Journal of Water Resource and Protection
Vol.08 No.05(2016), Article ID:66615,14 pages
10.4236/jwarp.2016.85049
Synthesis and Characterization of Pure and Ag-TiO2-Modified Diatomaceous Aluminosilicate Ceramic Membranes for Water Remediation
Emmanuel Ajenifuja1, John Adegbindin Ajao1, Samson Oluwagbemiga Alayande1, Mufutau Kolawole Bakare2, Bidini Alade Taleatu2, Ezekiel Oladele Bolarinwa Ajayi3
1Center for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
3Department of Physics, Obafemi Awolowo University, Ile-Ife, Nigeria

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 2 October 2015; accepted 17 May 2016; published 20 May 2016
ABSTRACT
Mesoporous ceramic membranes were prepared from raw and modified diatomaceous earth alumi- nosilicate mineral precursors. The main modification component of the ceramic membranes was Ag-loaded TiO2 nanoparticles (STOX). Chemical and microstructural characterizations of the raw materials and the modified precursors were carried out using Fourier Transform Infrared (FTIR) Spectroscopy, Particle Induced X-ray Emission (PIXE-IBA), Energy Dispersive X-ray Spectroscopy (EDX) and Scanning Electron Microscopy (SEM). The precursors and membranes were prepared and subsequently subjected to a high temperature sintering treatment for physico-chemical modification and stability. Remediation functionalities of the ceramic membranes on water samples were studied using Atomic Absorption Spectrophotometry (AAS), Total Bacterial Count Enumeration; Total Dissolved Solids (TDS), pH, and Electroconductivity (EC). Remediation experiments showed reductions in the concentration of certain cations such as Mg2+, Mn2+, Cd2+, Ni2+ and K+ by the modified ceramic membrane samples, while increased concentrations were observed for Ca2+, Na+ and Mg2+. The antimicrobial microfiltration process showed 100% bacterial removal and 70% fungi removal in most of the samples. Membranes exhibited good flux output from 5.607 L/hr∙m2 (STOX-Z) to 39.245 L/hr∙m2 (ZEO-T) under a pressure of 0.0196 MPa.
Keywords:
PIXE, Ceramics, Titanium Oxide, Remediation, Membranes, Anti-Microbial

1. Introduction
Ceramic membranes have a wide range of domestic, industrial and scientific uses, the most common of which is the use in separation processes. Organic membranes are often used in industry and scientific establishments for separation and purification processes, but ceramic membranes offer several advantages over organic membranes. Studies [1] [2] have shown that ceramic based membranes are more resistant to organic solvents, chlorine, and extremes of pH. Ceramic membranes have even been applied in extreme conditions such as oil fields for separation of produced water. At high temperature, ceramic membranes are inherently more stable, thus allowing more efficient sterilization of process equipment than is possible with polymeric membranes. In addition, they are generally quite resistant to microbial and biological degradation, which can occasionally be a problem with organic membranes. Ceramic membranes are also more mechanically stable under high pressures.
The production of macroporous and microporous ceramic materials is of considerable practical interest, because they make it possible to design heat-insulating materials and membranes for filtration of liquids, gases, and as catalyst supports [3] [4] for high-temperature and chemical processes [5] . The asymmetric multi-layer structure has often been used for preparation of ceramic membranes [6] . The support layer provides the mechanical strength with larger pore size and several millimeters in thickness, control layer is the last top layer with very tiny pore size, between them are transition layers. Membranes with asymmetric multi-layer structure have the following disadvantages, first, they are comparatively very costly due to the multi-layer forming and sintering process, and secondly, defects occur easily between layers because of the ceramic particle size changing suddenly and easy peel off from last support layer [6] . Those disadvantages especially the cost of production, limit their applications. Industry and research departments are therefore searching for new ceramic membranes which can be manufactured in one-step fabrication process. At the moment, studies are being carried out on industrial wastes, natural polymers and geological raw materials such as diatomite, kaolinite and natural zeolites to prepare low cost porous materials [7] - [10] , ceramic membranes [11] - [16] and adsorbents for various environmental remediation applications. At the same time, simpler and low-cost production methods [6] [7] are being sought for in ceramic membrane preparation. Studies on photocatalytic have shown that expensive TiO2 based chemicals such as titanium trichloride (TiCl3) and titanium tetra-isopropoxide (TTIP)are often used to prepare photo-reactive materials which may be expensive for large-scale practical applications [8] [9] [17] [18] . However, using readily available geological materials [3] [10] - [12] [19] [20] as the main constituent in photocatalytic degradation of toxic and microbial pollutants would be less expensive and highly productive.
The present study involves the preparation of Ag/TiO2-modified mesoporous ceramic membranes from locally available diatomaceous aluminosilicates materials. The prepared membrane possessed unique functional properties such as high thermal and mechanical strength, chemical stability and ion-exchange capabilities. The mesoporous ceramic was used successfully to remove some heavy metal and microbial contaminants from polluted local river effluent.
2. Materials and Methods
2.1. Raw Materials
The diatomaceous aluminosilicate raw material was sourced from North-East Nigeria. Typically, diatomaceous earth minerals occur in two types of geological environments, marine and lake. The raw material sample was obtained in North-East Nigeria, which is close to the Lake Chad basin. Therefore, it can be surmised to have originated from the lacustrine environment. As received dried sample is loosely cemented, porous and lightweight rock of sedimentary origin, mainly formed from skeleton of diatomea. Other chemicals used in this work are reagents grade materials. The modifying materials areanatase TiO2 nanoparticle and AgNO3, which is the silver ion source. Sodium carbonate (Na2CO3) was used to reduce the silver ions. The Ag-TiO2 material expectedly served as a photocatalyst enhancing the free radical and microbial degradation functionality of the main ceramic porous structure.
2.2. Modified Diatomaceous Aluminosilicate Preparation
Raw diatomaceous material was pulverized carefully in a mortar and fine powder (<200 µm) was obtained. The powder was mixed with 500 ml of distilled water in a beaker to form a colloid, which was agitated and left for a few minutes for heavier particles and other impurities to settle at the base of the beaker. Thereupon the top suspension was decanted into another beaker, and the process repeated till a homogeneous grey gelatinous separate was obtained. The collected pure diatomaceous gel was transferred into an oven for drying for 18 hours at 100˚C; and a soft white light-weight cake was formed. In order to enhance the physical and chemical properties of the raw aluminosilicate material, acid activation was carried out on the well-dried diatomaceous aluminosilicate powder using 30% dilute HNO3. The acid treatment was intended for dissolution of some amorphous materials in the microstructure in order to refine and chemically activate the pores surface area.
2.3. Modified TiO2 Preparation
For photo-activation of the diatomaceous membrane in the visible region, silver nanoparticles was intercalated into the TiO2 nanopowder to give Ag-TiO2 (STOX). Silver nitrate (AgNO3) was used as the silver ions source. Firstly, 10 g of TiO2 powder was placed into a 500 ml beaker with 100 ml ethanol as the dispersion medium. Then, 0.1 M solution of AgNO3 and 1% (w/v) solution of sodium carbonate (reducing agent) were prepared separately. Hence, 4.6 ml of the prepared silver nitrate solution and 5 ml of sodium carbonate were added into the beaker containing the dispersed TiO2. The mixture was magnetically stirred vigorously for 2 hours to form a slurry solution and afterwards the solid material (Ag-TiO2) was collected by centrifugation. The collected modified TiO2 nanoparticle was dried in the furnace at 100˚C for about 24 hours, thereafter; the powder was calcined at 400˚C for 12 hours to remove leftover organics and also for thermal diffusion of the material.
2.4. Ag-TiO2/Diatomaceous Aluminosilicate (ZEO) Precursor
The ceramic precursors were prepared using two procedural routes for membranesZEO-T and STOX-Z. For ZEO-T, the prepared Ag-TiO2 powder(STOX)wasring milled withthe acid treateddiatomaceous aluminosilicate powder (ZEO) in other to achieve mechano-chemical interactions and even distribution of constituents in the ceramic composite. This was followed by solid state fusion, the milled mixture was subjected to high tem- perature pressured treatment at 800˚C. To prepare membrane STOX-Z precursors, dispersion of modified Ag-modified TiO2 was prepared as earlier described. Then, depending on the quantity proposed, 25 g of acid treated diatomaceous aluminosilicate powder was added slowly into the continuously stirred Ag-TiO2 colloidal solution. The slurry solution formed was stirred and mildly heated simultaneously for 2 hrs on the hot plate. The final modified diatomaceous aluminosilicate precursor for membrane STOX-Z was recovered from the slurry solution. Conspicuous visible light induced colour change was observed for the recovered STOX-Z membrane precursor when exposed to daylight. The constituents of the ceramic precursors are highlighted in Table 1.
2.5. Ceramic Membrane Casting
Ceramic membranes disc with dead-end flow configurations were fabricated using the procedure highlighted in Figure 1. The selected precursors were pulverized accordingly to achieve homogenization. Hence, the precur- sors were compacted at an optimum pressure to form a disk. Generally ceramic structure could take any desired shape from simple to complex ones depending on the functionality of the ceramic object. In the present case, dead-end configuration is regarded as being most suitable for qualitative performance tests, which rarely depend on membrane shape. The cast ceramic membrane samples STOX-Z and ZEO-T were sintered at temperature between 900˚C and 1000˚C in the furnace for about 12 hours to attained prime mechanical stability.
2.6. Chemical and Thermal Characterization Methods
The elemental and oxide compositions of the raw diatomaceous aluminosilicate minerals was obtained with
Table 1. Constituents of the ceramic membrane precursor materials.
√ Present. - Not present.
Figure 1. Ag-TiO2 modified diatomaceous aluminosilicate ceramic membrane preparation steps.
PIXE technique using the 1.7 MV Pelletron Tandem Accelerator at the Center for Energy Research and Development (CERD), Obafemi Awolowo University, Ile-Ife, Nigeria. The data obtained were analyzed and fitted using GUPIXWIN software. Because the samples are primarily geological in nature, SOIL 7 was used as the reference material. Energy Dispersive X-ray spectroscopy (EDX) was carried out as a complementary analysis to the PIXE technique. Elemental characterization of the raw material before and after modification was done using the EDX. Chemical analyses of the materials before and after modification was done using multi-func- tional Fourier Transform Infra-red (FTIR) spectrophotometer (Thermo Scientific NICOLET iS5) equipped with iD3 AIR sample holder at CERD. Differential Thermal Analyzer (DTA 404 PC Ëos®) was used to study the thermal history of the precursors as they were subjected to different heat treatments. The pore size and morphology of the natural and modified materials were examined using scanning electron microscopy (SEM).
2.7. Functional Characterization Methods
The functional studies and characterizations such as mechanical strength, heavy metal and microbe removal from pollute driver and industrial effluents were carried out to ascertain the suitability of the prepared ceramic membrane samples for environmental remediation. The Atomic Absorption Spectrometry (AAS) analysis of the samples obtained from remediation experiments using the ceramic membranes was carried out. This analysis focused mainly on the change in concentrations of some beneficial elements such as calcium, potassium, sodium and magnesium, and also some known heavy metal contaminants in water samples before and after filtration. The microbiological analysis of the water samples was carried out using plate count method. This method relies on bacteria growing a colony on a nutrient medium so that it becomes visible to the naked eye and the number of colonies on a plate can be counted. The procedure involved making serial dilutions of the water samples in sterile water and then cultivated on nutritional medium (agar). Typical media include plate count agar for a general count or MacConkey agar to count Gram-negative bacteria such as E. coli. This approach is commonly used for the evaluation of the effectiveness of water treatment by the inactivation of representative microbial contaminants such as E. coli following ASTM D5465 [13] [14] . The relative acidic or basic level (pH) of the water samples before and after remediation was measured. Total dissolve solid (TDS) and Electroconductivity (EC) analyses were also carried out on the samples.
3. Results and Discussions
3.1. PIXE and EDX Analysis
The elemental concentrations of the raw diatomaceous aluminosilicateas determined with PIXEtechniques is given in Table 2. The EDX analysis showing the concentrations of the samples before and after modification is given in Table 3, while the spectra is shown in Figure 2. Different peaks are observed corresponding to the
Table 2. PIXE elemental concentrations of diatomaceous aluminosilicate.
Table 3. Elemental concentrations by energy dispersive X-ray spectroscopy.
Figure 2. EDX spectra for samples (a) ZEO (b) STOX-Z and (c) ZEO-T.
elemental concentrations of the samples, complementing the results obtained from the PIXE method for raw sample. The results obtained using EDX were useful for comparison between ZEO and the Ag-TiO2 modified diatomaceous aluminosilicates and also to confirm the more significant Si/Al ratio of the materials. Figure 2, which illustrates the EDX results confirmed the compositional relationship between aluminium and silicon in the geological material shown with the elemental peak intensities as obtained using the PIXE technique. The amounts of Si and Al detected in both samples ZEO and STOX-Z is also similar to results obtained using PIXE technique. It shows that silicon content is higher than Al content in the materials. The compositions of the raw materials in term of oxides show that the diatomaceous material is largely constituted by silica (76.05 wt. %); with aluminium oxide (Al2O3) being the next major compound at 15.33 wt. %. Other oxides except Na2O appear in very small concentrations, while elements such as Mn and Zn are detected in trace amounts, whereas Mn and Zn were not detected in any of the materials using EDX. Nonetheless, using the EDX technique, it was possible to know the concentrations of low Z elements;carbon and oxygen in the materials as shown in the spectra. The peak intensities shows that oxygen has the highest concentration. Ti is detected in the modified materials only, but not observed in the raw materials, though a trace amount of titanium was detected by PIXE technique as indicated in Table 2. The titanium peaks shown in STOX-Z and ZEO-T spectra confirmed the presence of TiO2 in the modified ceramic membrane precursor.
3.2. Thermal Properties of the Raw and Modified Aluminosilicate Materials
The DTA spectra of the ordinary and modified materials are presented in Figure 3 and Figure 4 for TiO2 (TOX, STOX) and diatomaceous aluminosilicate (ZEO, STOX-Z) respectively. The thermal analysis of TiO2 and
Figure 3. DTA spectra for samples STOX and TOX.
Figure 4. DTA Spectra for samples STOX-Z and ZEO.
Ag-TiO2 was carried out separately to understand the behavior and the effect of the modification on the physical and chemical nature of the natural aluminosilicate minerals. The spectra obtained for TiO2 are observed to show both exothermic and endothermic effects at temperature ranges peculiar to the behaviour of the minerals, thus showing the purity of the material. For instance, the pure and Ag-loaded TiO2 nanoparticles samples conspicuously exhibited an endothermic doublet peak at 798.4˚C and 848.6˚C (STOX) and 807.0˚C and 858.1˚C (TOX). These doublet endothermic peaks could be attributed to a successive decomposition and phase transitional recrystallization behaviour of the TiO2 nanoparticles. The slight shifting of the doublet endothermic peaks towards lower temperature for Ag-modified sample STOX is most likely due to the influence of melting silver nanoparticles (Ag-NP) in the matrix. Studies [15] have shown that metastable anatase and brookite TiO2 phases usually convert irreversibly to the equilibrium rutile phase upon heating above temperatures in the range 600˚C - 800˚C. Confirmed by the presence of the endothermic peaks, the pure and modified titanium oxide particles underwent drastic change in their crystal structures to form a rutile phase due to the application of heat. As stated earlier, slight left shifts in the successive peaks observed for sample STOX may not be unconnected with presence of Ag-nanoparticles in the pure materials. A broad exothermic peak observed at 989.1˚C for the modified sample STOX could be attributed to the Ag-TiO2 nanoparticles solid state reaction encouraged by oxidation. Such exothermic peak is not observed in the TiO2 thermal spectra.
An endothermic effect is observed at 125˚C for the raw diatomaceous aluminosilicate sample ZEO as shown in Figure 4. Endothermic effect exhibited at a lower temperature range indicated removal or desorption of surface water in the material. Nevertheless, such low temperature range behaviour is not observed for STOX-Z, maybe due to the acid activation effect which might have removed the molecular water locked in the pores and microstructure before modification with Ag-TiO2. The response to heat by STOX-Z at lower temperature range differs greatly from that of the pure mineral. Thehygroscopic surfacewater eliminated due to the chemical and thermal treatments seems to be irreversible in nature. Another low loop endothermic peak is observed for STOX-Z at 766.8˚C; and this clearly showed the presence of TiO2 in the modified sample structure as observed in the DTA spectra for the pure TiO2 nanoparticles, with similar thermal behaviour around the temperature range (726˚C - 858˚C). Based on the thermal behaviour of STOX-Z, it is shown that there is a phase conversion of the TiO2 in the STOX-Z, thus it changed to a rutile structure in the diatomaceous aluminosilicate which actually influence the performance of the material for remediation.
3.3. FT-IR Spectroscopy
Experimental FTIR spectra of raw and modified diatomaceous aluminosilicate samples can be categorized into three main absorption regions; adsorption within the hydroxyl region, OH-bending of physically adsorbed water and the low frequency range band caused by structural aluminosilicate frameworks. Precursors for ceramic membranes ZEO and STOX-Z exhibit transmission bands in the wavenumber range 4000 cm−1 to about 500 cm−1 as shown in Figure 5(a) and Figure 5(b) respectively. The broad band at 3416.65 cm−1 and 3416.98 cm−1 could be attributed to H-O-H vibration of absorbed water. The spectra for sample ZEO-T (Figure 5(c)) did not show any visible band in this region and the main reason behind this behaviour is its initial heat treatment which might have driven out the absorbed water, thus broken the H-O-H vibration due to water [16] . The band at 1628.60 cm−1 and 1635.69 cm−1 shown by ZEO-RAW and STOX-Z respectively could be attributed to OH bending vibration of the physically absorbed water, and also could be indicative of the presence of phyllosilicate mineral or illite [16] [21] . The band shown in the spectra exhibited by pure and modified samples at the low wavenumber range is obviously due to structural aluminosilicate frameworks. Bands 1077.98 cm−1 and 1077.61 cm−1 in spectra shown in Figure 5(a) and Figure 5(b) for STOX-Z and ZEO respectively are in the range within the 1093 cm−1 and 1039 cm−1 bands, attributed to vibration modes of siloxane (Si-O-Si) stretching, and Si-O stretching vibration [22] [23] . Considering sample ZEO-T with transmission band shown at 1076.88 cm−1 indicating vibration which corresponds to Si-O stretching, it showed that the main aluminosilicate framework was not destroyed by the heat treatment. It is observed that only sample ZEO (raw material) exhibited band at 796.89 cm−1 which indicates the presence of kaolinite constituent in the aluminosilicate raw material. Typically, in kaolinite, absorption band at 790.9 cm−1 are attributed to Si-O, Si-O-Al, (Al, Mg)-O-H and Si-O-(Mg, Al) stretching [24] . Generally, it is observed that the chemical properties of the modified precursors have changed principally due to the chemical and thermal treatments they have been subjected to. The bands that characterized the raw materials at the highest and the lowest wavenumber have all disappeared leaving the main broad band
Figure 5. FTIR spectra of (a) modified sample STOX-Z, (b) unmodified sample ZEO, (c) modified sample ZEO-T and (d) modified TiO2 (STOX).
corresponding to Si-O-Si stretching in the heat treated ZEO-T, which indicated that some of the bonds have broken. The spectra for TiO2 is given by Figure 5(d). The transmission bands shown at 1450.90 cm−1 and 3341.34 cm−1 are characteristic of Ti-O-Ti stretching.
3.4. Surface Morphology and Microstructural Analyses
The surface morphology and the microstructure analyses of the ceramic membranes were carried out using scanning electron microscope (SEM) equipment (VEGA 3 TESCAN and ZEISS DSM-940A). For the analysis, the specimens were made electrically conductive at the surface, and electrically grounded to prevent the accumulation of electrostatic charge at the surface.The images acquired for diatomaceous aluminosilicate materials are shown in Figures 6(a)-(c). The image for ZEO ceramic membrane is shown to contain well- arranged and neatly distributed network of pores which are very crucial in straining and size exclusion separation mechanism. However, for the Ag-TiO2 modified samplesZEO-T and STOX-Z, the images showed microstructures consisting of well dispersed particles and coagulates of the modifying TiO2nanoparticles in- terspersed in the natural pore structure. It is observedin Figure 6(b) that there is a conversion from the naturally arrange pore structure to a zeolite-like homogeneous crystal structure. The difference in morphology of the Ag-TiO2 modified and ZEO ceramic membranes shows that the chemical and thermal processes decomposed the original structure of the diatomaceous aluminosilicate, thereby forming a new crystalline structure interspersed in the main structure. The effect of the decomposition of the natural aluminosilicate structurereflected in the enhancementof the flow rate when used as a remediation membrane for polluted water samples. Additionally, the photocatalytic nature of the modifying material would be much helpful in eliminating microbes and
Figure 6. SEM micrographs for ceramic membranes (a) ZEO, (b) ZEO-T and (c) STOX-Z.
degrading contaminants induced by the formation of hydroxyl radical on the catalyst surface. TheSEM image for STOX-Z ceramic membrane showed a differentstructure from the others and this can be attributed to the chemical synthesis route which involved simultaneous stirring and heating which most likely broke down the natural pore structures of the natural material. The STOX-Z ceramic membrane microstructure is shown with well dispersed particles and coagulates, and with the main aluminosilicate materials forming spike-like structures.
3.5. Membranes Functional Physico-Chemical Studies
The presence of dissolved solids may affect the taste of water. Study [25] showed that the palatability of drinking water can be rated by taste, and relate to its total dissolved solids (TDS) level as follows: excellent, less than 300 ppm; good, between 300 and 600 ppm; fair, between 600 and 900 ppm; poor, between 900 and 1200 ppm; and unacceptable, greater than 1200 ppm [25] . The principal constituents are usually calcium, magnesium, sodium, and potassium cations and carbonate, hydrogen-carbonate, chloride, sulfate, and nitrate anions. Water with extremely low concentration of TDS may also be unacceptable for drinking due to its flat, insipid taste.
Results obtained from flame AAS analysis of the water samples are presented in Table 4, corresponding to the different aluminosilicate ceramic membranes. Water samples were obtained after filtration process using membrane discs, and were tested for common cationssuch as K+, Ca2+, Na+, and Mg2+ which usually serve as indicator for water hardness. Also, same samples were tested for the concentrations of heavy metal such as Ni2+, Mn2+, and Cd2+, which were present in the raw water samples. The sample designated as RAW corresponds to the source unfiltered water sample. It was observed that the concentrations of Mg2+, Na+ and Ca2+ ions increased slightly in water samples obtained from ceramic samples ZEO and STOX-Z. It indicated that hardness of the filtrates has increased based on increased concentrations of indicator cations. Though, the hardness is still very much within permissible limit for drinking. For filtrates from membrane ZEO-T, only Ca and Na ions are observed to have increased. Selective ion exchange behaviour of materials with open frame structure is usually responsible for the increase or decrease in the cations in the respective filtrate samples. An important property of aluminosilicate minerals is the ability to exchange ions relative to the surface charged of aluminosilicate minerals, and preference of the ion exchange material to attract one ion over another is termed its selective sequence. For heavy metals (Ni, Mn, and Cd) identified in the raw water, the result showed that their concentration reduced in filtrate samples from ZEO-T membrane.
Although, the pH of pure water is 7, drinking and natural water exhibit a pH range because it contains dissolved minerals and gases. Surface waters typically range from pH 6.5 to 8.5 while groundwater ranges from pH 6.0 to 8.5. Water with a pH less than 6.5 is regarded as being acidic. The results of the pH and total dissolved solid (TDS) analyses for samples obtained from ceramic membranes from raw and modified aluminosilicate materials showed that the pH of the filtrates slightly reduced especially ceramic membranes from pure raw materials, 7.60 for ZEO. The filtrate samples obtained from the treatment using ZEO-T ceramic membranes did not show any change in the pH. Whereas, for STOX-Z the pH of the samples collected was observed to be slightly higher. The pH values obtained in all samples are all within the limit of normal drinking water. Electroconductivity (EC) and TDS values are shown in Table 5. The data shown indicate that the samples obtained from membranes ZEO and ZEO-T have the lowest values ranging from 097 to 109. Considering TDS values with pH
Table 4. Atomic absorption spectroscopy result.
values, it is observed that there is a direct relationship between the parameters.
3.6. Membrane Functional Antimicrobial Studies
Results of the comparison of the water quality between feed water and the filtrate are shown in Table 6. The experimental results showed that for most of the samples, 100% bacterial population can be removed by the ceramic membranes. The total bacterial concentration might comprise one or more of the pathogenic bacterium such as Escherichia coli, Salmonellae, Staphylococcus and Pseudomonas etc. For most of the water samples, the results showed that there were 100% reductions in bacterial population as detected in different agar media used. Meanwhile, the untreated water sample designated as RAW obtained from a polluted river expectedly shows the highest presence of bacterial and fungi colonies as indicated. The main reference water sample used was the machine purified table water (OAU water) commercially produced by Obafemi Awolowo University Investment Company, Ile-Ife, Nigeria. The microbial result for OAU water returned nil for all the bacterial tests as shown in Table 6.
As presented under the result table, four different agar media were used for the microbial detection experiment namely; Nutrient Agar (NA), Eosin Methylene Blue Agar (EMB), MacConkey Agar (MAC) and Saborand Dextrose Agar (SDA). The first three media were for bacterial growth, while the SDA was prepared for fungi growth detection. Nutrient agar is a general purpose medium supporting growth of a wide range of non-fasti- dious organisms. For all sample plates, except the raw untreated water (designated as 100%), 0% colonies were detected on the agar plates. The “Levine’s formulation”, also called EMB, is a slightly selective strain for Gram-negative bacteria such E-coli, Salmonella, Shigella etc. On the EMB agar plates, as presented in Table 6, most of the sample returned 0% detection for the bacteria. However, 8 cfu/ml value was detected for the untreated (RAW) water samples. The MAC [26] agar, similar to EMB, is also used for the isolation and differentiation of Gram-negative enteric bacilli such as Enterococcus faecalis, Escherichia coli, Proteus mirabilis, Salmonella typhimurium etc. The medium was used to differentiate strains of Salmonella typhosa from members of the coliform group, but the modification of the formula improved growth of Shigella and Salmonella strains. For the fungi detection in the samples, Sabouraud dextrose agar (SDA) was used to culture fungi in petri dishes. It has a low pH that inhibits the growth of most bacteria, and also contains the antibiotic gentamicin to specifically inhibit the growth of Gram-negative bacteria. As shown in the table, the filtrate from unmodified ceramic membrane (ZEO) exhibit higher fungus growth count (2), while filtrates obtained from other photocatalytically modified membranes showed lower population count.
The plain untreated mesoporous ceramic membranes, though with the right pore sizes and structurefor size
Table 5. Total dissolved solid, electroconductivity and pH results of raw and treated water samples.
Table 6. Microbial population analysis.
Agar Media. NA: Nutrient Agar (General). EMB: Eosin Methylene Blue Agar. MAC: MacConkey Agar. SDA: Saborand Dextrose Agar.
Table 7. Output flux of the ceramic membranes.
exclusion and adsorption mechanisms, the surface is still very prone to microbial habitation with the formation of biofilm over time. Modifying with appropriate photocatalytic materialsas carried out in the present work, the effect of biofilm on ceramic is drastically reduced. The prepared mesoporous ceramic membranes are also very effective at reducing particulate matter and based on pore size was able to reduce water turbidity. The filtered water samples collected were visually observed and compared and were found to be clearer than the unfiltered raw water.
3.7. Flux Output Characteristics
The relations for the hydrostatic pressure due to the liquid alone and flux output are given respectively as:
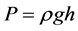 (1)
(1)
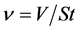 (2)
(2)
where ρ is the liquid density, g is the gravitational acceleration, h corresponds to the height of the liquid relative to the membrane, V is the filtrate volume, S is the surface area of the membrane filter, and t is the time. Using the relations (1) and (2), the transmembrane pressure was found to be 0.0196 MPa and the output flux at about 30˚C found to be 5.607 L/hr∙m2 for membrane sample STOX-Z and 39.245 L/hr∙m2 for ZEO-T as given in Table 7. The flow channels are mostly through the grain boundaries in sample ZEO-T. Theintercalation of titanium oxide nanoparticles in the activated diatomaceous material (ZEO) by thermal fusion appeared to have created more flow channels within the grain boundaries of membrane ZEO-T, and thereby enhanced its output flux.
4. Conclusion
Efficient mesoporous ceramic membranes were prepared with natural and abundantly available diatomaceous aluminosilicate minerals using less complicated procedures. The properties of the raw geological minerals and the modified materials were obtained via the elemental, chemical, thermal and microstructural characterizations of the materials. Functional analyses of the pure and TiO2-modified mesoporous ceramic membranes showed that the ceramic materials and membranes exhibited better remediation performance on polluted water. The ceramic membranes showed adsorption capacity for heavy metals ions such as K, Mg, Mn and Ni. Also, the ceramic membranes exhibited high capacity of up to 100% and 70% efficiencies in removal of bacteria and fungi respectively from polluted water samples.
Acknowledgements
The authors acknowledge the immense contribution of Dr. M. K. Bakare who passed away recently, may his soul rest in peace (amen).The support of Centre for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Nigeria is gratefully appreciated.
Cite this paper
Emmanuel Ajenifuja,John Adegbindin Ajao,Samson Oluwagbemiga Alayande,Mufutau Kolawole Bakare,Bidini Alade Taleatu,Ezekiel O. B. Ajayi, (2016) Synthesis and Characterization of Pure and Ag-TiO2-Modified Diatomaceous Aluminosilicate Ceramic Membranes for Water Remediation. Journal of Water Resource and Protection,08,594-607. doi: 10.4236/jwarp.2016.85049
References
- 1. Ebrahimi, M., Kovacs, Z., Schneider, M., Mund, P. and Bolduan, P. (2012) Multistage Filtration Process for Efficient Treatment of Oil-Field Produced Water Using Ceramic Membranes. Desalination and Water Treatment, 42, 17-23.
http://dx.doi.org/10.1080/19443994.2012.682964 - 2. Ebrahimi, M., Schmitz, O., Kerker, S., Liebermann, F. and Czermak, P. (2013) Dynamic Cross-Flow Filtration of Oilfield Produced Water by Rotating Ceramic Filter Discs. Desalination and Water Treatment, 51, 1762-1768.
http://dx.doi.org/10.1080/19443994.2012.694197 - 3. Jia, Y., Han, W., Xiong, G. and Yang, W. (2007) Diatomite as High Performance and Environmental Friendly Catalysts for Phenol Hydroxylation with H2O2. Science and Technology of Advanced Materials, 8, 106-109.
http://dx.doi.org/10.1016/j.stam.2006.10.003 - 4. Zhang, G.L., Cai, D.Q., Wang, M., Zhang, C.L., Zhang, J. and Wu, Z.Y. (2013) Microstructural Modification of Diatomite by Acid Treatment, High-Speed Shear, and Ultrasound. Microporous and Mesoporous Materials, 165, 106-112.
http://dx.doi.org/10.1016/j.micromeso.2012.08.005 - 5. Morozova, L.V., Lapshin, A.E. and Drozdova, I.A. (2008) Preparation and Investigation of Porous Aluminosilicate Ceramic Materials. Glass Physics and Chemistry, 34, 443-448.
- 6. Sui, X. and Huang, X. (2003) The Characterization and Water Purification Behavior of Gradient Ceramic Membranes. Separation and Purification Technology, 32, 73-79.
http://dx.doi.org/10.1016/S1383-5866(03)00057-1 - 7. Darcovich, K. and Cloutier, C.R. (1999) Processing of Functionally Gradient Ceramic Membrane Substrates for Enhanced Porosity. Journal of American Ceramic Society, 82.
- 8. Nainani, R., Thakur, P. and Chaskar, M. (2012) Syhthesis of Silver Doped TiO2 Nanoparticles for the Improved Photocatalytic Degradation of Methyl Orange. Journal of Materials Science and Engineering B, 2, 52-58.
- 9. Wang, H., Lin, C., Kuo, Y., Cheng, Y. and Yeh, Y. (2008) Synthesis and Photocatalysis of Mesoporous Anatase TiO2 Powder Incorporated Ag Nanoparticles. Journal of Physics and Chemistry of Solids, 69, 633.
http://dx.doi.org/10.1016/j.jpcs.2007.07.052 - 10. Zheng, S., Bai, C. and Gao, R. (2012) Preparation and Photocatalytic Property of TiO2/Diatomite-Based Porous Ceramics Composite Materials. International Journal of Photoenergy, 2012.
- 11. Ajenifuja, E., Akinwunmi, O.O., Bakare, M.K., Ajao, J.A., Adeniyi, I.F. and Ajayi, E.O.B. (2012) Remediation of Polluted Water Using Natural Zeolitic Aluminosilicates/Lateritic Clay Ceramic Matrix Membrane. ISRN Ceramics, 2012.
- 12. Ajenifuja, E., Ajao, J.A. and Ajayi, E.O.B. (2016) Equilibrium Adsorption Isotherm Studies of Cu (II) and Co (II) in High Concentration Aqueous Solutions on Ag-TiO2-Modified Kaolinite Ceramic Adsorbents. Applied Water Science.
- 13. Hanaor, D.A.H. and Sorrell, C.C. (2014) Sand Supported Mixed-Phase TiO2 Photocatalysts for Water Decontamination Applications. Advanced Engineering Materials, 16, 248-254.
http://dx.doi.org/10.1002/adem.201300259 - 14. Hanaor, D., Michelazzi, M., Leonelli, C. and Sorrell, C.C. (2011) The Effects of Firing Conditions on the Properties of Electrophoretically Deposited Titanium Dioxide Films on Graphite Substrates. Journal of the European Ceramic Society, 31, 2877-2885.
http://dx.doi.org/10.1016/j.jeurceramsoc.2011.07.007 - 15. Greenwood, N.N. and Earnshaw, A. (1984) Chemistry of the Elements. Pergamon Press, Oxford, 1117-1119.
- 16. Ilia, K.I., Stamatakis, G.M. and Perraki, T.S. (2009) Mineralogy and Technical Properties of Clayey Diatomites from North and Central Greece. Open Geosciences, 1, 393-403.
http://dx.doi.org/10.2478/v10085-009-0034-3 - 17. Kondo, M.M. and Jardim, W.F. (1991) Photodegradation of Chloroform and Urea Using Ag-Loaded Titanium Dioxide as Catalyst. Water Research, 25, 823-827.
http://dx.doi.org/10.1016/0043-1354(91)90162-J - 18. Page, K. (2009) Photocatalytic Thin Films: Their Characterizations and Antimicrobial Properties. London University College, London, 74.
- 19. Yamashita, T., Aketo, T., Minowa, N., Sugimoto, K., Yokoyama, H., Ogino, A. and Tanaka, Y. (2013) Simultaneous Removal of Colour, Phosphorus and Disinfection from Treated Wastewater Using an Agent Synthesized from Amorphous Silica and Hydrated Lime. Environmental Technology, 34, 1017-1025.
http://dx.doi.org/10.1080/09593330.2012.733417 - 20. Eturki, S., Ayari, F., Kallali, H., Jedidi, N. and Dhia, H.B. (2012) Treatment of Rural Wastewater by Infiltration Percolation Process Using Sand-Clay Fortified by Pebbles. Desalination and Water Treatment, 49, 65-73.
http://dx.doi.org/10.1080/19443994.2012.708200 - 21. Wolf, R.G. (1963) Structural Aspects of Kaolinite Using Infrared Absorption. American Mineralogist, 48, 390-399.
- 22. Madejova, J., Janek , M., Komadel, P., Herbert, H.J. and Moog, H.C. (2002) FTIR Analyses of Water in MX-80 Bentonite Compacted from High Salinary Salt Solution Systems. Applied Clay Science, 20, 255-271.
http://dx.doi.org/10.1016/S0169-1317(01)00067-9 - 23. Van Jaarsveld, J.G.S., Van Deventer, J.S.J. and Luckey, G.C. (2002) The Effect of Composition and Temperature on the Properties of Fly Ash and Kaolinite-Based Geopolymers. Chemical Engineering Journal, 89, 63-73.
http://dx.doi.org/10.1016/S1385-8947(02)00025-6 - 24. Nayak, P.S. and Singh, B.K. (2007) Instrumental Characterization of Clay by XRF, XRD and FTIR. Bulletin of Materials Science, 30, 235-238.
http://dx.doi.org/10.1007/s12034-007-0042-5 - 25. Bruvold, W.H. and Ongerth, J.H. (1969) Taste Quality of Mineralized Water. Journal of the American Water Works Association, 61, 170-174.
- 26. MacConkey, A. (1905) Lactose-Fermenting Bacteria in Faeces. Journal of Hygiene, 5, 333-379.
http://dx.doi.org/10.1017/S002217240000259X








