Technology and Investment
Vol.07 No.03(2016), Article ID:69705,13 pages
10.4236/ti.2016.73010
Open Innovation and Involvement of End-Users in the Medical Device Technologies’ Design & Development Process: End-Users’ Perspectives
Selim Hani1, Nathalie de Marcellis-Warin2
1ÉcolePolytechnique de Montréal, Montreal, Canada
2Harvard T Chan School of Public Health, Boston, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 12 June 2016; accepted 9 August 2016; published 12 August 2016
ABSTRACT
Literature and Regulatory bodies growing interest in End-Users’ implication in Medical Device Technologies (MDTs) development processes are a clear proof of the importance of this involvement and the positive impacts it can have on the development, implementation and use of MDTs, thus subsequent improvements in healthcare services’ delivery. However, existing research has mainly been focused on the theoretical importance of this involvement, and the manufacturers’ views and attitudes, with little attention focused on End-Users’ concerns and thoughts concerning this process. The aim of this paper is to identify the perspectives of Nurses and Doctors as the best representatives of MDT End-Users, regarding their own involvement in MDT development processes. The results of 49 semi-structured interviews conducted with End-Users, helped identify a number of high-level themes: 1) End-Users’ conflicting perspective with that of manufacturers regarding the impact of their involvement in MDT development; 2) End-Users’ concerns regarding the nature of their contribution, its level and their suggestions for a potential amelioration. These results reveal the importance End-Users attach to their involvement in MDT development processes, and the added value they perceive for the proper development as well as upgrade of MDTs. It also underlines many concerns they have regarding the current patterns of involvement, and suggests their recommendations for a standardization of this process, with input on forms and levels of involvement.
Keywords:
Medical Device Technologies, Open Innovation, End-Users’ Involvement

1. Introduction
End-user involvement in the development of medical device technologies (MDTs) has been the focus of many recent researches [1] - [6] . As a matter of fact this involvement has proved beneficial at many levels: improved patient safety [7] , increased user satisfaction [8] , reduction is development costs while limiting redesign [9] ―a finding contested by other authors [10] [11] ―and increased likelihood of commercial success [2] . This phenomenon became an imperative in MDTD (Medical Device Technology Development) after international bodies amongst which the US Food and Drug Administration [12] and the European Union [10] [13] started requiring user participation as well as user-focused development of medical device technologies as regulatory requirements that would lead to licensing and subsequent commercialization of the MDTs in the USA and the EU.
1.1. A Form of Open-Innovation?
The idea of open innovation comes in contrast with the traditional innovation development processes [14] - [16] , following more iterative processes, involving different internal but also external stakeholders and following unconventional methods of development [17] . A particular matter of interest that comes in contrast with the traditional in-house approach (Figure 1) is the involvement of new parties in the development process (Figure 2) like outsiders, competitors, universities and end-users more particularly [18] . Active implication of end-users is therefore proven of substantial advantage to the innovation itself and to its subsequent commercialization and market-acceptance [18] - [20] .
1.2. Where Is the End-User Involved?
Based on the seven phases of industrial product development [21] : 1) Idea, 2) Preliminary Assessment, 3) Concept, 4) Development, 5) Testing, 6) Trial, 7) Launch [21] , Biemans (1991) [22] tried to identify where in this 7-phase process was the role of the end-user most significant. On a study performed on 17 MDTs of 13 different
Figure 1. Traditional (or Closed) Innovation (Chesbrough, 2003) [16] .
Figure 2. Open Innovation (Chesbrough, 2003) [16] .
manufacturers it was shown that “end-users dominated the initial stages of the product development process while the manufacturers became the dominant party during the development of the prototype”. Indeed, numerous works have emphasized the role of the end-users in the more preliminary phases of development: idea generation and conceptualization [2] [3] [6] [22] , a role that can be of significant advantage to the manufacturer [22] . Literature however is strongly based on studies performed on manufacturers and developers [1] - [3] [6] [10] - [12] [22] and there is an absence [6] of works concentrated on the end-users’ perception of their own participation, and their personal subsequent satisfaction. In this study, we will concentrate our efforts to identify the end-users’ perceptions on this phenomenon, and thus evaluate end-users’ beliefs as compared to the manufacturers’ thoughts. In this study we will concentrate on Cooper [21] , 7-step approach as opposed to the 4-step development process suggested by Shah et al. [11] . By basing our study on 7 steps instead of 4 we allow the participants a broader range of choice and decision in regards to the level of involvement they believe they have been involved in, or think their involvement would be advantageous. Shah et al. [11] ’s Step 1 merges the idea generation phase, the preliminary assessment as well as the conceptualization phases pointed by Biemans [22] (Phases 1, 2 & 3). Step 2 corresponds to the Development phase (Phase 4), Step 3 regroups in-house testing and field trial, phases 5 & 6 respectively and Step 4 contains the Launch phase but also a “market & user-feedback” phase absent in Cooper’s [21] and Biemans’ [22] works.
1.3. Different Types of End-Users: Whom to Include in Our Study?
According to the literature the end-user in the case of MDTD can be one of the following: healthcare professionals, patients and lay caregivers [1] [3] [12] . Literature though tends to emphasize the contributions of “lead-users” [15] portraying them as representatives of the major-users, or of high-professional healthcare individuals [11] and thus limiting the investigations concerning the role of the patients or of more down-the-line healthcare professionals like nurses and family-members caregivers. There is evidence that involving end-users in the planning and development of healthcare leads to more acceptable and satisfactory services, and this idea is growing and has been defended by many previous works [4] [5] , some of which went to recommend involving patients and nurses as a mean of improving the quality of medical device technologies in particular [6] . Patients with disabilities and special needs are less likely to be included in development processes according to the literature [2] [23] , but healthcare and homecare technologies developed to assist them in mobility, activities of daily living, medical care, nursing care, ventilation are significantly improving [24] [25] , as are nurses and Healthcare Workers lower in the Institution’s hierarchy. Nurses’ relationship as well as interactions with MDTs is substantial and significant as they can be considered some of the main users of these technologies in providing healthcare services [26] , and actively participate in the adoption and implementation of new systems within healthcare facilities [27] [28] . Another interesting point is the impact MDTs are shown to have on improving caregivers’ and nurses’ quality of life [29] [30] not limiting it to patients’ survival. Our definition of an end-user for the sake of this present research will cover HCWs (mainly Doctors and Nurses) with a special emphasis on nurses because of their frequent exclusion from previous studies [3] .
1.4. Channels of Involvement
Manufacturers’ “search of excellence” is based on their best understanding of end-users’ needs [31] [32] which usually happens through need assessment methods consisting of involving the end-user and underlining his specific needs and expectations so that the development can happen accordingly. However, users’ needs are often bad interpreted [33] given they happen through intermediates between the end-user and the manufacturer, experts or sales executives who have direct access to the user with significant bias however in understanding his needs. Some authors underline the role of third-parties in the development [21] which is often characterized of the role of an intermediate between the manufacturer and the end-user which will render this latter relationship as an indirect relationship. In this study we will try to identify, through the eye of the end-user, the form of their implication (direct or indirect) and the extent of potential error in the translation of their comments and needs.
Overall, the aim of our study is to identify the involvement of end-users in the development of MDTs’ innovations as well as contribute to the optimization of this involvement. Unlike previous studies, we will gather our data from end-users and not manufacturers pointing out their opinion concerning the actual degree of their involvement, what they think their impact is and what they think it could be, their recommendations on how their participation could be more beneficial following the “how” and “when” of a probable involvement. Our works are thus based on their opinion and their suggestions in contrast with previous studies exclusively concentrated on the manufacturers’ perspectives [3] . Based on our findings, we will suggest a development process that would include end-users’ input at important stages, rendering this participation to a regulated pattern, as opposed to the unregulated ad-hoc steps it actually follows [21] [23] .
1.5. Research Question
As required in an exploratory qualitative study, our first step was to develop a main research question followed by objectives based on the literature [37] . The aim of this research is to understand the process of End-Users’ involvement in the development processes of Healthcare Technologies (HTs) and medical equipment technologies (MDTs), based on the End-Users’ perspective and understanding. The main research question we intend to answer is the following: What is the End-Users’ perception regarding their involvement in the Design & Development of MDTs and what are the ways to improve this implication?
Under the banner of this main research question we can identify the following objectives:
・ Evaluate End-Users’ perception of Manufacturers commitment to their implication in MDT Design & Development
・ Identify the different levels of their current involvement
・ Identify aspects of their current involvement
・ Identify the channels of involvement, and evaluate direct contact with the manufacturer v/s through a third-party
・ Identify End-Users’ rate of satisfaction regarding this involvement and the ways they believe it can be improved
・ Contribute by the translation of end-users’ input to the development of a universal regulated process of involvement
2. Methods
2.1. Semi-Structured Interviews
Due to the significant interaction between nurses and MDTs [26] [27] [29] [30] , and little existing literature studying their involvement as end-users in MDTD [3] [14] we have decided to focus our research on nurses as the main end-user and identify their concerns, thoughts, ideas and recommendations. We have also decided to add Medical Doctors in our sample because of the frequent interactions between Doctors and Nurses [34] [35] as part of a medical team, as well as the significance of the Doctor-Technology relationship [36] .
We have decided to conduct semi-structured interviews as opposed to our first thoughts of using a survey by questionnaire to ensure [38] : first the flexibility and liberty given to the participant to express himself and expose his thoughts, second the relevance of the participation and the focus on main points of interest. Given our previous understanding of the topic thus ability to develop relevant semi-structured question to guide our interview [39] and the fact that we will not have more than one chance to interview each participant [40] semi- structured interviews appeared as the best way to address our needs. Also [41] they 1) allow for clarification of interesting and relevant issues [42] , 2) can elicit valuable and complete information [43] , and 3) enable the interviewer to explore and clarify inconsistencies with the respondents’ accounts [44] .
2.2. Contacting Healthcare Professionals & Sampling Technique
In June 2015, we participated to the SIDIIEF congress (6ème Congrès mondial des infirmières et infirmiers francophones) in Montreal, Canada, for four consecutive days soliciting participation to our study during the poster sessions [45] . This event can be considered as a good opportunity to meet and encounter HCWs in general and nurses as well as doctors in particular, but also a good opportunity to meet attendees from different countries and thus different cultures. One of our ideas was to involve participants from different nationalities in order to study the cultural effect, if any in this case. Cultural differences have been shown to have significant impact in the field of healthcare technology mostly in regards to MDTs implementation, acceptance and resistance to change [46] [47] . Participants were quickly informed of the nature & background of our study, they were handed a consent form and asked to sign it should they agree to be part of our study. The survey being completely anonymous, we faced no objection in this regard from any of the participants. The semi-structured interviews were conducted in the form of 15 to 20 min audiences.
Limited existing literature is available concerning this subject thus restricting our knowledge in developing a sampling strategy. We have decided to conduct our research using a convenience sample. This sample requires us to develop a sampling strategy based on our knowledge of the research area and the existing literature even if limited [48] so we decided to target Nurses, Doctors, look at different cultural backgrounds. We also asked participants to recommend useful potential candidates (snowball sample) [49] . This technique however forces us to satisfy the following criteria from an analytical point of view: the relevance of the sample which we have justified previously in this paragraph, and the sample size in order to lead stability in the results [50] .
2.3. Interview Guide and Questions
We will expose in this section some of the questions we asked during the semi-structured interviews, which helped us guide our meetings and structure the flow of the conversation with each participant. Only some of the questions will be included in this paragraph. This pre-developed guide that addressed the main points of interest was developed based on the literature and previous works, on numerous brainstorming sessions between the authors of this article, and on the consulting of peers.
Given the important impact the order of the questions can have on the quality of responses [51] have used a specific order going from general questions preceding specific ones. In order to minimize inter-variability, we have developed this guide with a particular attention to the design and layout. We did not have much concern about the saliency of the guide―apparent relevance, importance and interest of the survey to the respondent― health-related surveys being considered to be salient more than the average, and given the initial interest expressed by some end-users (patients and/or healthcare professionals) concerning our work which may contribute, in a way or another, to the advancements in the quality of healthcare services they receive. Our questions were subsequently reviewed and validated by peers at the “Center for Interuniversity Research and Analysis of Organizations” (CIRANO) and by a statistical expert to guarantee the credibility of the used scales and the formulations of the questions.
Inquiries were made with participants in the two cases of MDT Development and MDT Amelioration. A total of eight (8) mains questions were developed to inquire about End-Users’ perception some of which with a number of sub-questions to help us refine our inquiry.
1) How many times have you been involved in the development of a new healthcare innovation?
2) How many times have you been involved in the improvement of a healthcare innovation?
3) At which specific level or levels of development have you been involved?
4) What is your degree of agreement with the below?
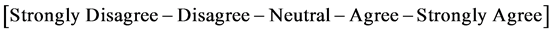
・ I was in contact with the manufacturer directly (through direct contact with a professional hired and/or employed by the manufacturer)
・ A direct contact with the manufacturer would make me feel more involved
・ My involvement & recommendations can be beneficial to all users of the device and I believe mass production should involve my modifications
・ My participation was in order to develop a new healthcare technology that was previously non-existent
・ My participation was in order to develop an improved version of the original first version of the innovation
・ My participation was in order to develop an improved version of an existing product that was already upgraded
5) How many times have you been asked by the manufacturer:
・ about your satisfaction concerning the purchased device?
・ about the technical performance of the device?
・ to suggest improvements to the purchased device
6) How often do you think your comments were taken into consideration?
7) Have many times have you been contacted after you have given your comments in order to follow-up on your suggestions?
8) Participants have also been asked to rate their implication (if any) in each of the phases of MDT development (as in Biemans [22] ) and to suggest at which steps they prefer to be involved as a recommendation for future implication.
3. Results and Discussion
Healthcare professionals interviewed totaled 49 participants, with ages varying from 25 to above 75 years and experience with MDTs ranging from 3 to 51 years. SIDIIEF being an international congress regrouping HCWs (Healthcare Workers) from francophone countries all over the world, we were able to gather a pool of participants from different nationalities that gave a sense of unbiaseness to our study, mainly from the following territories: Canada (within Quebec), Canada (outside Quebec), France, Belgium, Switzerland, Lebanon, Madagascar, Cameroun, Mexico, Morocco, Tunisia, Algeria, Niger, Ivory Coast, Senegal and Mali.
Thirty-nine (39) of the participants answered being professional nurses practicing in Hospitals or Medical Centers and ten (10) answered being practicing Medical Doctors.
The results of our work give rise to different themes that we will explore through different subsections each concentrating on a specific subject. From the End-User satisfaction concerning the actual involvement, to the stages of implication, followed by the aim of the involvement, the communication schemes, the forms of involvement, the impact of the cultural factor and finally the End-Users’ recommendations, the following section regroups all the above titles and examines each carefully, based on the findings of our research works.
3.1. Satisfaction Concerning Implication
As previously seen and expected, a significant number of participants declared having never been implicated or approached for implication in MDTD processes. Twenty-one (21) out of the forty-nine (49) participants said to have never been involved nor approached for involvement in a MDTD process, Fifteen (15) or 30.6% said to have been implicated once or twice in MDTD processes, six (6) or 12.3% said three to five times, four (4) or 8% talked about having been involved on 6 to 8 occasions and three (3) or 7% mentioned 9 times or more. It is to mention these last 3 participants are all above 75 years of age, and in interaction with MDTs for over 50 years. They revealed that most of these occasions happened in “recent years”, when they became renowned names in the field, persons of reference or in key positions within their organizations. This is an argument supporting the idea that “the richest understanding of products’ needs is held only by a few users” [52] , especially that most selected end-users satisfy certain criteria of experience, education and hierarchical position.
3.2. Stages of Implication
The 28 participants already involved in MDTD were asked about the stages of their implication (Table 1).
With 20 saying being implicated at the level of the Idea or of the Preliminary assessment, 17 at the level of the testing, 5 at the trial and 5 at the launch compared to only 2 at the Concept level and 2 at the Development level, we notice an involvement of end-users more focused on the preliminary stages of the MDTD process or towards the end of it. The role they appear to have is of consultancy nature and advisory aspect, with little implication in the actual design and engineering processing at the conceptualization or the development.
Interestingly, when the 49 participants were asked to define the stages they believe their involvement would best benefit the project (Table 2), the patterns were to some extent a contrast to those of the actual involvement patterns.
A significant attention exceeding 60% was given to the stages of Concept and Development, where the actual involvement was shown to be the lowest (Table 1). This insistence to be involved at the design and engineering stages is aimed to ensure an optimal translation of the end-users’ needs: “The best guarantee that our exact needs will be well understood, well reported and perfectly translated in an optimal product, is that we be involved in the engineering and the design missions with the R&D people. We will be able to comment, alter, discuss and advise before the design is made and then it is too late to make major modifications” stated a participant, which comes in opposition to Manufacturers’ opinion limiting the end-users’ role to the early stages of the MDTD process, or to the later testing levels [3] [21] , giving them strictly the power of suggestion and of feedback, with no interference in the technical conceptualization. Furthermore, 5 participants expressed an interest in being involved at each and every one of the seven steps of MDTD, to ensure what they defined as a “delicate & precise, but efficient& advantageous input”. With their presence at each step of the MDTD process, participants seem confident that the developed product will answer perfectly their needs in all aspects of engineering ranging from technical and ergonomics, to performance and friendliness of use.
According to the participants, a planned and better structured involvement would significantly alter the final developed product (Table 3): 23% believe a proper involvement would lead to a MDT that is more than 50% improved than traditional methods, 20% expected an amelioration of 20% to 30% and 32% evaluate the end-users’ involvement to bring a 10% to 20% refinement.
Only one of the 49 participants believes that the added value of his involvement would only benefit him as a user as it will lead the product to an over-specialization and customization to his own use, which might not be the preference of other users, whereas all the 48 other participants stated that their input would be beneficial for all other users, thus towards a “mass production” of the resulting MDT.
3.3. Upgrade and Improvement
Participants also emphasized that their involvement should not only be limited to MDTD processes, but involve MDTAU (Medical Device Technology Amelioration & Upgrading). Forty-three out of the 49 participants said they would be interested in reporting technical deficiencies in MDTs thus contributing to the amelioration of the technology, and 97% of them believe these technical deficiencies to often be life-threatening to the patient. “Any technical problem, shutdown, or performance error at the level of the technology can be life-threatening to the patient we are treating. But any discomfort or difficulty of use of the MDT linked to poor ergonomics or design of the unit can also be of major circumstances on patients’ lives” said one participant, stating the importance of finding HCWs satisfaction and acceptance of the technology to contribute to a smooth treatment environment and an enhanced safety culture. Participants believe their involvement in MDTAU is not limited to failure reporting but is beneficial at numerous levels with recommendations that can attain different aspects of the MDT: 75% are confident their involvement will contribute to the increase in the performance of the MDT, 92% believe they have recommendations that will substantially improve the software aspect, 93% said they have suggestions to ameliorate ergonomics of the unit, increase user interface, thus facilitate its use and acceptance. Resistance to technology being a major preoccupation nowadays and a matter of increasing interest [53] , end-users’ involvement in its conception or amelioration seems to constitute an alternative for a smooth acceptation of MDTs in established healthcare environments.
3.4. Contact of Involvement
According to most of the participants, an ideal involvement would be in a direct contact with the manufacturer, and with members of the R&D team handling the project development. Literature has however showed us there
Table 1. MDTD Stages of actual involvement of end-users.
Table 2. MDTD Stages of preferential involvement of end-users.
Table 3. End-users’ expected improvement of MDTs in case of proper involvement in MDTD processes.
are different ways of involvement of End-Users in the MDTD processes [23] . Out of the 28 participants who said they were previously approached and involved in MDTD, only 9 said to have cooperated directly with the manufacturer’s team and with employees of the manufacturer. Five were involved via Government Agencies, appointed by Governmental certification bodies in order to ensure end-users’ participation and the subsequent obtaining of certifications, a criteria that became a requirement in different areas of the world [10] [12] [13] . Four mentioned being involved by partnering Universities. In this case, they were members of a HCWs committee within a University Hospital, and external manufacturers were conducting the development of the device in cooperation with the University for the use of its laboratories and student expertise in a special biomedical engineering program. Three mentioned being involved through their employers, whom they say, was a sort of representative taking their opinion to the R&D team. Seven mentioned being involved through Distributors for the manufacturer. Many reasons were stated justifying this fact: 1) the fact that the manufacturers’ premises were in different countries or territories making communication difficult, 2) distributors played the role of a buffer between the end-users and the manufacturers, and the distributors representatives, very often biomedical engineering, were very knowledgeable technically and could translate best the end-users concerns, requirements and recommendations to the R&D team, 3) communication management techniques between manufacturers and their distributors are already established, thus using these channels means significantly less costs that having to establish new channels directly with end-users.
However, participants appear to favor a direct contact with the manufacturer without going through a third- party intermediary. When asked about if a direct connection with the manufacturer would make the participant feel more involved in the MDTD processes, 88% stated they are totally supportive of this affirmation, their argument being: 1) the reduction in the errors reporting their opinions and thoughts, 2) a faster interaction, 3) and an opportunity to better understand the manufacturer’s arguments and technical reasons behind taken decisions. Last but not least, a significant concern that a direct End-user―Manufacturer interaction would help to reduce, is the serious consideration of End-users’ opinions and suggestions. Of the 28 participants who had already been involved in MDTD or MDTAU processes, 13 expressed skepticism whether their involvement was taken into consideration at all and 11 said their opinion was taken into consideration only once or twice (Table 4).
These confirmations were made based on modifications or upgrades visible in the final version launched by the manufacturer and not through feedback or through subsequent correspondence between the Manufacturer (or third-party) and participant end-users: 75% of the participants involved in MDTD or MDTAU stated to have never been contacted again by the manufacturer or third-party.
3.5. Forms of Involvement
Concerns raised by the participants were not limited to the quality of the contact with the manufacturer, but also to the aspects and forms of this involvement. Many methods and ways of involving end-users in MDTD or MDTAU processes are followed by manufacturers [1] - [3] [11] [29] . This can be done through Questionnaires aiming to identify end-users’ needs, focus groups between R&D representatives and end-users, Individual Interviews, Cognitive task analysis as well as Trial Periods and feedback. Three participants expressed an interest in being involved in all these five activities, which they judged necessary as a step to ensure proper end-user implication. However, processing of all five activities for MDTD or MDTAU processes might raise some concerns from the manufacturers in regards of management, time as well as financial resources, constraints that have already been pointed out in previous works [11] [21] [23] . Other participants expressed an interest in being part of focus groups―14 participants―where they would be invited to sit with R&D team members and follow-up on the development processes, 11 stated the best way of involvement would be through precise and concise questionnaires aiming to understand their needs and concerns regarding the technology, 12 said that individual interviews would be the best way for them to explain their opinion and contribute positively to the development or amelioration of the MDT. Six participants prefer the traditional method of trial and feedback, where they would
Table 4. Number of times their opinion was taken into consideration in MDTAU processes (End-users’ perception).
be using a prototype during a defined period of time with periodic reviews with the manufacturer’s team, and three mentioned an interest in cognitive task analysis as a preferred way of implication.
3.6. Culture
Even though the bigger part of participants were from developed countries (34 out the total 49), and only 15 participants or 30% from developing nations, it was clear that a significant difference existed in their approach. Professionals from Canada, France, Belgium and Switzerland appeared to have more precise, more concise requirements, larger experience in MDTD implication, and richer knowledge in the ways and impacts of this implication. They answered our questions with a higher focus on MDT processing details, came up with recommendations for the improvement of their implication at the micro-level of the development with suggestions of level implication, ways, sometimes examples. HCWs from developing countries are less exposed to MDTD processes and their implication is scarce. Poor MDTD industry in their countries might be a reason for their poor and less frequent involvement. Many of their comments were focused on a simple adaptation of the MDT to the environment of their country unlike micro-customization other participants had mentioned. An example of that can be their needs of basic hydraulic, mechanical or even electrical backups to the MDT that would allow their use reference to major power cutouts in their countries, a need that is rarely significant in the developed nations. It seemed to us that MDT manufacturers are less keen on implicating users originating from these parts of the world, but this reality doesn’t necessarily evolve from a culture effect rather from the overall conjuncture: 1) one reason might be the difficulty of reach to end-users in these areas from manufacturers most of whom are based in North America or Western Europe, 2) a second more viable reason can be the small representative market developing countries constitute. The U.S. accounts alone for more than 50% of the global MDT market, and the EU for an additional 25% leaving the rest of the world to share the remaining quarter [54] . This might explain the focus of manufacturers in their choice of end-users to involve, wanting to enhance their market chances in more significant world-markets thus raising their sales probabilities.
4. Recommendations and Conclusion
Many previous works have been developed around the involvement of end-users in MDTD processes [3] [21] [23] , most of them focusing however on the manufacturers’ perspectives and very siding on the end-users’ views of the matter. This is in part is due to the difficulty in identifying proper participants, and most importantly research methods. Having started with a questionnaire, we ended up having a 15min semi-structured interview heavily based on the questionnaire with willing participants. As a form of conclusion, it is clear that the end-users’ perspective regarding their actual involvement in MDTD or MDTAU processes differs from that of manufacturers [3] [21] . First, it is important to point out that the levels of MDTD where the end-users see an interest in being involved are not the same as the steps where manufacturers perceive and plan this involvement. It is to note that end-users show an actual interest in being involved in all the steps of an MDTD (or MDTAU) ranging from the idea to the trial, even to the launching where they can contribute to the organization of the launching event, the deployment of the first units, first trainings to the staff, or simply share their experiences with the first prototypes as success stories to facilitate technology acceptance and subsequently reduce resistance to change [29] . It would be interesting to add an 8th step to the 7-steps of MDTD or MDTAU as suggested by Biemans [22] , this 8th step being a sub-part of Shah et al. [11] ’s Step 4, focusing on the implication of users in the feedback after deployment of MDTs in the market for the continuous improvement & upgrading of the technology in relation to the market needs (Figure 3). This after-deployment implication has been accused by many participants to be completely absent in the cases where no major breakdown is occurring, thus rendering the MDTAU process reactive and not proactive, with manufacturers interested in upgrading the MDT only after significant issues start appearing. Our suggestion would be an active implication of end-users at all steps of the MDTD, but also in a continuous manner to ensure a further research and evaluation.
Participant end-users understand the concerns and of their involvement and some of them even pointed out time and financial constraints of their potential involvement, as limitations manufacturers have, however, they believe their participation in MDTD (or MDTAU) processes would facilitate technology acceptance thus increase device deployment, limit need for reengineering thus reducing costs and increasing benefits. Furthermore, and even when implicated, end-users are not always properly listened to as a result of the complexity of communication channels with the manufacturers’ R&D teams. Another concern is the form of discrimination they
Figure 3. Suggestion of End-User Involvement in MDTD and subsequently continuous MDTAU processes. Inspired by Chesbrough [16] , Biemans [22] & Cooper [23] .
experience relatively to lead-users, professionals with higher qualifications, experience or hierarchical positions whom are often not the real end-users of the MDTs. There lies another controversial issue, concerning the procurement decisions of MDTs within Healthcare institutions where the decision-takers are frequently not the HCWs who will be using the machine: HCWs in some instances feel the chosen MDT is imposed on them in a top-down manner, fueling significant resistance to technology adoption [53] .
5. Further Research
Time and sample constraints have limited the extent of our research, constituting a potential source of errors. This work should be considered as exploratory. It would be interesting to reproduce this work with a bigger sample, with a focus on one specific form of MDT so as to compare relatively different opinion as well as validate our findings [54] . Another idea would be to organize panel-shaped focus groups comprising manufacturers as well as end-users in order to compare arguments and counter-arguments and validate the conclusions of this research work. It goes without mentioning that one better way of understanding what is actually going on in MDTD processes would be through action research [55] [56] , with the involvement of researchers in an actual MDTD process alongside manufacturers and end-users. This option is however highly challenged by the availability of resources and opportunity.
Cite this paper
Selim Hani,Nathalie de Marcellis-Warin, (2016) Open Innovation and Involvement of End-Users in the Medical Device Technologies’ Design & Development Process: End-Users’ Perspectives. Technology and Investment,07,73-85. doi: 10.4236/ti.2016.73010
References
- 1. Grocott, P., Weir, H. and Bridgelal Ram, M. (2007) A Model of User Engagement in Medical Device Development. International Journal of Health Care Quality Assurance, 20, 484-493.
http://dx.doi.org/10.1108/09526860710819422 - 2. Martin, J.L., Murphy, E., Crowe, J.A. and Norris, B.J. (2006) Capturing User Requirement in Medical Device Development: The Role of Ergonomics. Physiological Measurement, 27, R49-R62
http://dx.doi.org/10.1088/0967-3334/27/8/R01 - 3. Money, A.G., Barnett, J., Kuljis, J., Craven, M.P., Martin, J.L. and Young, T. (2011) The Role of the User within the Medical Device Design and Development Process: Medical Device Manufacturers’ Perspective. BMC Med Inform Decision Making, 11, 15.
http://dx.doi.org/10.1186/1472-6947-11-15 - 4. Beresford, P. and Croft, S. (1993) Citizen Involvement: A Practical Guide for Change. Macmillan, Basingstoke.
http://dx.doi.org/10.1007/978-1-349-22544-6 - 5. Barker, J., Bullen, M. and de Ville, J. (1997) Reference Manual for Public Involvement. Bromley, West Kent, Lambeth, Southwark, and Lewisham Health Authorities.
- 6. Crawford, M., Rutter, D., Manley, C., Weaver, T., Bhui, K., Fulop, N. and Tyrer, P. (2002) Systematic Review of Involving Patients in the Planning and Development of Healthcare. BMJ, 325, 1263.
http://dx.doi.org/10.1136/bmj.325.7375.1263 - 7. Gosbee, J. (2002) Human Factors Engineering and Patient Safety. Quality & Safety in Health Care, 11, 352-354.
http://dx.doi.org/10.1136/qhc.11.4.352 - 8. Harrison, S. (2002) Guest Editorial: Public and User “Involvement” in the UK National Health Service. Health and Social Care in the Community, 10, 63-66.
- 9. Buhler, C., Hoelper, R., Hoyer, H. and Humann, W. (1995) Autonomous Robot Technology for Advanced Wheelchair and Robotic Aids for People with Disabilities. Robotics and Autonomous Systems, 14, 213-222.
- 10. Martin, J.L. and Barnett, J. (2012) Integrating the Results of User Research into Medical Device Development: Insights from a Case Study. BMC Medical Informatics and Decision Making, 12, 74.
http://dx.doi.org/10.1186/1472-6947-12-74 - 11. Shah, S.G., Robinson, I. and AlShawi, S. (2009) Developing Medical Device Technologies from Users’ Perspectives: A Theoretical Framework for Involving Users in the Development Process. International Journal of Technology Assessment in Healthcare, 25, 514-521.
http://dx.doi.org/10.1017/S0266462309990328 - 12. American National Standards Institute (2009) ANSI HE75: Human Factors Engineering—Design of Medical Devices. American National Standards Institute, Washington DC.
- 13. International Electrotechnical Commission (2007) IEC 62366 Medical Devices—Application of Usability Engineering to Medical Devices. International Electrotechnical Commission, Geneva.
- 14. Fernald, K., Weenen, T., Sibley, K. and Claassen, E. (2013) Limits of Biotechnological Innovation. Technology and Investment, 4, 168-178.
http://dx.doi.org/10.4236/ti.2013.43020 - 15. de Jong, J.P.J. and von Hippel, E. (2009) Transfers of User Process Innovations to Process Equipment Producers: A Study of Dutch High-Tech Firms. Research Policy, 38, 1181-1191.
http://dx.doi.org/10.1016/j.respol.2009.04.005 - 16. Chesbrough, H. (2003) The Logic of Open Innovation: Managing Intellectual Property. California Management Review, 45, 33-58.
http://dx.doi.org/10.2307/41166175 - 17. Coates, M. and Bals, L. (2013) External Innovation Implementation Determinants and Performance Measurement: A Case Study from the Pharmaceutical Industry. Technology and Investment, 4, 131-143.
http://dx.doi.org/10.4236/ti.2013.42016 - 18. Von Hippel, E. (1976) The Dominant Role of Users in the Scientific Instrument Innovation Process. Research Policy, 5, 212-239.
http://dx.doi.org/10.1016/0048-7333(76)90028-7 - 19. Abeele, P.V. and Christiaens, I. (1986) Strategies of Belgian High-Tech Firms. Industrial Marketing Management, 15, 299-308.
http://dx.doi.org/10.1016/0019-8501(86)90022-2 - 20. Shaw, B. (1986) The Role of the Interaction between the Manufacturer and the User in the Technological Innovation Process. DPhil Thesis, Science Policy Research Unit, University of Sussex, Brighton.
- 21. Cooper, R.G. (1983) A Process Model for Industrial New Product Development. IEEE Transaction on Engineering Management, EM-30, 2-11.
http://dx.doi.org/10.1109/TEM.1983.6448637 - 22. Biemans, W.G. (1991) User and Third-Party Involvement in Developing Medical Equipment Innovations. Technovation, 11, 163-182.
http://dx.doi.org/10.1016/0166-4972(91)90032-Y - 23. Shah, S.G.S. and Robinsons, I. (2006) User Involvement in Medical Device Technology Development and Assessment: A Structured Literature Review. International Journal of Health Care Quality Assurance Incorporating Leadership in Health Services, 19, 500-515.
http://dx.doi.org/10.1108/09526860610687619 - 24. Sathasivam, S. (2009) Managing Patients with Amyotrophic Lateral Sclerosis. European Journal of Internal Medicine, 20, 355-358.
http://dx.doi.org/10.1016/j.ejim.2008.09.002 - 25. Krivickas, L.S., Shockley, L. and Mitsumoto, H. (1997) Home Care of Patients with Amyotrophic Lateral Sclerosis (ALS). Journal of Neurological Sciences, 152, S82-S89.
http://dx.doi.org/10.1016/s0022-510x(97)00251-7 - 26. Locsin, R.C. (2007) Machine Technologies and Caring in Nursing. The Journal of Nursing Scholarship, 27, 201-203.
http://dx.doi.org/10.1111/j.1547-5069.1995.tb00859.x - 27. Ball, M., Weaver, C. and Abbott, P. (2003) Enabling Technologies Promise to Revitalize the Role of Nursing in an Era of Patient Safety. International Journal of Medical Informatics, 69, 29-38.
http://dx.doi.org/10.1016/S1386-5056(02)00063-1 - 28. Ma, C., Kuo, K. and Alexander, J. (2016) A Survey-Based Study of Factors That Motivate Nurses to Protect the Privacy of Electronic Medical Records. BMC Medical Informatics and Decision Making, 16, 13.
http://dx.doi.org/10.1186/s12911-016-0254-y - 29. McGuire, D., Garrison, L., Armon, C., Barohn, R.J., Bryan, W.W., Miller, R., Parry, G.J., Petajan, J.H., Ross, M.A. and The Syntex-Synergen ALS/CNTF Study Group (1997) A Brief Quality-of-Life Measure for ALS Clinical Trials Based on a Subjset of Items from the Sickness Impact Profile. Journal of Neurological Sciences, 152, S18-S22.
http://dx.doi.org/10.1016/S0022-510X(97)00239-6 - 30. Trail, M., Nelson, N.D., Van, J.N., Appel, S.H. and Lai, E.C. (2003) A Study Comparing Patients with Amyotrophic Lateral Sclerosis and Their Caregivers on Measures of Quality of Life, Depression, and Their Attitudes toward Treatment Options. Journal of the Neurological Sciences, 209, 79-85.
http://dx.doi.org/10.1016/S0022-510X(03)00003-0 - 31. Myers, S. and Marquis, D.G. (1969) Successful Industrial Innovations: A Study of Factors Underlying Innovation in Selected Firms. National Science Foundation.
- 32. Peters, T. and Waterman, R. (1982) In Search of Excellence: Lessons from America’s Best-Run Companies. APA 6th Edition, Harper & Row, New York.
- 33. Holt, K. (1988) The Role of the User in Product Innovation. Technovation, 7, 249-258.
http://dx.doi.org/10.1016/0166-4972(88)90023-5 - 34. Reeves, S., Nelson, S. and Zwarenstein, M. (2008) The Doctor-Nurse Game in the Age of Interprofessional Care: A View from Canada. Nursing Inquiry, 15, 1-2.
http://dx.doi.org/10.1111/j.1440-1800.2008.00396.x - 35. Keddy, B., Jones Gillis, M., Jacobs, P., Burton, H. and Rogers, M. (1986) The Doctor-Nurse Relationship: A Historical Perspective. Journal of Advanced Nursing, 11, 745-753.
http://dx.doi.org/10.1111/j.1365-2648.1986.tb03393.x - 36. Bennet, G. (1987) The Wound and the Doctor Healing, Technology and Power in Modern Medicine. Journal of the Royal Society of Medicine, 81, 60.
- 37. Mayer, R. and Ouellet, F. (1991) Méthodologie de recherche pour les intervenants sociaux. Gaëtan Morin éditeur, Boucherville, 537.
- 38. Martel, V. (2007) L’inédite portée de la méthodologie qualitative en sciences de l’éducation: réflexion sur les dfis de l’observation et de l’analyse de la vie cognitive de jeunes apprenants. Recherches Qualitatives—Hors Série—numéro 3, Actes du colloqie Bilan et Prospectives de la recherche Qualitative.
- 39. Bernard, H.R. Ed. (1998) Person-Centered Interviewing and Observation. AltaMira Press, Walnut Creek.
- 40. Cohen, D. and Crabtree, B. (2006) Qualitative Research Guidelines Project.
http://www.qualres.org/HomeEval-3664.html - 41. Barriball, L.K. and While, A. (1994) Collecting Data Using a Semi-Structured Interview: A Discussion Paper. Journal of Advanced Nursing, 19, 328-335.
http://dx.doi.org/10.1111/j.1365-2648.1994.tb01088.x - 42. Hutchison, S. and Skodol-Wilson, H. (1992) Validity Threats in Scheduled Semi-Structured Research Interviews. Nursing Research, 41, 117-119.
- 43. Bailey, K.D. (1987) Methods of Social Research. 3rd Edition, the Free Press, New York.
- 44. Smith, L. (1992) Ethical Issues in Interviewing. Journal of Advanced Nursing, 17, 98-103.
http://dx.doi.org/10.1111/j.1365-2648.1992.tb01823.x - 45. Hani, S. and de Marcellis-Warin, N. (2015) L’implication des utilisateurs dans le design et développement des technologies médicales: deux perspectives, manufacturiers v/s utilisateurs. Poster session presented at SIDIIEF 6th International Congress, Montreal, June 2015.
- 46. Bandyopadhyay, K. and Fraccastoro, K. (2007) The Effect of Culture on User Acceptance of Information Technology. Communications of the Association for Information Systems, 19, Article 23.
- 47. Brown, C. (2012) Healthcare Data Protection and Biometric Authentication Policies: Comparative Culture and Technology Acceptance in China and in the United States. Review of Policy Research, 29, 141-159.
http://dx.doi.org/10.1111/j.1541-1338.2011.00546.x - 48. Ellison, S., Farrant, T. and Barwick, V. (2009) Practical Statistics for the Analytical Sciences: A Bench Guide. Royal Society of Chemistry.
- 49. Marshall, M. (1996) Sampling for Qualitative Research. Vol. 13, No. 6, Family Practice, Oxford University Press, Oxford.
- 50. Ferber, R. (1977) Research by Convenience. Journal of Consumer Research, 4, 57-58.
http://dx.doi.org/10.1086/208679 - 51. McColl, E., Jacoby, A., Thomas, L., Soutter, J., Bamford, C., Steen, N., Thomas, R., Harvey, E., Garratt, A. and Bond, J. (2001) Design and Use of Questionnaires: A Review of Best Practice Applicable to Surveys of Health Service Staff And Patients. Health Technology Assessment, 5, 1-256.
http://dx.doi.org/10.3310/hta5310 - 52. Herstatt, C. and Von Hippel, E. (1992) From Experience: Developing New Product Concepts via the Lead-User Method: A Case Study in a “Low-Tech” Field. Journal of Product Innovation Management, 9, 213-221.
http://dx.doi.org/10.1016/0737-6782(92)90031-7 - 53. Al Salman, J.M., Hani, S., de Marcellis-Warin, N., Isa, S.F. (2015) Effectiveness of an Electronic Hand Hygiene Monitoring System on Healthcare Workers’ Compliance to Guidelines. Journal of Infection and Public Health, 8, 117-126.
http://dx.doi.org/10.1016/j.jiph.2014.07.019 - 54. International Trading Agency. Medical Device Industry Assessment.
http://ita.doc.gov/td/health/medical%20device%20industry%20assessment%20final%20ii%203-24-10.pdf - 55. Yin, K.-R. (1993) Application of Case Study Research. Sage Publication, California, 33-35.
- 56. Jewkes, C. (1995) What Is Participatory Research? Social Science and Medicine, 41, 1667-1676.
http://dx.doi.org/10.1016/0277-9536(95)00127-S





