 Vol.5, No.11A, 35-41 (2013) Health http://dx.doi.org/10.4236/health.2013.511A1005 Renal function after laparoscopic cholecystectomy and analgesia with tramadol and dipyrone or ketorolac Tiago Pechutti Medeiros1, Pedro Thadeu Galvão Vianna2, Leopoldo Muniz da Silva3, Lídia Raquel de Carvalho4, Gilberto Elias Wady5, Leandro Gobbo Braz2, Yara Marcondes Machado Castiglia2* 1Graduate Program in Anesthesiology, Botucatu Medical School, UNESP-Universidade Estadual Paulista, Botucatu, Brazil 2Department of Anesthesiology, Botucatu Medical School, UNESP-Universidade Estadual Paulista, Botucatu, Brazil; *Corresponding Author: yarac@fmb.unesp.br 3São Luiz Hospital, São Paulo, Brazil 4Department of Biostatics, Institute of Biosciences, UNESP-Universidade Estadual Paulista, Botucatu, Brazil 5Mercy Hospital, São Carlos, Brazil Received 4 September 2013; revised 12 October 2013; accepted 28 October 2013 Copyright © 2013 Tiago Pechutti Medeiros et al. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Laparoscopic cholecystectomy (LC) reduces surgical trauma and hospital stay, but requires effective and safe postoperative anal- gesia. This prospective and double-blind study investigated the effects of analgesia with tra- madol combined with either dipyrone or keto- rolac on the postoperative renal function of pa- tients submitted to LC. Methods: Pre- and post- operatively (PO), estimated glomerular filtration rates (GFR), obtained by two formulas depend- ent on blood Cr and one on blood cystatin C values, and tubular enzymuria—alkaline phos- phatase (AP), γ-glutamiltransferase (γ-GT)— were determined in well hydrated patients who underwent LC and analgesia with tramadol com- bined with either dipyrone (Dipyrone, n = 63) or ketorolac (Ketorolac, n = 63). Upon discharge from the post-anesthetic care unit (PACU), pain (through Verbal Numerical Scale—VNS) and need for rescue analgesia with morphine were evaluated. Results: There was hemodilution PO, which made GFR profile analysis more diffi- cult—those dependent on Cr increased and sta- tistically correlated, but those dependent on cystatin C did not change. There was a signifi- cant PO increase in AP in the Dipyrone and Ke- torolac groups, and in the product of the both enzymes in the Ketorolac group. Upon PACU discharge, the Dipyrone group showed signifi- cantly higher VNS scores than the Ketorolac group. All patients received morphine PO, and the total dose needed for pain control differed between groups, but without statistical signifi- cance. Conclusions: The association of trama- dol with dipyrone or ketorolac in well hydrated patients submitted to LC had similar analgesic effectiveness in the PACU. Postoperatively, the effect on GFR may have been masked by hemo- dilution, and enzymuria was discreetly enhanced when ketorolac was used. Keywords: Kidney Function Tests; Pneumoperitoneum; Biological Markers; Cystatin C; Ketorolac; Analgesia 1. INTRODUCTION Renal venous pressure, hydrostatic pressure and glo- merular filtration rate (GFR) are adversely affected by pneumoperitoneum. However, expansion of the extra- cellular volume can be beneficial to renal function [1-3]. Pain after laparoscopic surgery is multifactorial and may be quite intense. Current pain management relies on a number of strategies, including the concomitant use of opioids and anti-inflammatory drugs [4]. Remifentanil, a synthetic opioid used in anesthesia, has a predictable duration of action (elimination half-life of 8 - 20 minutes) because it is metabolized via plasma esterases [5]. While reducing opioid-induced adverse ef- Copyright © 2013 SciRes. OPEN ACCESS  T. P. Medeiros et al. / Health 5 (2013) 35-41 36 fects, remifentanil does not provide residual analgesia at the immediate postoperative period. Tramadol is a cen- trally acting analgesic that consists of two enantiomers, both of which contribute to analgesic activity via dif- ferent mechanisms: (+)-tramadol and the metabolite (+)-0-desmethyl-tramadol are agonists of μ opioid re- ceptor. (+)-tramadol inhibits serotonin re-uptake, and (−)-tramadol inhibits norepinephrine re-uptake, increas- ing the inhibitory effects on pain transmission in the spinal cord [6]. It is excreted via the kidney (over 30%), and in bolus doses and various injections it does not affect renal blood flow in normal rats [7]. Tramadol and non- steroidal anti-inflammatory drugs (NSAID) have often been combined for the clinical treatment of post-operative pain. However, the stimulation of opioid receptors may increase pain sensitivity immediately after opioid admi- nistration (opioid-induced hyperalgesia). Therefore, in- vestigating the effects of combining opioids with non- opioid analgesics, including NSAIDs, would be of espe- cial interest for anesthesia researchers [8]. NSAIDs block the enzyme cyclooxygenase (COX) and, in consequence, the synthesis of renal vasodilator prostaglandins (PG) [9], which help maintain renal blood flow and GFR, modulating the vasoconstrictor effects of angiotensine II or norepinephrine [10]. However, in rats anesthetized with sodium pentobarbital, the NSAID ke- toprofen, administered early after hypotension due to hemorrhage, caused fewer changes in renal function and hystology than the barbituric [11]. Serum cystatin C level, an early marker of mild GFR deterioration [12], is a useful endogenous alternative to estimate GFR. However, in a study including 8058 individuals aged 25 - 75 years, besides not being superior to creatinine in measuring GFR, serum cystation C seemed to be influenced by factors other than kidney function alone [13]. To measure the integrity of tubular cells, assessing urinary concentrations of renal enzymes is a sensitive non-invasive method. These renal enzymes are located on specific sites of the kidney: γ-gluta- myltransferase (γ-GT) is mainly found in the tubules near the loop of Henle, while alkaline phosphatase (AP) is found in the epithelial cells of the proximal tubule [14]. As such, once serum creatinine, cystatin C and urinary enzymes are determined, results that would possibly quantify perioperative injury to the kidneys may be obtained. Thus, the aim of this clinical prospective trial was to assess the effects of two analgesia regimens on post- operative renal function in patients submitted to general anesthesia for laparoscopic cholecystectomy. 2. METHODS This was a randomized, placebo-controlled, double- blind trial. After Institutional Review Board approval, written informed consent was obtained from a total of 126 patients scheduled for general anesthesia for elective laparoscopic cholecystectomy with CO2 pneumoperito- neum at 13 mmHg for chronic cholecystitis due to cholelithiasis. Patients older than 60 years, allergic to NSAIDs or opioids, with an ASA physical status of more than III, plasma creatinine higher than 1.5 mg/dl or heart failure, and users of nephrotoxic drugs were excluded. Oral 15 mg midazolam was given as pre-medication to all patients, one hour before anesthesia. Patients were included in one of two groups: Dipyrone group—re- ceived placebo (saline) intravenously (iv) at the time of pre-medication, and 100 mg tramadol with 2 g dipyrone, iv, approximately 30 min before the end of anesthesia for analgesia; Ketorolac group—received 30 mg ketorolac, iv, at the time of pre-medication, and 100 mg tramadol with 30 mg ketorolac, iv, approximately 30 min before the end of anesthesia for analgesia. The placebo and ketorolac solutions were administered by a nurse, who prepared them in an identical volume (10 ml). The same nurse prepared the drugs for postoperative analgesia, so that the double-blind condition of the study was main- tained. In the operating theater, all patients were monitored with electrocardioscopy, pulse oxymeter and non-inva- sive arterial pressure measurement. All patients were given 10 ml/kg/h Ringer lactate solution. At this time point (T1), 20 ml of blood were collected for the labora- tory assessment of cystatin C, by the immunonephe- lometric method using Dade Behring® reagents and cali- brators (N Latex Cystatin C, Dade Behring, Deerfield, USA), urea and creatinine (dry-chemical method), and albumin by protein electrophoresis. Additionally, 80 ml of urine (after bladder catheterization) were collected to dose AP, γ-GT, and creatinine, by the Vitros 950— Johnson & Johnson® (USA) automation system. Urinary creatinine concentrations in mg/dl were multiplied by 0.0884 for conversion to mmol/l, and used to eliminate the effect of urinary dilution: AP⁄creatinine (U/mmol); γ-GT/creatinine (U/mmol); AP × γ-GT/creatinine (U/mmol). Twenty four hours after the end of anesthesia and sur- gery (time point T2), 20 ml of blood and 80 ml of urine were once more collected, and the same laboratory as- says were repeated. 2.1. Anesthesia Procedures Anesthesia was induced with intravenous 0.5 μg/kg/min remifentanil, 2 mg/kg propofol, and 0.6 mg/kg rocur- onium. After tracheal intubation to perform general anesthesia, intermittent positive pressure ventilation was continued, and end-tidal carbon dioxide pressure was monitored to remain around 33 mmHg. Anesthesia was maintained with 0.25 μg/kg/min remifentanil, sevoflu- rane adjusted according to hemodynamic parameters, 4 Copyright © 2013 SciRes. OPEN ACCESS  T. P. Medeiros et al. / Health 5 (2013) 35-41 37 liters of fresh gas comprising N2O in 50% of O2, con- trolled breathing and rebreathing with a semiclosed sys- tem. Analgesia in patients from both the Dipyrone and the Ketorolac groups was assessed by the Verbal Numerical Scale (VNS) at admission to the post-anesthesia care unit (PACU), and at every 15 minutes until discharge, at least two hours after admission to the PACU. According to instructions received before anesthesia and surgery, pa- tients rated pain on a zero-ten scale where: zero = absence of pain, one (1) = minimum existing pain, and 10 = the worst pain imaginable. In the PACU, when values were above three in the VNS, 1mg morphine hydrochloride was administered, iv, every 10 minutes until pain cessation or VNS = 3. Level of sedation (awake and cooperative, asleep and cooperative after stimulus, asleep and uncooperative) and quantity of morphine used were also assessed in the PACU. GFR was estimated by the following formulas: GFR- Larsson (ml/min) [15,16] = 77.24 × [cystatin C−1.2623 (mg/l)]; GFR-MDRD (“Modification of Diet in Renal Disease”) (ml/min/1.73m2) [17] = 170 × (creatinine)−0.999 × (age)−0.176 × [0.762 if female] × [1.18 if black] × (urea)−0.17 × (albumin)0.318 and GFR-CG (ml/min) [18] = (140 – age) × weight/serum creatinine x 72 × [0.85 if female]. 2.2. Statistical Analysis The exact Fisher test was used to study the association between group and gender. The Student t test was used for comparisons between groups regarding age, pre- and postoperative cystatin C level (Δ cystatin C), and the amount of morphine used in the PACU. Profile analysis was used to study the effect of group, time and time × group interactions. Correlations between variables were analyzed by Pearson’s correlation coefficient. Statistical significance was set at p < 0.05. 3. RESULTS Duration of anesthesia was 70 min ± 10 in the Dipyrone group, and 68 min ± 13 in the Ketorolac group (p = 0.42). Age was 40.9 years ± 12.1 in the Dipyrone group, and 40.2 years ± 11.5 in the Ketorolac group (p = 0.75). In the Dipyrone and Ketorolac groups, females represented 73% (46) and 82.5% (52) of the patients, respectively (p = 0.28). Weight was 71.5 kg ± 13.5 in the Dipyrone group, and 73.6 kg ± 14.9 in the Ketorolac group (p = 0.31). It can be said that the groups were homogeneous (Table 1). Cystatin C values did not differ between groups (p = 0.30), and time points (p = 0.09), and there was no time points x groups interaction (p = 0.44) (Tab l e 1 ). Serum creatinine values were not different between groups (p = 0.62), nor was there time points × groups interaction (p = 0.10). However, there was a difference between time points (p = 0.00001) (Table 1). Plasma albumin pre- sented the same profile with no difference between groups (p = 0.42), without time points × groups interac- tion (p = 0.27), but showing a difference between time points (p = 0.0005) (Table 1). The GFR-Larsson was not different between groups (p = 0.33), and time points (p = 0.07), and there was no time points × groups interaction (p = 0.66) (Table 2). GFR-CG was not different between groups (p = 0.79), and did not exhibit time points × groups interaction (p = 0.55). However, it was different between time points (p = 0.001) (Table 2). GFR-MDRD was not different between groups (p = 0.72), and did not exhibit time points × groups interaction (p = 0.58), but it differed between time points (p = 0.003) (Table 2). A significant cor- relation was observed only between GFR-MDRD and GFR-CG values at T1 (r = 0.48 and p = 0.000) and T2 (r = 0.36 and p = 0.000). AP results significantly differed between groups (p = 0.02) and time points (p = 0.03), and showed time points × groups interaction (p = 0.001). Thus, Dipyrone group > Ketorolac group and T1 < T2 (Tab le 2 ). γ-GT presented a statistically significant difference between groups (p = 0.001), and time points × groups interaction (p = 0.00002), with no difference between time points (p = 0.07). The product of the two enzymes significantly differed between groups (p = 0.005) and time points (p = 0.008), and showed time points × groups interaction (p = 0.0002) (Table 2). Table 1. Clinical data of study group patients (Means ± SD). Parameter Dipyrone gr oup (n = 63) Ketorolac group (n = 63) p Age (years) 40.9 ± 12.1 40.2 ± 11.5 0.75 Weight (kg) 71.5 ±13.5 73.6 ± 14.9 0.31 Gender F:M 46:17 52:11 0.28 Duration of anesthesia (min) 70 ± 10 68 ± 13 0.42 T10.83 ± 0.19 0.78 ± 0.18 *0.31 Plasma cystatin C (mg/l) T20.84 ± 0.18 0.81 ± 0.17 #0.24 T10.78 ± 0.14 0.77 ± 0.16 *0.62 Blood creatinine (mg/dl) T20.70 ± 0.16 0.73 ± 0.19 #0.00001 T14.1 ± 0.7 4.1 ± 0.5 *0.42 Plasma albumin (g/dl)T23.3 ± 0.7 3.7 ± 2.5 #0.0005 Δ cystatin C (mg/ml) 0.0017 ± 0.14 0.0030 ± 0.15 *0.23 * = p between Dipyrone and Ketorolac groups; # = p between time point 1 (T1—at the arriving in operating theater) and time point 2 (T2—24 h after the end of anesthesia and surgery). Copyright © 2013 SciRes. OPEN ACCESS 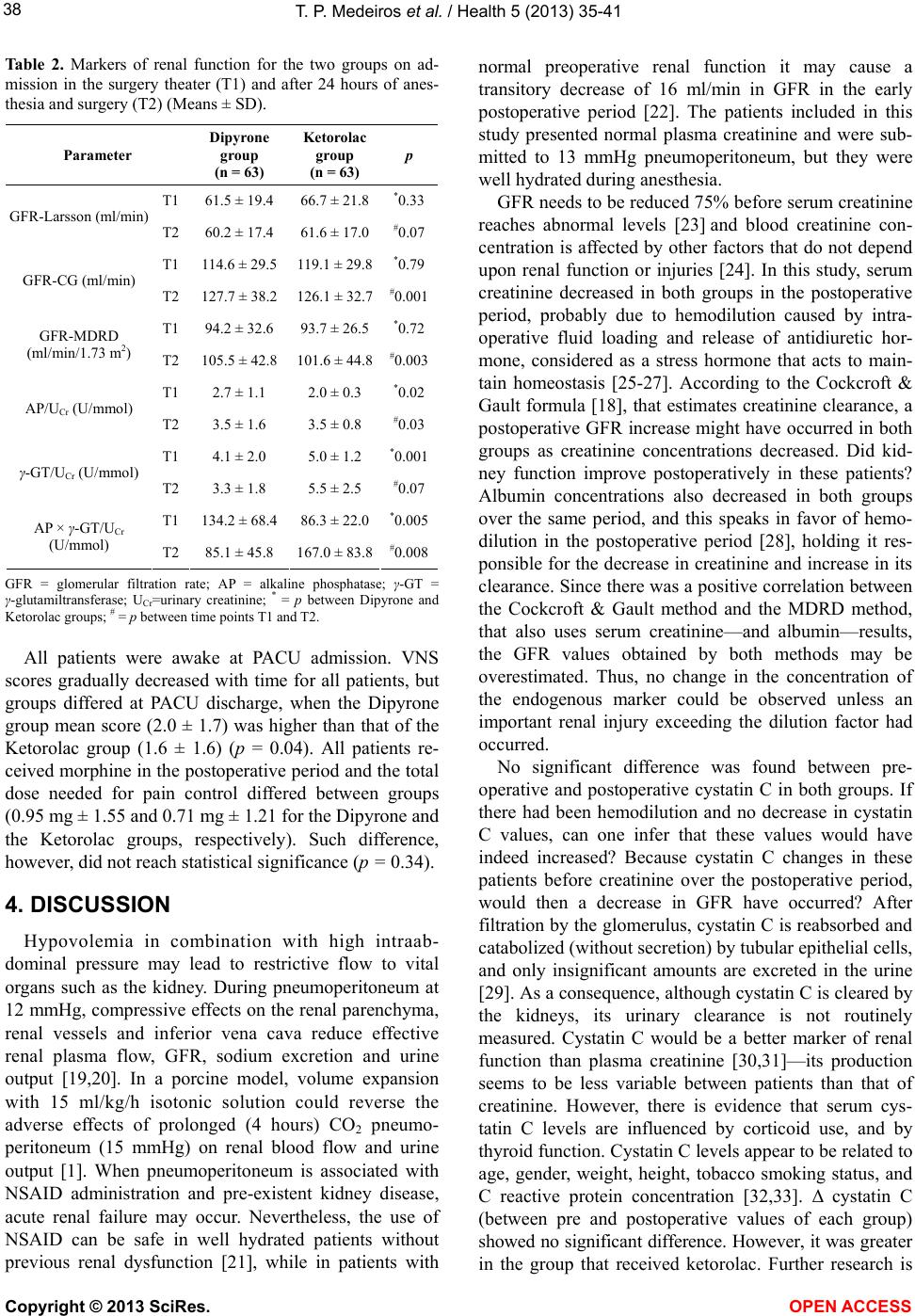 T. P. Medeiros et al. / Health 5 (2013) 35-41 38 Table 2. Markers of renal function for the two groups on ad- mission in the surgery theater (T1) and after 24 hours of anes- thesia and surgery (T2) (Means ± SD). Parameter Dipyrone group (n = 63) Ketorolac group (n = 63) p T1 61.5 ± 19.4 66.7 ± 21.8*0.33 GFR-Larsson (ml/min) T2 60.2 ± 17.4 61.6 ± 17.0#0.07 T1 114.6 ± 29.5 119.1 ± 29.8*0.79 GFR-CG (ml/min) T2 127.7 ± 38.2 126.1 ± 32.7#0.001 T1 94.2 ± 32.6 93.7 ± 26.5*0.72 GFR-MDRD (ml/min/1.73 m2) T2 105.5 ± 42.8 101.6 ± 44.8#0.003 T1 2.7 ± 1.1 2.0 ± 0.3 *0.02 AP/UCr (U/mmol) T2 3.5 ± 1.6 3.5 ± 0.8 #0.03 T1 4.1 ± 2.0 5.0 ± 1.2 *0.001 γ-GT/UCr (U/mmol) T2 3.3 ± 1.8 5.5 ± 2.5 #0.07 T1 134.2 ± 68.4 86.3 ± 22.0*0.005 AP × γ-GT/UCr (U/mmol) T2 85.1 ± 45.8 167.0 ± 83.8#0.008 GFR = glomerular filtration rate; AP = alkaline phosphatase; γ-GT = γ-glutamiltransferase; UCr=urinary creatinine; * = p between Dipyrone and Ketorolac groups; # = p between time points T1 and T2. All patients were awake at PACU admission. VNS scores gradually decreased with time for all patients, but groups differed at PACU discharge, when the Dipyrone group mean score (2.0 ± 1.7) was higher than that of the Ketorolac group (1.6 ± 1.6) (p = 0.04). All patients re- ceived morphine in the postoperative period and the total dose needed for pain control differed between groups (0.95 mg ± 1.55 and 0.71 mg ± 1.21 for the Dipyrone and the Ketorolac groups, respectively). Such difference, however, did not reach statistical significance (p = 0.34). 4. DISCUSSION Hypovolemia in combination with high intraab- dominal pressure may lead to restrictive flow to vital organs such as the kidney. During pneumoperitoneum at 12 mmHg, compressive effects on the renal parenchyma, renal vessels and inferior vena cava reduce effective renal plasma flow, GFR, sodium excretion and urine output [19,20]. In a porcine model, volume expansion with 15 ml/kg/h isotonic solution could reverse the adverse effects of prolonged (4 hours) CO2 pneumo- peritoneum (15 mmHg) on renal blood flow and urine output [1]. When pneumoperitoneum is associated with NSAID administration and pre-existent kidney disease, acute renal failure may occur. Nevertheless, the use of NSAID can be safe in well hydrated patients without previous renal dysfunction [21], while in patients with normal preoperative renal function it may cause a transitory decrease of 16 ml/min in GFR in the early postoperative period [22]. The patients included in this study presented normal plasma creatinine and were sub- mitted to 13 mmHg pneumoperitoneum, but they were well hydrated during anesthesia. GFR needs to be reduced 75% before serum creatinine reaches abnormal levels [23] and blood creatinine con- centration is affected by other factors that do not depend upon renal function or injuries [24]. In this study, serum creatinine decreased in both groups in the postoperative period, probably due to hemodilution caused by intra- operative fluid loading and release of antidiuretic hor- mone, considered as a stress hormone that acts to main- tain homeostasis [25-27]. According to the Cockcroft & Gault formula [18], that estimates creatinine clearance, a postoperative GFR increase might have occurred in both groups as creatinine concentrations decreased. Did kid- ney function improve postoperatively in these patients? Albumin concentrations also decreased in both groups over the same period, and this speaks in favor of hemo- dilution in the postoperative period [28], holding it res- ponsible for the decrease in creatinine and increase in its clearance. Since there was a positive correlation between the Cockcroft & Gault method and the MDRD method, that also uses serum creatinine—and albumin—results, the GFR values obtained by both methods may be overestimated. Thus, no change in the concentration of the endogenous marker could be observed unless an important renal injury exceeding the dilution factor had occurred. No significant difference was found between pre- operative and postoperative cystatin C in both groups. If there had been hemodilution and no decrease in cystatin C values, can one infer that these values would have indeed increased? Because cystatin C changes in these patients before creatinine over the postoperative period, would then a decrease in GFR have occurred? After filtration by the glomerulus, cystatin C is reabsorbed and catabolized (without secretion) by tubular epithelial cells, and only insignificant amounts are excreted in the urine [29]. As a consequence, although cystatin C is cleared by the kidneys, its urinary clearance is not routinely measured. Cystatin C would be a better marker of renal function than plasma creatinine [30,31]—its production seems to be less variable between patients than that of creatinine. However, there is evidence that serum cys- tatin C levels are influenced by corticoid use, and by thyroid function. Cystatin C levels appear to be related to age, gender, weight, height, tobacco smoking status, and C reactive protein concentration [32,33]. Δ cystatin C (between pre and postoperative values of each group) showed no significant difference. However, it was greater in the group that received ketorolac. Further research is Copyright © 2013 SciRes. OPEN ACCESS  T. P. Medeiros et al. / Health 5 (2013) 35-41 39 needed to find out whether this is clinically relevant. In this study, the endogenous biomarkers of renal injury AP and γ-GT were dosed in urine. The increased secretion of both markers, that takes place before serum creatinine increases, indicates injury in the brush border membrane and loss of the microvilli structure. In rats submitted to an excessive dose of paracetamol (leading to acute proximal tubular injury), the urinary levels of these enzymes significantly increased in the first 24 hours, and returned to nearly baseline values after 48 - 72 hours, while GFR drastically decreased [34]. Our results show that urinary AP increased 24 h after surgery in both groups, indicating alteration in the tu- bular cells after surgery, apparently not related to ke- torolac use. In the group that did not receive NSAID, AP values were higher at both time points studied. The concentration of γ-GT did not significantly change in the postoperative period in both groups, and in contrast with AP, it remained higher in the Ketorolac group. The product of both urinary enzymes showed a different profile in both groups and time points. In the Dipyrone group, the product was significantly higher preoperatively, but it de- creased in the postoperative period. In patients receiving ketorolac, urinary enzyme product was significantly in- creased postoperatively. Whether such increase indicates some degree of injury at the brush border membrane of the tubular cell is not clear. Further studies are necessary to substantiate and better explain these results. The difference in preoperative enzyme results observed between groups may be due to individual differences in creatinine excretion (the common denominator). This may be affected by various factors such as age, gender, race, body habits, obesity, chronic disease (poor nutrition, inflammation, lack of conditioning when bedridden, neu- romuscular disease) and diet [35]. On the other hand, it is noteworthy that patients in the Ketorolac group at T1 had already received ketorolac at least 60 minutes earlier. The consequences of preoperative stress in this group would then be milder, hence less urinary enzyme released. The cross-talk mechanism involving the afferent and efferent arterioles and the renal tubules has been de- monstrated. Such relation would be more intense than expressed by the anatomical contact between these struc- tures, and has been known for a long time as the jux- taglomerular apparatus. Thus, decreased stress and stress hormones may indirectly influence the tubules by acting on these renal capillaries [36]. Ketorolac given as a pre-medication drug (0.5 mg/kg) in gynecological laparoscopy has been reported to influence the response of white blood cells, which is usually affected by surgical stress [37]. Administered intravenously, 15 mg ketorolac followed by 7.5 mg/h, in elective cesarean section, eased hemodynamic response to stress of tracheal intubation, improving the quality of analgesia and determining lower concentrations of plas- ma cortisol [38]. Ketorolac reaches maximum plasma concentration in 45 minutes and analgesic peak in one or two hours with an increased peak in aged patients with impaired renal function. In a study of 40 dogs submitted to hysterectomy and oophorectomy under general anes- thesia, ketorolac was administered as one of the anal- gesics for treatment of postoperative pain (0.5 mg/kg). The analysis of serum creatinine and urea and renal function biomarkers such as γ-GT and AP showed that ketorolac is safe for this purpose [39]. In this study, good results were achieved using ana- lgesia for pain control in both groups. According to VNS, only a small amount of rescue analgesia (morphine) was needed. Treatment with ketorolac as a premedication may ease renal response to preoperative stress. Addi- tionally, low-dose ketorolac instillation into wounds has been demonstrated to modulate local inflammatory events, decrease postoperative pain, and reduce opioid con- sumption, suggesting that the administration of NSAIDs into surgical wounds may be an analgesic alternative to higher systemic dosing of NSAIDs [40]. In conclusion, both analgesia regimens used in this study that combined tramadol with either dipyrone or ketorolac, in well hydrated patients submitted to laparo- scopic cholecystectomy, showed similar results in the PACU. Postoperatively, the effect on GFR might have been masked by hemodilution, but there was a discreet increase in the release of renal tubular enzymes when ketorolac was used, a response that requires further clari- fication. 5. ACKNOWLEDGEMENTS Grant 07/51101-0—São Paulo Research Foundation (FAPESP); TP Medeiros was granted a scholarship from CAPES. REFERENCES [1] London, E.T., HO, H.S., Neuhaus, A.M., Wolfe, B.M., Rudich, S.M. and Perez, R.V. (2000) Effect of intravas- cular volume expansion on renal function during pro- longed CO2 pneumoperitoneum. Annals of Surgery, 231, 195-201. http://dx.doi.org/10.1097/00000658-200002000-00007 [2] Lindström, P., Wadström, J., Ollerstam, A., Johnsson, C. and Persson, A.E. (2003) Effects of increased intra-ab- dominal pressure and volume expansion on renal function in rat. Nephrology Dialysis Transplantation, 18, 2269- 2277. http://dx.doi.org/10.1093/ndt/gfg362 [3] Demyttenaere, S., Feldman, L.S. and Fried, G.M. (2007) Effect of pneumoperitoneum on renal perfusion and func- tion: A systematic review. Surgical Endoscopy, 21, 152- 160. http://dx.doi.org/10.1007/s00464-006-0250-x [4] Michaloliakou, C., Chung, F. and Sharma, S. (1996) Pre- operative multimodal analgesia facilitates recovery after Copyright © 2013 SciRes. OPEN ACCESS  T. P. Medeiros et al. / Health 5 (2013) 35-41 40 ambulatory laparoscopic cholecistectomy. Anesthesia & Analgesia, 82, 44-51. [5] Burkle, H., Dunbar, S. and Van Aken, H. (1996) Re- mifentanil: A novel, short-acting μ-opioid. Anesthesia & Analgesia, 83, 646-651. [6] Grond, S. and Sablotzki, A. (2004) Clinical pharmacol- ogy of tramadol. Clinical Pharmacokinetics, 43, 879-923. http://dx.doi.org/10.2165/00003088-200443130-00004 [7] Nagaoka, E., Minmi, K., Shiga, Y., Uezono, Y., Shiraishi, M., Aoyama, K. and Shigematsu, A. (2002) Tramadol has no effect on cortical renal blood flow despite increased serum catecholamine levels in anesthetized rats: Implica- tions for analgesia in renal insufficiency. Anesthesia & Analgesia, 94, 619-625. http://dx.doi.org/10.1097/00000539-200203000-00026 [8] Koppert, W. and Scmelz, M. (2007) The impact of opi- oid-induced hyperalgesia for postoperative pain. Best Practice & Research Clinical Anaesthesiology, 21, 65-83. http://dx.doi.org/10.1016/j.bpa.2006.12.004 [9] Kurumbail, R.G., Stevens, A.M., Gierse, J.K., McDonald, J.J., Stegeman, R.A., Pak, J.Y., Gildehaus, D., Miyashiro, J.M., Penning, T.D., Seibert, K., Isakson, P.C. and Stal- lings, W.C. (1996) Structural basis for selective inhibition of cuclooxygenase-2 by anti-inflammatory agents. Nature, 384, 644-648. http://dx.doi.org/10.1038/384644a0 [10] Dunn, M.J. and Zambraski, E.J. (1980) Renal effects of drugs that inhibit prostaglandin synthesis. Kidney Inter- national, 18, 609-622. http://dx.doi.org/10.1038/ki.1980.179 [11] De Souza Silva, M., Castiglia, Y.M., Vianna, P.T., Viero, R.M., Braz, J.R. and Cassetari, M.L. (2006) Rat model of depending prostaglandin renal state: Effect of ketoprofen. Renal Failure, 28, 77-84. http://dx.doi.org/10.1080/08860220500461294 [12] Coll, E., Botey, A., Alvarez, L., Poch, E., Quinto, L., Saurina, A., Vera, M., Piera, C. and Darnell, A. (2000) Serum cystatin C as a new marker for noninvasive esti- mation of glomerular filtration rate and as a marker for early renal impairment. American Journal of Kidney Dis- eases, 36, 29-34. http://dx.doi.org/10.1053/ajkd.2000.8237 [13] Knight, E.L., Verhave, J.C., Spiegelman, D., Hillege, H.L., Zeeuw, D.D., Curhan, G.C. and de Jong, P.E. (2004) Factors influencing serum cystatin C levels other than renal function and the impact on renal function measure- ment. Kidney International, 65, 1416-1421. http://dx.doi.org/10.1111/j.1523-1755.2004.00517.x [14] Jung, K. and Burchardt, U. (1987) Urinary enzymes in research and in clinical medicine. Report on a symposium of the Humboldt-Universität Berlin and the Bezirkskran- kenhaus Frankfurt/Oder in Frankfurt/Oder, 22-25 April 1987. Journal of Clinical Chemistry & Clinical Biochem- istry, 25, 823-828. [15] Larsson, A., Malm, J., Grubb, A. and Hansson, L.O. (2004) Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scandi- navian Journal of Clinical & Laboratory Investigation, 64, 25-30. http://dx.doi.org/10.1080/00365510410003723 [16] Madero, M., Sarnak, M.J. and Stevens, L.A. (2006) Se- rum cystatin C as a marker of glomerular filtration rate. Current Opinion in Nephrology and Hypertension, 15, 610-616. http://dx.doi.org/10.1097/01.mnh.0000247505.71915.05 [17] Levey, A.S., Bosch, J.P., Lewis, J.B., Greene, T., Rogers, N. and Roth, D. (1999) A more accurate method to esti- mate glomerular filtration rate from serum creatinine: A new prediction equation. Annals of Internal Medicine, 30, 461-470. http://dx.doi.org/10.7326/0003-4819-130-6-199903160-0 0002 [18] Cockcroft, D.W. and Gault, M.H. (1976) Prediction of creatinine clearance from serum creatinine. Nephron, 16, 31-41. http://dx.doi.org/10.1159/000180580 [19] Ost, M.C., Tan, B.J. and Lee, B.R. (2005) Urological laparoscopy: Basic physiological considerations and im- munological consequences. Journal of Urology, 174, 1183- 1188. http://dx.doi.org/10.1097/01.ju.0000173102.16381.08 [20] Miki, Y., Iwase, K., Kamiike, E., Taniguchi, E., Sakagu- chi, K., Sumimura, J., Matsuda, H. and Nagai, I. (1997) Laparoscopic cholecystectomy and time-course changes in renal function. Surgical Endoscopy, 11, 838-841. http://dx.doi.org/10.1007/s004649900466 [21] Yuksel, H., Darcan, S., Kabasakal, C., Cura, A., Mir, S. and Mavi, E. (1998) Effect of enalapril on proteinuria, phosphaturia, and calciuria in insulin-dependent diabetes. Pediatric Nephrology, 12, 648-650. http://dx.doi.org/10.1007/s004670050520 [22] Lee, A., Cooper, M.G., Craig, J.C., Knight, J.F. and Ke- neally, J.P. (2007) Effects of nonsteroidal anti-inflam- matory drugs on postoperative renal function in adults with normal renal function. Cochrane Database of Sys- tematic Reviews, 2, CD002765. [23] Kellen, M., Aronson, S., Roizen, M.F., Barnard, J. and Thisted, R.A. (1994) Predictive and diagnostic tests of renal failure: A review. Anesthesia & Analgesia, 78, 134- 142. http://dx.doi.org/10.1213/00000539-199401000-00022 [24] Westhuyzen, J., Endre, Z.H., Reece, G., Reith, D.M., Saltissi, D. and Morgan, T.J. (2003) Measurement of tu- bular enzimuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrology Dialy- sis Transplantation, 18, 543-551. http://dx.doi.org/10.1093/ndt/18.3.543 [25] Goldsmith, S.R. (1988) Baroreceptor-mediated suppres- sion of osmotically stimulated vasopressin in normal hu- mans. Journal of Applied Physics, 65, 1226-1230. [26] Julier, K., Silva, R., Garcia, C., Bestmann, L., Frascarolo, P., Zollinger, A., Chassot, P.-G., Schmid, E.R., Turina, M.I., von Segesser, L.K., Pasch, T., Spahn, D.R. and Zaugg, M. (2003) Preconditioning by sevoflurane de- creases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. An- esthesiology, 98, 1315-1327. http://dx.doi.org/10.1097/00000542-200306000-00004 [27] Youssef, M.A. and saleh Al-Mulhim, A. (2007) Effects of different anesthetic techniques on antidiuretic hormone Copyright © 2013 SciRes. OPEN ACCESS  T. P. Medeiros et al. / Health 5 (2013) 35-41 Copyright © 2013 SciRes. OPEN ACCESS 41 secretion during laparoscopic cholecystectomy. Surgical Endoscopy, 21, 1543-1548. http://dx.doi.org/10.1007/s00464-006-9166-8 [28] Meyer, P., Pernet, P., Hejblum, G., Baudel, J.L., Maury, E., Offenstadt, G. and Guidet, B. (2008) Haemodilution in- duced by hydroxyethyl starches 130/0.4 is similar in sep- tic and non-septic patients. Acta Anaesthesiologica Scan- dinavica, 52, 229-235. http://dx.doi.org/10.1111/j.1399-6576.2007.01521.x [29] Herget-Rosenthal, S., Feldkamp, T., Volbracht, L. and Kribben, A. (2004) Measurement of urinary cystatin C by particle-enhanced nephelometric immunoassay: Precision, interferences, stability, and reference range. Annals of Clinical Biochemistry, 41, 111-118. http://dx.doi.org/10.1258/000456304322879980 [30] Dharnidharka, V.R., Kwon, C. and Stevens, G. (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. American Journal of Kidney Diseases, 40, 221-226. http://dx.doi.org/10.1053/ajkd.2002.34487 [31] Roos, J.F., Doust, J., Tett, S.E. and Kirkpatrick, C.M. (2007) Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—A meta-analysis. Clinical Biochem- istry, 40, 383-391. http://dx.doi.org/10.1016/j.clinbiochem.2006.10.026 [32] Randers, E. and Erlandsen, E.J. (1999) Serum cystatin C as an endogenous marker of the renal function—A review. Clinical Chemistry and Laboratory Medicine, 37, 389- 395. http://dx.doi.org/10.1515/CCLM.1999.064 [33] Coll, E., Botey, A., Alvarez, L., Poch, E., Quinto, L., Saurina, A., Vera, M., Piera, C. and Darnell, A. (2000) Serum cystatin C as a new marker for noninvasive esti- mation of glomerular filtration rate and as a marker for early renal impairment. American Journal of Kidney Dis- eases, 36, 29-34. http://dx.doi.org/10.1053/ajkd.2000.8237 [34] Da Silva Melo, D.A., Saciura, V.C., Poloni, J.A., Oliveira, C.S., Filho, J.C., Padilha, R.Z., Reichel, C.L., Neto, E.J., Oliveira, R.M., D’avila, L.C., Kessler, A. and de Oliveira, J.R. (2006) Evaluation of renal enzymuria and cellular excretion as an marker of acute nephrotoxicity due to an overdose of paracetamol in Wistar rats. Clinica Chimica Acta, 373, 88-91. http://dx.doi.org/10.1016/j.cca.2006.05.006 [35] Stevens, L.A., Coresh, J., Greene, T. and Levey, A.S. (2006) Medical progress: Assessing kidney function— Measured and estimated glomerular filtration rate. New England Journal of Medicine, 354, 2473-2483. http://dx.doi.org/10.1056/NEJMra054415 [36] Ren, Y., Garvin, J.L., Liu, R. and Carretero, O.A. (2009) Cross-talk between arterioles and tubules in the kidney. Pediatric Nephrology, 24, 31-35. http://dx.doi.org/10.1007/s00467-008-0852-8 [37] Hong, J.Y. (2005) The effect of preoperative ketorolac on WBC response and pain in laparoscopic surgery for en- dometriosis. Yonsei Medical Journal, 46, 812-817. http://dx.doi.org/10.3349/ymj.2005.46.6.812 [38] El-Tahan, M.R., Warda, O.M., Yasseen, A.M., Attallah, M.M. and Matter, M.K. (2007) A randomized study of the effects of preoperative ketorolac on general anaesthesia for caesarean section. International Journal of Obstetric Anesthesia, 16, 214-220. http://dx.doi.org/10.1016/j.ijoa.2007.01.012 [39] Lobetti, R.G. and Joubert, K.E. (2000) Effect of admini- stration of nonsteroidal anti-inflammatory drugs before surgery on renal function in clinically normal dogs. Ame- rican Journal of Veterinary Research, 61, 1501-1507. http://dx.doi.org/10.2460/ajvr.2000.61.1501 [40] Carvalho, B., Lemmens, H.J., Ting, V. and Angst, M.S. (2013) Postoperative subcutaneous instillation of low-dose ketorolac but not hydromorphone reduces wound exudate concentrations of interleukin-6 and interleukin-10 and im- proves analgesia following cesarean delivery. Journal of Pain, 14, 48-56. http://dx.doi.org/10.1016/j.jpain.2012.10.002
|