2192 (m), 2024 (w), 1673 (s), 1627 (w), 1599 (w), 1506 (s), 1410 (m), 1233 (m), 1126 (m), 881 (w), 850 (w); HRMS (ESI) calcd for C22H16N2OF2S+ [M + Na]+: 417.0844, found: 417.0843. 4.1.7. (E)-2 - A mi no- 8-(4-Fluorobenzylidene)- 4- (4-Fluorophenyl)-5,6,7,8-Tetrahydro-4H- Chromene-3-Carbonitrile (2g) [10] White solid, yield 99%, mp 211˚C - 213˚C: 1H NMR (400 MHz, CDCl3): δ = 7.25 - 7.20 (4H, m, ArH), 7.06 - 7.02 (4H, m, ArH), 6.83 (1H, s, HC=C), 4.56 (2H, s, NH2), 3.97 (1H, s, CH), 2.74 - 2.70 (1H, m, CH2), 2.60 - 2.56 (1H, m, CH2), 2.04 - 1.92 (2H, m, CH2), 1.74 - 1.52 (2H, m, CH2); HRMS (ESI) calcd for C23H18N2OF2 + [M + Na]+: 399.1279, found: 399.1261.. 4.2. Typical Procedure for the Synthesis of Homochiral Compounds (S)-2a, (S)-2d, (R)-2a, and (R)-2d A mixture of (3E, 5E)-3,5-bis(4-fluorobenzylidene)-1- methylpiperidin-4-one 1a (66.1 mg, 0.2 mmol), malononi- trile (19.8 mg, 0.3 mmol) and 3a (9.0 mg, 0.02 mmol) in toluene (2 mL) were stirred for 28 h at room temperature. The white precipitate (S)-2a was collected by the centri- fugalization. 4.2.1. (S,E)- 2 -Amino-8- (4 -Fluorobenz yli dene)-4- (4-Fluorophenyl)-6-Methyl-5,6,7,8-Tetrahydro- 4H-Pyrano-[3,2-c]Pyridine-3-Carbonitrile (S-2a) [11] White solid, yield 94%, mp 197˚C - 198˚C; 1H NMR (400 MHz, CDCl3): δ = 7.24 - 7.17 (4H, m, ArH), 7.08- 7.02 (4H, m, ArH), 6.86 (1H, s, HC=C), 4.55 (2H, s, NH2), 4.03 (1H, s, CH), 3.52 (1H, d, J = 13.4 Hz, CH2), 3.36 (1H, d, J = 13.0 Hz, CH2), 2.95 (1H, d, J = 15.9 Hz, CH2), 2.72 (1H, d, J = 15.4 Hz, CH2), 2.28 (3H, s, CH3). Enantiomeric excess was determined by HPLC with a CHIRALPAK AD-H column (i-PrOH/hexane = 30:70,  C. X. CHEN ET AL. Open Access OJMC 133 254 nm, 0.8 mL/min), tr (major) = 10.6 min, tr (minor) = 7.5 min, 99%ee. 4.2.2. (R,E) -2 -Amino-8- (4 -Fluorobenz yli dene)-4- (4-Fluorophenyl)-6-Methyl-5,6,7,8-Tetrahydro- 4H-Pyrano-[3,2-c]Pyridine-3-Carbonitrile (R-2a) [11] White solid, yield 94%, mp 197˚C - 198˚C; 1H NMR (400 MHz, CDCl3): δ = 7.24 - 7.17 (4H, m, ArH), 7.08 - 7.02 (4H, m, ArH), 6.86 (1H, s, HC=C), 4.55 (2H, s, NH2), 4.03 (1H, s, CH), 3.52 (1H, d, J = 13.4 Hz, CH2), 3.36 (1H, d, J = 13.0 Hz, CH2), 2.95 (1H, d, J = 15.9 Hz, CH2), 2.72 (1H, d, J = 15.4 Hz, CH2), 2.28 (3H, s, CH3). Enantiomeric excess was determined by HPLC with a CHIRALPAK AD-H column (i-PrOH/hexane = 30:70, 254 nm, 0.8 mL/min), tr(major) = 7.5 min, tr (minor) = 10.6 min. 98%ee. 4.2.3. (S,E)-2- Amino-8- (4-Chlorob enzylidene)-4- (4-Chlorophe-nyl)-6-Methyl-5,6,7,8- Tetrahydro-4H-Pyrano[3,2-c]Pyridine-3- Carbonitrile (S-2d) [11] White solid, yield 96%, mp 205˚C - 207˚C; 1H NMR (400 MHz, CDCl3): δ = 7.35 - 7.32 (4H, m, ArH), 7.21- 7.13 (4H, m, ArH), 6.84 (1H, s, HC=C), 4.57 (2H, s, NH2), 4.02 (1H, s, CH), 3.51 (1H, d, J = 13.8 Hz, CH2), 3.35 (1H, d, J = 13.6 Hz, CH2), 2.94 (1H, d, J = 16.0 Hz, CH2), 2.72 (1H, d, J = 16.0 Hz, CH2), 2.27 (3H, s, CH3). Enantiomeric excess was determined by HPLC with a CHIRALPAK AD-H column (i-PrOH/hexane = 30:70, 254 nm, 0.8 mL/min), tr (major) = 9.7 min, tr (minor) = 7.6 min, 98%ee. 4.2.4. (R,E) -2 - Ami no - 8- (4 -C hl oro b enz yl i dene)- 4 - (4-Chlorophe-nyl)-6-Methyl-5,6,7,8- Tetrahydro-4H-Pyr-ano[3,2-c]Pyridine-3- Carbonitrile (R-2d) [11] White solid, yield 96%, mp 205˚C - 207˚C; 1H NMR (400 MHz, CDCl3): δ = 7.35 - 7.32 (4H, m, ArH), 7.21 - 7.13 (4H, m, ArH), 6.84 (1H, s, HC=C), 4.57 (2H, s, NH2), 4.02 (1H, s, CH), 3.51 (1H, d, J = 13.8 Hz, CH2), 3.35 (1H, d, J = 13.6 Hz, CH2), 2.94 (1H, d, J = 16.0 Hz, CH2), 2.72 (1H, d, J = 16.0 Hz, CH2), 2.27 (3H, s, CH3). Enantiomeric excess was determined by HPLC with a CHIRALPAK AD-H column (i-PrOH/hexane = 30:70, 254 nm, 0.8 mL/min), tr (major) = 7.6 min, tr (minor) = 9.7 min, 99%ee. 4.3. Typical Procedure for the Synthesis of Compounds 2h-2j A mixture of (E)-2-(4-fluorobenzylidene)-3,4-dihy-dro- naphthalen -1(2H)-one 1h (50.5 mg, 0.2 mmol), malono- nitrile (19.8 mg, 0.3 mmol) and triethylamine (20.2 mg, 0.2 mmol) in ethanol (2 mL) were stirred for 12 h at room temperature. The precipitate was filtered to provide 2h as a yellow solid. 4.3.1. 2-Amino-4-(4-Fl u orophenyl)- 5, 6-Dihydro- 4H-Benzo[h]Chrom ene-3-Ca rbonitrile (2h) [12] Yellow solid, yield 94%, mp 184˚C - 186˚C; 1H NMR (400 MHz, CDCl3): δ = 7.46 (1H, d, J = 7.2 Hz, ArH), 7.26 - 7.19 (4H, m, ArH), 7.11 (1H, d, J = 6.8 Hz, ArH), 7.01 (2H, t, J = 8.2 Hz, ArH), 4.58 (2H, s, NH2), 4.07 (1H, s, CH), 2.81 (1H, dd, J = 15.9, 8.1 Hz, CH2), 2.69 (1H, dt, J = 15.7, 7.8 Hz, CH2), 2.16 (1H, dt, J = 15.9, 7.9 Hz, CH2), 2.09 - 1.96 (1H, m, CH2); HRMS (ESI) calcd for C20H15N2OF+ [M + Na]+: 341.1061, found: 341.1054.. 4.3.2. 2-Amino-4-(4-Fl u orophenyl)- 4, 5- Dihydropyrano[3,2-c]Chromene-3-Carbonitrile (2i) Yellow solid, yield 75%, mp 175˚C - 177˚C; 1H NMR (400 MHz, CDCl3): δ = 7.34 (1H, d, J = 7.6 Hz, ArH), 7.30 - 7.14 (3H, m, ArH), 7.05 (2H, dd, J = 12.0, 5.0 Hz, ArH), 6.96 (1H, t, J = 7.5 Hz, ArH), 6.80 (1H, d, J = 8.1 Hz, ArH), 4.67 (2H, s, NH2), 4.62 - 4.55 (1H, d, J = 13.6 Hz, CH2), 4.41 (1H, d, J = 13.7 Hz, CH2), 4.03 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δ = 163.61, 161.17, 158.86, 154.14, 138.16, 136.81, 130.46, 129.55, 129.48, 121.36, 121.13, 116.58, 116.08, 116.00, 115.76, 104.75, 66.37, 60.87, 39.07; IR (KBr) v/cm−1: 3327 (m), 2195 (m), 1709 (s), 1656 (s), 1600 (s), 1506 (m), 1356 (m), 1230 (s), 1157 (m), 1101 (w), 1036 (w), 836 (m), 755 (w); HRMS (ESI) calcd for C19H13N2O2F+ [M + Na]+: 343.0853, found: 343.0857.. 4.3.3. 2-Amino-4-(4-Fl u orophenyl)- 4, 5- Dihydrothiochromeno[4,3-b]Pyran-3- Carbonitrile (2j) Yellow solid, yield 76%, mp 158˚C - 160˚C; 1H NMR (400 MHz, CDCl3): δ = 7.53 (1H, d, J = 16.8 Hz, ArH), 7.36 - 7.29 (2H, m, ArH), 7.28 - 7.14 (3H, m, ArH), 7.04 (2H, dd, J = 12.1, 4.9 Hz, ArH), 4.63 (2H, s, NH2), 4.13 (1H, s, CH), 3.29 (1H, d, J = 15.1 Hz, CH2), 3.09 (1H, d, J = 15.1 Hz, CH2); 13C NMR (100 MHz, CDCl3): δ = 163.63, 161.18, 158.63, 142.14, 137.53, 132.76, 129.77, 129.70, 129.11, 127.15, 125.58, 123.28, 119.20, 116.03, 115.81, 107.77, 60.99, 42.39, 27.00; IR (KBr) v/cm−1: 3475 (w), 2360 (w), 2197 (s), 2024 (w), 1692 (s), 1635 (w), 1598 (s), 1506 (w), 1407 (s), 1343 (w), 1258 (w), 1219 (w), 1121 (s), 848 (w), 728 (w); HRMS (ESI) calcd for C19H13N2OFS+ [M + Na]+: 359.0625, found: 359.0631. 4.4. Typical Procedure for the Synthesis of Compounds 2k-2n A mixture of (E)-2-benzoyl-3-(4-fluorophenyl)acryloni-  C. X. CHEN ET AL. Open Access OJMC 134 itrile 1k (50.2 mg, 0.2 mmol), malononitrile (19.8 mg, 0.3 mmol), and piperidine (17.0 mg, 0.2 mmol) in tolu- ene (2 mL) was stirred at room temperature for 24 h. After the solvent was evaporated, the residue was puri- fied by flash column chromatography (ethyl acetate/pe- troleum ether = 1:5) to give 2k. 4.4.1. 2- Ami no-4-(4-Fluorophenyl) - 6-Phenyl- 4H-Pyran-3,5-Dicarbonitrile (2k) Yellow solid, yield 85%, mp 61˚C - 63˚C; 1H NMR (400 MHz, CDCl3): δ = 7.74 (2H, dd, J = 7.0, 1.5 Hz, ArH), 7.56 - 7.40 (3H, m, ArH), 7.38 - 7.27 (2H, m, ArH), 7.10 (2H, ddd, J = 8.6, 5.0, 2.7 Hz, ArH), 4.89 (2H, s, NH2), 4.35 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δ = 163.98, 161.48, 157.86, 157.70, 136.49, 131.96, 129.71 , 129.60, 129.51, 128.81, 127.80, 117.76, 116.83, 116.34, 116.12, 90.70, 59.91, 40.11; IR (KBr) v/cm−1: 3333 (w), 2198 (w), 1673 (s), 1602 (w), 1508 (m), 1401 (m), 1341 (s), 1263 (w), 1081 (m), 743 (w); HRMS (ESI) calcd for C19H12N3OF+ [M + Na]+: 340.0857, found: 340.0891. 4.4.2. 2-Amino-6-Me thyl-4,5- Di phenyl-4H- Pyran-3-Carbonitrile (2l) [12] White solid, yield 78%, 1H NMR (400 MHz, CDCl3): δ = 7.23 - 7.13 (6H, m, ArH), 7.09 - 7.07 (2H, m, ArH), 6.90 - 6.87 (2H, m, ArH), 4.49 (2H, s, NH2), 4.18 (1H, s, CH), 1.80 (3H, s, CH3). 4.4.3. 2-Amin o-4-(4-Chlorophenyl)- 5, 6 -Diphenyl- 4H-Pyran-3-Carbonitrile (2m) [12] White solid, yield 80%, 1H NMR (400 MHz, CDCl3): δ = 7.26 - 7.07 (12H, m, ArH), 6.84 - 6.82 (2H, m, ArH), 4.56 (2H, s, NH2), 4.35 (1H, s, CH). 4.4.4. 2- Ami no-5,6-Dipheny l -4-(Thiophen-2-yl) - 4H-Pyran-3-Carbonitrile (2n) [12] White solid, yield 86%, 1H NMR (400 MHz, CDCl3): δ = 7.25 - 7.10 (9H, m, ArH), 6.95 - 6.93 (2H, m, ArH), 6.87 - 6.85 (1H, m, ArH), 6.79 (1H, d, J = 2.8, Hz, ArH), 4.66 (1H, s, CH), 4.58 (2H, s, NH2). 4.5. Typical Procedure for the Synthesis of Compounds 4a and 4j A mixture of compound 2a (39.1 mg, 0.1 mmol), Ac- ONH4 (92 mg, 1.2 mmol), and AcOH (1.0 mL) in EtOAc (1.0 mL) was refluxed for 24 h. After cooled to room temperature, EtOAc (10 mL) was added. The mixture was washed with saturated aqueous NaHCO3 (10 mL) and brine (5 mL), and dried over anhydrous Na2SO4. After the solvent was evaporated under vacuum, the re- sidue was purified by flash column chromatography (ethyl acetate/petroleum ether = 2:5) to give the products 4a. 4.5.1. (E)-2 - A mi no- 8-(4-Fluorobenzylidene)- 4- (4-Fluorophenyl)-6-Methyl-5,6,7,8-Tetrahydro- 1,6-Naphthyridine-3-Carbonitrile (4a) Yellow solid, yield 80%, mp 208˚C - 210˚C; 1H NMR (400 MHz, CDCl3): δ = 8.02 (1H, s, HC=C), 7.31 - 7.10 (8H, m, ArH), 5.15 (2H, s, NH2), 3.61 (2H, s, CH2), 3.27 (2H, s, CH2), 2.35 (3H, s, CH3); 13C NMR (100 MHz, CDCl3): δ = 164.50, 163.38, 162.20, 161.18, 157.36, 153.20, 152.20, 132.66, 131.78, 131.48, 131.40, 130.21, 130.13, 129.39, 116.55, 116.22, 116.00, 115.58, 115.37, 90.78, 55.84, 55.27, 45.52; IR (KBr) v/cm−1: 3427 (s), 2215 (w), 2024 (w), 1627 (m), 1602 (m), 1558 (s), 1506 (s), 1423 (w), 1224 (m), 1160 (w), 1103 (s), 917 (w), 829 (w), 558 (m); HRMS (ESI) calcd for C23H18N4F2 + [M + H]+: 389.1572, found: 389.1569. 4.5.2. 2-Amin o- 4- ( 4-Fl uorophenyl )- 5H -Thiochromeno [4,3-b]Pyridine-3-Carbonitrile (4j) Yellow solid, yield 85%, mp 202˚C - 204˚C; 1H NMR (400 MHz, CDCl3): δ = 8.32 (1H, d, J = 6.6 Hz, ArH), 7.51-7.11 (7H, m, ArH), 5.26 (2H, s, NH2), 3.65 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δ = 164.51, 162.03, 158.00, 154.68, 151.51, 136.62, 133.51, 131.06, 130.57, 130.53, 130.50, 128.39, 127.84, 126.26, 116.94, 116.39, 116.17, 90.92, 27.21; IR (KBr) v/cm−1: 3446 (s), 2360 (w), 2213 (w), 2023 (w), 1626 (s), 1559 (m), 1427 (m), 1224 (w), 1074 (s), 843 (w), 767 (w), 546 (m); HRMS (ESI) calcd for C19H12N3FS+ [M + H]+: 334.0809, found: 334.0804. 4.6. Evaluation of Antitubercular Activity Autoluminescent M. tuberculosis H37Ra was constructed as previously reported [14] and was inoculated in a 50 mL centrifuge tube containing 5 mL 7H9 with 0.1% Tween80 and 10% ODAC, then incubated at 37˚C with shaking. When the culture reached an OD600 nm of 0.7, the culture was diluted. 50 L diluted H37Ra were in- oculated in sterile 384 well plate. The RLU of each well should be between 8000 - 12000 and was recorded as the basic luminescence of Day 0. The test compounds and the positive drugs were added to the 384 well plate in triplicate by the Echo520 with the final concentration 1 or 10 g/mL. The luminescent values were detected for the following three days. The data were analyzed with the Excel compared to the DMSO control to estimate the inhibition activity of the compounds. The antitubercular activities against M. tuberculosis H37Rv were determined by standard agar dilution me- thod [15]. 5. Acknowledgements Financial supports from National Natural Science Foun- dation of China (Nos. 20972195, 21172270) and Guang-  C. X. CHEN ET AL. Open Access OJMC 135 dong Engineering Research Center of Chiral Drugs are gratefully acknowledged. REFERENCES [1] World Health Organization, “Global Tuberculosis Control Report 2013,” World Health Organization, Geneva. http://www.who.int/tb/publications/global_report/en/ [2] L. Christian, G. Philippe, U. Mukund, L. Knut, G. Hai- leyesus and R. Mario, “Global Tuberculosis Control: Les- sons Learnt and Future Prospects,” Nature Reviews Mi- crobiology, Vol. 10, No. 6, 2012, pp. 407-416. http://dx.doi.org/10.1038/nrmicro2797 [3] World Health Organization, “Multidrug-Resistant Tuber- culosis (MDR-TB),” World Health Organization, Geneva. http://www.who.int/tb/challenges/mdr/en/index.html [4] M. P. Grobusch, “Drug-Resistant and Extensively Drug- Resistant Tuberculosis in Southern Africa,” Current Opi- nion in Pulmonary Medicine, Vol. 16, No. 3, 2010, pp. 180-185. http://dx.doi.org/10.1097/MCP.0b013e3283378680 [5] J. L. Mainardi, J. E. Hugonnet, L. Gutmann and M. Ar- thur, “Fighting Resistant Tuberculosis with Old Com- pounds: The Carbapenem Paradigm,” Clinical Microbio- logy and Infection, Vol. 17, No. 12, 2011, pp. 1755-1756. http://dx.doi.org/10.1111/j.1469-0691.2011.03699.x [6] A. G. Nikalje and P. Mudassar, “Multidrug-Resistant My- cobacterium Tuberculosis: A Brief Review,” Asian Jour- nal of Biological Sciences, Vol. 4, No. 2, 2011, pp. 101- 115. http://dx.doi.org/10.3923/ajbs.2011.101.115 [7] R. Shi and I. Sugawara, “Development of New Anti- Tuberculosis Drug Candidates,” Tohoku Journal of Ex- perimental Medicine, Vol. 221, No. 2, 2010, pp. 97-106. http://dx.doi.org/10.1620/tjem.221.97 [8] S. T. Cole and G. Riccardi, “New Tuberculosis Drugs on the Horizon,” Current Opinion in Microbiology, Vol. 14, No. 5, 2011, pp. 570-576. [9] R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, “An Atomefficient, Solvent-Free, Green Synthesis and Antimycobacterial evaluation of 2-amino-6- Methyl-4-Aryl-8-[(E)-Arylmethylidene]-5,6,7,8-Tetrahy-Dro- 4H-Pyrano[3,2-c]Pyridine-3-Carbonitriles,” Bioorganic & Me- dicinal Chemistry Letters, Vol. 17, No. 23, 2007, pp. 6459-6462. http://dx.doi.org/10.1016/j.bmcl.2007.09.095 [10] R. R. Kumar, S. Perumal, J. C. Menendez, P. Yogeeswari and D. Sriram, “Antimycobacterial Activity of Novel 1,2,4- Oxadiazole-Pyranopyridine/Chromene Hybrids Generated by Chemoselective 1,3-Dipolar Cycloadditions of Nitrile Oxides,” Bioorganic & Medicinal Chemistry Letters, Vol. 19, No. 11, 2011, pp. 3444-3450. http://dx.doi.org/10.1016/j.bmc.2011.04.033 [11] Z. P. Hu, C. L. Lou, J. J. Wang, C. X. Chen and M. Yan, “Organocatalytic Conjugate Addition of Malononitrile to Conformationally Restricted Dienones,” The Journal of Organic Chemistry, Vol. 76, No. 10, 2011, pp. 3797- 3804. [12] Z. P. Hu, W. J. Wang, X. G. Yin, X. J. Zhang and M. Yan, “Enantioselective Synthesis of 2-Amino-4H-Pyrans via the Organocatalytic Cascade Reaction of Malononitrile and A-Substituted Chalcones,” Tetrahedron: Asymmetry, Vol. 23, No. 22-23, 2012, pp. 461-467. [13] H. F. Wang, P. Li, H. F. Cui, X. W. Wang, J. K. Zhang, W. Liu and G. Zhao, “Highly Enantioselective Synthesis of A-Trichloromethyldihydropyrans Catalyzed by Bifunc- tional Organocatalysts,” Tetrahedron, Vol. 67, No. 10, 2011, pp. 1774-1780. http://dx.doi.org/10.1016/j.tet.2011.01.043 [14] T. Y. Zhang, S. Y. Li and E. L. Nuermberger, “Autolu- minescent Mycobacterium Tuberculosis for Rapid, Real- Time, Non-Invasive Assessment of Drug and Vaccine Ef- ficacy,” PLoS One, Vol. 7, No. 1, 2012, Article ID: e29774. http://dx.doi.org/10.1371/journal.pone.0029774 [15] National Committee for Clinical Laboratory Standards, “Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Approved Standard,” 2nd Edition, CLSI Document M24-A2, Vol. 31, No. 5, 2011, pp. 3-19.
|