Journal of Cancer Therapy
Vol.5 No.1(2014), Article ID:41644,10 pages DOI:10.4236/jct.2014.51004
Safety and Therapeutic Efficacy of the Lewis Y Carbohydrate Specific Humanized Antibody MB311 in Patients with Malignant Effusion
![]()
1Medical University Graz, Graz, Austria; 2Currently: Hospital Leoben, Leoben, Austria; 3Igeneon, Vienna, Austria; 4Currently: AGES MEA, Vienna, Austria; 5Currently: Apeiron, Vienna, Austria, 6Currently: Hookipa, Vienna, Austria; 7Currently: Meridian Biopharmaceuticals, Vienna, Austria; 8Currently: ViroLogik, Erlangen, Germany.
Email: *a.nechansky@meridian-biopharm.at
Received November 7th, 2013; revised December 6th, 2013; accepted December 14th, 2013
ABSTRACT
Purpose: Investigation of safety and tolerability as well as therapeutic efficacy of the LeY specific humanized mAb MB311 in cancer pts with malignant effusions in a Phase II clinical trial. Experimental Design: An openlabel, single treatment arm, uncontrolled study with MB311 (100 mg per dose, intravenous infusion on day 1 and 7) in pts with malignant effusion (ascites or pleural effusion) was conducted with the primary objective to examine safety and tolerability as well as pharmacokinetics. Secondary objectives were assessment of pharmacodynamics, volumetric measurement of the malignant effusion and obtaining data for several immunological parameters. Results: Five pts (2 pts with gastric cancer and malignant ascites, 3 pts with breast cancer and malignant pleural effusion/ascites) have completed the study. MB311 was well tolerated with only two pts showing the easily manageable side effects nausea, vomiting (up to grade 2) and one episode of skin rash (grade 2) after the first application. Data of 4 pts were available for evaluating immunologic results and efficacy. In all pts significant levels of MB311 could be detected in the systemic blood circulation and the effusion leading to increased infiltration of CD45 positive immune cells (4/5 pts) and resulting in a reduction of tumor cell counts as detected by immunocytochemistry of effusion samples in 3/5 pts). Most interestingly, the pt with the highest LeY positive tumor showed a significant reduction of effusion volume after treatment—this decrease was also evident for Her2/neu positive tumor cells which were dramatically reduced after MB311 treatment in this breast cancer pt. Conclusion: MB311 was well tolerated in patients with malignant effusions, permeated into malignant effusion and attracted immune cells leading to decreased tumor cell counts in the effusion. In the case of strong LeY expression of malignant cells in the effusion a pronounced decrease in LeY, EpCAM and Her2/neu positive tumor cells and a significant reduction of the effusion volume could be demonstrated.
Keywords:Passive Immunotherapy; Therapeutic Antibody; Circulating Tumor Cells; Malignant Effusion; Lewis Y Carbohydrate; Ascites
1. Introduction
Today passive cancer immunotherapy with therapeutic monoclonal antibodies (mAbs) that have the ability to directly mediate anti-tumor responses belongs to the standard portfolio in cancer treatment [1]. The LeY carbohydrate antigen (CD174) is expressed on the majority of human cancers of epithelial origin whereas expression on normal tissue is limited to epithelial cells of the esophagus, stomach, the proximal small intestine, some acinar cells of the pancreas and resting granulocytes [1-5]. Regarding tumor cells, predominantly adenocarcinomas of the lung, breast, colorectal, gastric, pancreatic, prostate and ovarian cancers have been tested positive for LeY [2,6-9]. Pronounced expression of LeY in different tumors is associated with decreased survival and higher metastatic potential [10-14]. In the past, a variety of murine mAbs have been generated against LeY, their antitumor activities have been explored and have demonstrated anti-tumor activity in tumor cell models in vitro, animal models and clinical studies [1,2,6,15-22].
Based on the promising effects found with murine mAbs, humanized LeY specific mAbs have been designed which have shown improved pharmacokinetics in vivo and in humans [23-25].
A Phase I bio-distribution and pharmacokinetic trial with the humanized IgG3/κ mAb 3S193 in pts with LeY positive, advanced epithelial cancer have demonstrated selective targeting of tumors with no evidence of any consistent normal tissue uptake [26,27]. For another humanized anti-LeY mAb, IGN311 (now renamed to MB311), a Phase I dose escalation study was recently conducted and safety, tolerability, pharmacokinetic data, anti-tumor activity and immunologic parameters were assessed [28,29]. Intravenous application of MB311 in this study enabled the patients’ serum to lyse tumor cells by two mechanisms, Complement Dependent Cytotoxicity (CDC) and Antibody Dependent Cellular Cytotoxicity (ADCC). Moreover, disseminated tumor cells detectable in two patients before treatment were eliminated after treatment with MB311 [28]. In animal models, MB311 was also shown to block signal transduction through LeY glycosylated growth factor receptors such as EGFR and Her2-neu on tumor cells [24,30].
In the present study MB311 was tested for the first time in pts with malignant effusion (ascites or pleura) with the primary objective to examine safety and tolerability as well as pharmacokinetics. Secondary objectives were assessment of pharmacodynamics, immunological parameters, and—as a clinically relevant parameter regarding efficacy—the volume of the malignant effusion. MB311 was well tolerated, permeated into malignant effusion and attracted immune cells leading to decreased tumor cell counts in the effusion. About 20 years ago, disseminated tumor cells were regarded as important marker [31] and have gained recently increasing attention and relevance as a diagnostic and prognostic factor in cancer therapy [32-36]. Notably, in the pt with the strongest LeY expression as well as concomitant Her2/ neu expression on the malignant cells in the effusion a dramatic effect on LeY, EpCAM (CD326) and Her2/neu positive tumor cells and a clinically significant reduction of the effusion volume were found following application of MB311.
2. Patients and Methods
2.1. Study Synopsis
The primary objective of the study was to assess safety, tolerability and pharmacokinetics of two doses of MB311 administered by intravenous bolus infusion to pts with malignant effusion (ascites or pleural effusion). Secondary objectives were assessment of the response based on the malignant effusion volume by volumetric measurement at baseline and after therapy (via computer tomography). X-ray or ultrasound examination of effusion was to be performed after 4 weeks—only in case of response an additional CT scan was to be performed. Also the number of LeY expressing cells in pleural effusion/ ascites was determined. Additionally, the functional activity of MB311 in the pleural effusion/ascites by CDC and ADCC reactivity; the amount of disseminated tumor cells in peripheral blood and the tumor response (other than tumor effusion; e.g. stage and size of the primary tumor) were measured.
2.2. Study Design and Treatment
Open-label, multiple dose, uncontrolled study enrolling 5 pts evaluable for safety and tolerability assessment. The study was performed at the department of oncology of the Medical University Clinic Graz, 8036 Graz, Austria. All subjects received two administrations of 100 mg MB311. The mAb was administered intravenously by prolonged infusion for two hours on day 1 and 7. Pts were followed up for a period of 4 weeks. Volumetric measurements of effusion at baseline and at the end of study at week four were performed. Effusion-related symptoms and adverse events were assessed weekly. Pts with significant progression of effusion causing symptomatic deterioration would have been removed from the trial and treated at the discretion of the investigator. Also if concomitant systemic anti-tumor therapy was required the pt would have been removed from the evaluation. All pts who received study medication were included in the safety/olerability analysis. The study was conducted in accordance with the latest revision of the Declaration of Helsinki, the requirements of Good Clinical Practice of the European Community (CPMP/ICH/135/95) and European Clinical Trials Directive 2001/20/EC. Written, voluntary informed consent to participate in this study was obtained prior to enrolment into the study and for performing of any study specific evaluations.
2.3. Eligibility Criteria
All included patients had a histologically proven Lewis Y positive primary tumor (stage IV) as well as Lewis Y positive tumor cells detectable in the effusion sample prior to start of treatment. The median age of the pts was 62 (range of 45 - 74) years. Gastric cancer was the primary diagnosis in two male pts (pat. 1 and 3) and breast cancer in three female pts (pat. 2, 4 and 5). All pts had prior treatment for metastatic disease either with chemotherapy for median 2 lines (range 1 - 4) and/or with anti-hormonal therapy (median 3 lines) in 2/3 pts with receptor-positive breast cancer. In addition, patient 4 had a prior history of herceptin treatment for metastatic disease due to HER2/neu2 positive breast cancer. Furthermore, no concomitant systemic anti-tumor therapy was allowed and any previous systemic anti-cancer therapy ended more than 4 weeks before study entry. The Karnofsky Performance Score (KPS) had to be higher than 70 with a life expectancy of more than 3 months. Measurable (CT based volumetric) but asymptomatic malignant effusion (ascites or pleural effusion) was required as inclusion criteria.
2.4. Pharmacokinetic (PK)
Sera obtained at different time points after infusion of MB311 were incubated in 96-well plates coated with MMA383 (anti-idiotypic antibody to MB311 mimicking the LeY antigen) in appropriate dilutions. After washing, a mouse anti-human IgG1 HRP conjugate was added. After washing, color was developed using OPD as substrate, stopped with H2SO4 and read at 492/620 nm. A standard curve of MB311 spiked into normal human serum was used for calibration.
2.5. Human Anti-Human Antibody (HAHA) Response
HAHA reactivity was assessed using a surface plasmon resonance based assay as described previously [37]. This highly sensitive assay monitors binding to immobilized MB311 and to an isotype matched control antibody. A positive HAHA response was defined as at least two-fold increase in response units (RUs) compared to the matched control antibody and absolute value >50 RUs.
2.6. Complement Dependent Cytotoxicity (CDC)
SKBR3 (LeY positive adenocarcinoma breast cancer cell line) cells were incubated with 51Cr, washed and incubated for 60 minutes at 37˚C with serial dilutions of patient sera, thereby using patient’s complement. Supernatants were collected and counted for released 51Cr (“Cs”) using a Packard γ-counter. Values for spontaneous release (“Sr”) and maximum release (“Mr”) were measured after incubation of representative samples with medium alone and with detergent (20% SDS) respectively. Cytotoxicity was calculated as percentage of cell lysis by the formula 100 × (Cs-Sr)/(Mr-Sr). The percentage cytotoxicity was plotted against the logarithm of the antibody concentration (ng/ml).
2.7. Antibody Dependent Cellular Cytotoxicity (ADCC)
SKBR3 cells were incubated for 2 hours with 51Cr, washed and plated into 96-well microplates. Effector cells (peripheral blood mononuclear cells from healthy volunteers) were freshly prepared and added to the target cells at a E:T ratio of 40:1. Heat inactivated serum samples were diluted 1:20 and incubated at 37˚C for 16 hours in a CO2-incubator, cell supernatants were collected and counted for released 51Cr (“Cs”) using a Packard γ- counter. Values for spontaneous release (“Sr”) and maximum release (“Mr”) were measured after incubation of representative samples with medium alone and with detergent (SDS) respectively. Cytotoxicity was calculated as percentage of cell lysis by the formula 100 × (Cs-Sr)/ (Mr-Sr). The percentage cytotoxicity was plotted against the logarithm of the antibody concentration (ng/ml).
2.8. Microscopic and Immuno-Histological Analysis
Effusion specimens were embedded in agarose gel and subsequently formalin fixed and paraffin embedded following standard procedures. Serial 4 μm sections were cut, and incubated with anti-EpCAM (Epithelial Cell Adhesion Molecule), LeY, calretinin, CD45, HER2/neu and mouse-anti-human IgG mAbs followed by detection using a streptavidin biotin method on an autostainer (DAKO, Denmark).
2.9. Analysis of Effusion Sample Using Flow Cytometry
Cells obtained by drainage of ascites or pleurocentesis were washed, treated for 30 minutes on ice with blocking buffer (PBS + 10% FCS), and incubated on ice for 30 minutes with PEor FITC labeled antibodies directed against LeY, EpCAM or CD45. Samples were counted after an incubation with 7-AAD (20 minutes) using a BD FACSCalibur®. Results were measured in percentage of gated cells on the basis of all viable cells after correction for unstained control and isotype control.
3. Results
All included pts (n = 5) had a histologically proven LeY positive primary tumor as well as LeY positive tumor cells detectable in the effusion sample prior to start of treatment (Table 1). As a second tumor marker EpCAM, typically expressed in tumors of epithelial origin, was used, and found to be positive in 4/5 pts. The median age of the pts was 62 (range of 45 - 74) years. Gastric cancer was the primary diagnosis in two pts (pat. 1 and 3) and breast cancer in three pts (pts 2, 4 and 5), respectively.
Analysis of effusion samplesby flow cytometry. Data are based on single staining of viable cells and are corrected with isotype control values. Cells obtained by drainage of ascites or pleurocentesis were washed, treated for 30min on ice with blocking buffer (PBS + 10% FCS) and incubated with PEor FITC labelled antibodies directed against LeY, EpCAM or CD45 on ice for 30 min. Samples were counted with a BD FACSCalibur after incubation with 7-AAD (20 min). Results were measured in percentage of gated cells on the basis of all viable cells after correction for unstained control and isotype control. n.a. = data not available.
All pts received prior treatment for metastatic disease either with chemotherapy for median 2 lines (range 1 - 4) and/or with anti-hormonal therapy (median 3 lines) in 2/3 pts with receptor-positive breast cancer. In addition, pt 4 had a history of treatment with trastuzumab for metastatic disease due to HER2/neu2 positive breast cancer (Table 2).
3.1. Safety and Tolerability (n = 5)
MB311 was well tolerated with mild to moderate nausea and vomiting in 2 pts which could be successfully managed with 5HT3 blocker and metoclopramid. Furthermore, one episode of skin rash was observed in pt 2 after the first cycle which could be resolved and subsequently prevented with the administration of H1 and H2 blockers.
3.2. Pharmacokinetics & Pharmacodynamics (n = 4)
Systemic antibody levels in serum were about 30 µg/ml after the first and about 55 µg/ml after the second administration of 100 mg of MB311 (Figure 1, blue symbols). Volume of distribution corresponded therefore to the whole average plasma volume, after an apparent saturation of internalization and recycling mechanisms following the first administration. ADCC and—to a lesser extent—CDC reactivity against LeY positive tumor cells was induced in all pts (Figure 1, red and green symbols, respectively).
Penetration of MB311 into the effusion sample was quantified by anti-idiotypic ELISA and was found in all four evaluable pts (Figure 2(a)). Moreover, binding of MB311 mAb to tumor cells could be detected in 2/4 pts
Abbreviations: Le Y = Lewis Y; M = Mesothel, T = Tumor cells, Ly = Lymphocytes; SiC. = single cells; n.d. = not determined. Staining intensities: +/−: marginal, +: weak, ++: medium, +++: strong.

Figure 1. Pharmacokinetics (Titer) and Pharmacodynamics (CDC and ADCC) of MB311. Pharmacokinetics: Sera obtained from the patients at different time points after infusion of MB311 were incubated in 96-well plates coated with the anti-idiotypic antibody MMA383, followed by incubation with mouse anti-human IgG1 HRP conjugate. Color was developed using OPD as substrate. A standard curve of MB311 spiked into normal human serum was used for calibration. CDC: 51Cr-labelled SKBR3 cells were incubated for 60 minutes at 37˚C with serial dilutions of patient’s sera, thereby using patient’s complement. Released 51Cr in the supernatants was counted using a Packard γ-counter. The percentage of cytotoxicity was plotted against the logarithm of the antibody concentration (ng/ml). ADCC: 51Cr-labelled SKBR3 cells (target cells) were incubated for 2 hours with freshly prepared effector cells (PBMC from healthy volunteers) at a E:T ratio of 40:1, followed by addition of heat inactivated patient sera (diluted 1:20) and incubation for another 16 hours. Released 51Cr in the supernatants was counted using a Packard γ-counter. The percentage of cytotoxicity was plotted against the logarithm of the antibody concentration (ng/ml).
 (a)
(a) (b)
(b)
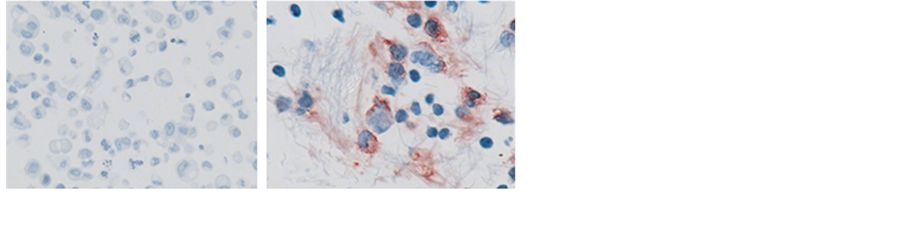
Figure 2. (a) Permeation of MB311 into the effusion volume (% of serum titer) and (b) Binding of MB311 to tumor cells in the effusion (Pat 4). (a) Effusion aspirates obtained at Day 1 and Day 21 after infusion of MB311 were incubated in 96-well plates coated with the anti-idiotypic antibody MMA383 followed by incubation with mouse anti-human IgG1 HRP conjugate. Color was developed using OPD as substrate. Amount of MB311 found in the effusion is shown as percentage of the amounts of MB311 found in the corresponding patient’s serum. No material from pt 5 was available. (b) IHC staining for human IgG of samples from effusion aspirates of patient 4 before (left) and after (right) treatment with MB311. Magnification 400×.
in the effusion sample (Figure 2(b)). Furthermore, a significant increase in ADCC in the effusion sample was found in 4/4 pts (Figure 3). A mild to moderate HAHA response was detected in all patients (data not shown), similarly as described previously [29].
3.3. Clinical Efficacy (n = 5)
Reduction of effusion volume was observed in 1/5 pts (pt 4) and a stabilisation of effusion volume and/or tumor marker in two out of five pts (pts 2, 5) (Table 3). A de-

Figure 3. Comparison of ADCC against LeY positive tumor cells in serum and effusion before treatment and at the end of study. 51Cr-labelled SKBR3 cells (target cells) were incubated for 2 hours with freshly prepared effector cells (PBMC from healthy volunteers) at a E:T ratio of 40:1, followed by addition of heat inactivated patient’s serum or patient’s effusion sample (diluted 1:20, respectively) and incubated for another 16 hours. Released 51Cr in the supernatants was counted using a Packard γ-counter. CDC activity in the serum compared to the CDC activity in the effusion samples before (white bars: serum day 1, black bars: effusion day 1) and after treatment (light gray bars: serum day 21, dark gray bars: effusion day 21) with MB311, respectively, is shown. No material from pt 5 was available.
Table 3. Volumetry, circulating tumor cells (CTC) and tumor markers.
Abbreviations: CEA = Carcino-Embryonic Antigen, n.a. = not analysed.
crease in the number of circulating tumor cells (determined by LeY and/or EpCAM staining) was shown in three out of five patients (pts 2, 4, 5) by immunohistochemistry and/or flow cytometry (Tables 1 and 2, Figures 4(c) and (d)). In addition, a significant infiltration of CD45 positive lymphocytes was noted by immunohistochemistry (IHC) in four out of five pts (pts 1, 3, 4, 5) in the effusion sample, which in two patients (pts 4, 5) correlated with a reduction of tumor cells in the same samples (Tables 1 and 2, Figures 4(e) and (f)). Flow cytometry results of available effusion samples confirmed an increased infiltration of CD45 positive cells for pts 1, 3 and 4 (Table 1). Most interestingly, in pt 4—with strong Her2/neu positive tumor cells in the effusion before treatment—a dramatic decrease of Her2/neu positive tumor cells following MB311 treatment was found (Figures 4(g) and (h)).
4. Discussion
Targeted cancer immunotherapy remains in the focus of cancer treatment [38] with the passive approaches belonging to the standard clinical treatment scheme. So far primarily monoclonal antibody therapies targeting protein based tumor associated antigens achieved approval, but passive immunotherapies targeting tumor associated carbohydrate antigens may turn out of being attractive development candidates. The anti-LeY mAb MB311 shows a favourable safety profile with manageable and reversible side effects like nausea, vomiting and skin rash. With the exception of pt 2 four out of five pts showed an increase of CD45 positive cells as detected by immunocytochemistry of effusion samples after i.v. application of MB311 confirming the attraction of effector cells to malignant cells of the effusion or ascites. Permeation of MB311 after systemic i.v. application into the malignant effusion volume of all pts evaluable for immunological and clinical analysis (n = 4) indicating effective distribu-
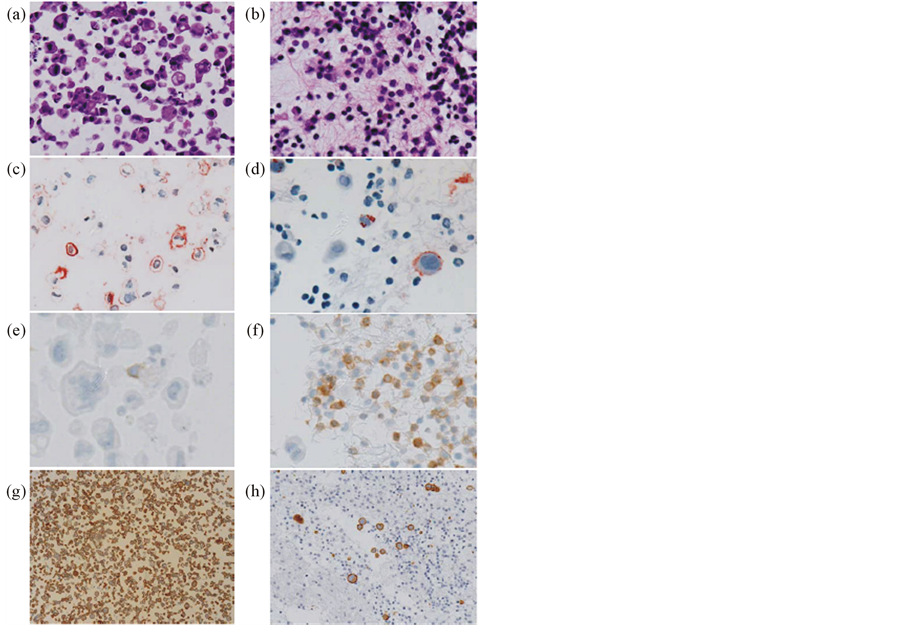
Figure 4. IHC stainings of cell blocks prepared from effusion aspirates of patient 4 before and after treatment with MB311. Effusion specimens were embedded in agarose gel and subsequently formalin fixed and paraffin embedded. Serial 4 μm sections were cut, and incubated with antiEpCAM, anti-LeY, anti-calretinin, anti-CD45, anti-HER2/ neu and mouse-anti-human IgG mAbs followed by detection using a streptavidin biotin method on an autostainer (DAKO, Denmark). Hematoxilin Eosin Staining (magnification 100x) before (a) and after (b) treatment. LeY staining before ((c), magnification 100×) and after ((d), magnification 200×) treatment. CD45 staining before ((e), magnification 400×) and after ((f), magnification 200×) treatment. HER-2/neu staining (magnification 40×) before (g) and after (h) treatment.
tion of MB311 through the blood stream. The results of ex-vivo measurements showed the induction of a lytic potential: Cytotoxicity against LeY positive tumor cells in the blood as well as in the effusion after i.v. application of MB311 indicates a dose level which might be sufficient to eradicate disseminated tumor cells, thereby preventing dissemination of disease and preventing metastatic disease. Noteworthy, particularly the patient with the highest LeY expression in the malignant ascites showed a significant reduction of the ascites volume after treatment with MB311 which goes along with eradication of tumor cells in the ascites (ex-vivo measurement). In contrast, CTCs the in peripheral circulation for patient 4 increased from 9 to 30, paralleled by an increase in tumor markers CEA and CA-15.3. This increase is most likely a consequence of the massive destruction of tumor cells in the effusion and thus only transient. Interestingly, the tumor cells of this breast cancer patient were (beside LeY and EpCAM positivity) also strongly Her2/neu positive and had been previously treated with repeated courses of Trastuzumab without sustained therapeutic effect. It has been published that LeY can be expressed as glyco-determinant on Her2/neu and related growth factor receptors on tumor cells (40). In the present study the number of Her2/neu positive tumor cells in the effusion was dramatically reduced following treatment with MB311 correlating also with a significant reduction in effusion volume.
Measuring the puncture-free survival time is an accepted and relevant parameter of assessing the clinical treatment efficacy in patients suffering from malignant ascites [39]. Also, the clinical response (disappearance of ascites accumulation) mediated by catumaxomab was found to correlate with the elimination of tumor cells [40]. Catumaxomab is a bispecfic (EpCAM/CD3) mAb that is approved in Europe for the intraperitoneal treatment of patients with ascites originating from epithelial tumors. Regarding MB311 (which was applied intravenously) the reduction of the effusion volume which were strongly positive for LeY at week 1 is in accordance with our previous data showing the eradication of disseminated tumor cells in the blood of two pts with high LeY expression of their primary tumor [28]. Regarding the anti-tumor activity of MB311 effector mechanisms, CDC and ADCC have been demonstrated against LeY positive tumor cells in ex vivo studies [28]. Furthermore, MB311 has been also shown to block signal transduction through LeY glycosylated growth factor receptors such as EGFR and Her2-neu on tumor cells as demonstrated in cell culture and in in vivo studies [24,30]. The combination of immunological effector mechanisms of MB311 together with the cytotoxic and cytostatic effects on EGFR and Her2-neu expressing tumor cells may underline the antitumor effect of MB311 in pts with LeY expressing tumor cells in effusion.
5. Conclusions
The presented study demonstrates that MB311 has the potency to eradicate LeY positive tumor cells in ascites which was also translated into a reduction of the ascites volume. Besides the potential of MB311 to eradicate disseminating tumor cells of a certain LeY expression status it might also lie in the combination with already established therapeutic mAbs like Trastuzumab. The present study was an exploratory study with a very specific setting providing an early idea about immediate in vivo effects against malign tumor cells in patients. As a next step, the identification of an appropriate indication, where the clinical relevance of attacking disseminating tumor cells is expected to directly influence overall survival, has to be selected.
Recently, it has been shown [41] that LeY also plays an essential role in ovarian cancer. Additionally, further studies may help select favourable tumor entities by examinations on differences in cell promoting effect if LeY is expressed as part of Her2/neu in contrast to expression of LeY on other backbone structures or expressed as soluble LeY [42]. With regard to Trastuzumab it has been shown that—in vivo—ADCC is one of the main anti-tumor mechanisms besides CDC and anti-proliferative effects [43]. The effusion ex-vivo results with MB311 suggest a more prominent role for ADCC when compared to the CDC activity, which would also point towards a pivotal role of ADCC in MB311 mediated cytotoxicity to facilitate induction of immune effectors suppressed by the tumor microenvironment. To confirm this observation, additional studies are required. Interestingly, an ADCC improved (de-fucosylated) version of MB311, designated MB314, has shown increased ADCC reactivity in vitro [44] and also an interesting cytokine release profile that might further increase its efficacy [45].
Acknowledgements
We thank Norbert Eller and Andrea Mayer for coordinating the study and Monika Chabicovsky for regulatory support.
REFERENCES
- A. M. Scott and S. Welt, “Antibody-Based Immunological Therapies,” Current Opinion in Immunology, Vol. 9, No. 5, 1997, pp. 717-722. http://dx.doi.org/10.1016/S0952-7915(97)80054-4
- I. Hellstrom, H. J. Garrigues, U. Garrigues, et al., “Highly Tumor-Reactive, Internalizing, Mouse Monoclonal Antibodies to Le(y)-Related Cell Surface Antigens,” Cancer Research, Vol. 50, No. 7, 1990, pp. 2183-2190.
- S. Zhang, H. S. Zhang, C. Cordon-Cardo, et al., “Selection of Tumor Antigens as Targets for Immune Attack Using Immunohistochemistry: II. Blood Group-Related Antigens,” International Journal of Cancer, Vol. 73, No. 1, 1997, pp. 50-56. http://dx.doi.org/10.1002/(SICI)1097-0215(19970926)73:1<50::AID-IJC9>3.0.CO;2-0
- Y. S. Kim, M. Yuan, I. S. Htzkowitz, et al., “Expression of LeY and Extended LeY Blood Group-Related Antigens in Human Malignant, Premalignant, and Nonmalignant Colonic Tissues,” Cancer Research, Vol. 46, No. 11, 1986, pp. 5985-5992.
- M. Dettke, G. Palfi and H. Loibner, “Activation-Dependent Expression of the Blood Group-Related Lewis Y Antigen on Peripheral Blood Granulocytes,” Journal of Leukocyte Biology, Vol. 68, No. 4, 2000, pp. 511-514.
- Z. Steplewski, M. Blaszczyk-Thurin, M. Lubeck, et al., “Oligosaccharide Y Specific Monoclonal Antibody and Its Isotype Switch Variants,” Hybridoma, Vol. 9, No. 2, 1990, pp. 201-210. http://dx.doi.org/10.1089/hyb.1990.9.201
- H. Inagaki, J. Sakamoto, H. Nakazato, et al., “Expression of Lewis(a), Lewis(b), and Sialated Lewis(a) Antigens in Early and Advanced Human Gastric Cancers,” Journal of Surgical Oncology, Vol. 44, No. 4, 1990, pp. 208-213. http://dx.doi.org/10.1002/jso.2930440404
- J. Sakamoto, K. Furukawa, C. Cordon-Cardo, et al., “Expression of Lewis a, Lewis b, X, and Y Blood Group Antigens in Human Colonic Tumors and Normal Tissue and in Human Tumor-Derived Cell Lines,” Cancer Research, Vol. 46, No. 3, 1986, pp. 1553-1561.
- K. Murata, H. Egami, Y. Shibata, et al., “Expression of Blood Group-Related Antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in Early Cancer, Intestinal Metaplasia, and Uninvolved Mucosa of the Stomach,” American Journal of Clinical Pathology, Vol. 98, No. 1, 1992, pp. 67-75.
- M. Miyake, T. Taki, S. Hitomi, et al., “Correlation of Expression of H/Le(y)/Le(b) Antigens with Survival in Patients with Carcinoma of the Lung,” New England Journal of Medicine, Vol. 327, No. 1, 1992, pp. 14-18. http://dx.doi.org/10.1056/NEJM199207023270103
- A. Larena, M. Vierbuchen, S. Schroder, et al., “Blood Group Antigen Expression in Papillary Carcinoma of the Thyroid Gland. An Immunohistochemical and Clinical Study of Expression of Lewis, ABO and Related Antigens,” Langenbecks Archiv für Chirurgie, Vol. 381, No. 2, 1996, pp. 102-113.
- K. Steplewska-Mazur, A. Gabriel, W. Zajecki, et al., “Breast Cancer Progression and Expression of Blood Group-Related Tumor-Associated Antigens,” Hybridoma, Vol. 19, No. 2, 2000, pp. 129-133. http://dx.doi.org/10.1089/02724570050031167
- H. Inufusa, Y. Nakatani, T. Adachi, et al., “Correlation of Prognosis of Breast Cancer Patients and Expression of Ley Which Acts as a Cofactor of Tumor Procoagulant,” International Journal of Oncology, Vol. 13, No. 3, 1998, pp. 481-487.
- Z. Madjd, T. Parsons, N. F. Watson, et al., “High Expression of Lewis y/b Antigens Is Associated with Decreased Survival in Lymph Node Negative Breast Carcinomas,” Breast Cancer Research, Vol. 7, No. 5, 2005, pp. R780- R787. http://dx.doi.org/10.1186/bcr1305
- K. Kitamura, E. Stockert, P. Garin-Chesa, et al., “ Specificity Analysis of Blood Group Lewis-y (Le(y)) Antibodies Generated against Synthetic and Natural Le(y) Determinants,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 91, No. 26, 1994, pp. 12957-12961. http://dx.doi.org/10.1073/pnas.91.26.12957
- D. Scholz, M. Lubeck, H. Loibner, et al., “Biological Activity in the Human System of Isotype Variants of Oligosaccharide-Y-Specific Murine Monoclonal Antibodies,” Cancer Immunology Immunotherapy, Vol. 33, No. 3, 1991, pp. 153-157. http://dx.doi.org/10.1007/BF01756135
- Z. Steplewski, M. D. Lubeck, D. Scholz, et al., “Tumor Cell Lysis and Tumor Growth Inhibition by the Isotype Variants of MAb BR55-2 Directed against Y Oligosaccharide,” In Vivo, Vol. 5, No.2, 1991, pp. 79-83.
- G. Schlimok, K. Pantel, H. Loibner, et al., “Reduction of Metastatic Carcinoma Cells in Bone Marrow by Intravenously Administered Monoclonal Antibody: Towards a Novel Surrogate Test to Monitor Adjuvant Therapies of Solid Tumours,” European Journal of Cancer, Vol. 31A, No. 11, 1995, pp. 1799-1803. http://dx.doi.org/10.1016/0959-8049(95)00317-C
- R. A. Stahel, H. Lacroix, J. P. Sculier, et al., “Phase I/II Study of Monoclonal Antibody against Lewis Y Hapten in Relapsed Small-Cell Lung Cancer,” Annals of Oncology, Vol. 3, No. 4, 1992, pp. 319-320.
- G. Schlimok, G. Riethmüller, K. Pantel, et al., “Tumor Cell Cytotoxicity Induced in Patients by a Murine Monoclonal Antibody against the Lewis Y Antigen,” Annual Meeting of the American Society of Clinical Oncology, 1991.
- I. Pastan, E. T. Lovelace, M. G. Gallo, et al., “Characterization of Monoclonal Antibodies B1 and B3 That React with Mucinous Adenocarcinomas,” Cancer Research, Vol. 51, No. 14, 1991, pp. 3781-3787.
- G. J. Schreiber, K. E. Hellström and I. Hellström, “An Unmodified Anticarcinoma Antibody, BR96, Localizes to and Inhibits the Outgrowth of Human Tumors in Nude Mice,” Cancer Research, Vol. 52, No. 12, 1992, pp. 3262-3266.
- M. S. Co, J. Baker, K. Bednarik, et al., “Humanized AntiLewis Y Antibodies: In Vitro Properties and Pharmacokinetics in Rhesus Monkeys,” Cancer Research, Vol. 56, No. 5, 1996, pp. 1118-1125.
- H. Farhan, C. Schuster, M. Klinger, et al., “Inhibition of Xenograft Tumor Growth and Down-Regulation of ErbB Receptors by an Antibody Directed against Lewis Y Antigen,” Journal of Pharmacology and Experimental Therapeutics, Vol. 319, No. 3, 2006, pp. 1459-1466. http://dx.doi.org/10.1124/jpet.106.107318
- A. M. Scott, D. Geleick, M. Rubira, et al., “Construction, Production, and Characterization of Humanized AntiLewis Y Monoclonal Antibody 3S193 for Targeted Immunotherapy of Solid Tumors,” Cancer Research, Vol. 60, No. 12, 2000, pp. 3254-3261.
- A. M. Scott, N. Tebbutt, F. T. Lee, et al, “A Phase I Biodistribution and Pharmacokinetic Trial of Humanized Monoclonal Antibody Hu3s193 in Patients with Advanced Epithelial Cancers That Express the Lewis-Y Antigen,” Clinical Cancer Research, Vol. 13, No. 11, 2007, pp. 3286-3292. http://dx.doi.org/10.1158/1078-0432.CCR-07-0284
- L. M. Krug, D. T. Milton, A. A. Jungbluth, et al., “Targeting Lewis Y (Le(y)) in Small Cell Lung Cancer with a Humanized Monoclonal Antibody, hu3S193: A Pilot Trial Testing Two Dose Levels,” Journal of Thoracic Oncology, Vol. 2, No. 10, 2007, pp. 947-952. http://dx.doi.org/10.1097/JTO.0b013e3181560dcc
- D. V. Oruzio, G. Waxenecker, C. Aulmann, B. Märkl, T. Wagner, C. Mudde, M. Schuster, N. Eller, A. Mayer, S. Stranner, G. Himmler, H. Loibner, G. Schlimok, R. Kircheis and A. Nechansky, “Phase I Dose Escalation Study with LeY Specific Humanized Mab IGN311,” Journal of Cancer Therapy, Vol. 2, No. 5, 2011, pp. 760-771. http://dx.doi.org/10.4236/jct.2011.25102
- A. Nechansky, S. Stranner, O. Scheiber, N. Halanek and R. Kircheis, “Induction of Human Anti-Human Antibody Responses (Ab2) after Application of a Humanized Lewis Y Carbohydrate Specific Antibody (Ab1): Connection of Prolonged Disease Stabilization with Ab3 Induction?” Journal of Cancer Therapy, Vol. 3, No. 4, 2012, pp. 269- 277. http://dx.doi.org/10.4236/jct.2012.34038
- M. Klinger, H. Farhan, H. Just, H. Drobny, G. Himmler, G. C. Mudde, M. Freissmuth and V. Sexl, “Antibodies Directed against Lewis-Y Antigen Inhibit Signaling of Lewis-Y Modified ErbB Receptors,” Cancer Research, Vol. 64, No. 3, 2004, pp. 1087-1093. http://dx.doi.org/10.1158/0008-5472.CAN-03-2435
- G. Schlimok, K. Pantel, H. Loibner, et al., “Reduction of Metastatic Carcinoma Cells in Bone Marrow by Intravenously Administered Monoclonal Antibody: Towards a Novel Surrogate Test to Monitor Adjuvant Therapies of Solid Tumours,” European Journal of Cancer, Vol. 31, No. 11, 1995, pp 1799-1803. http://dx.doi.org/10.1016/0959-8049(95)00317-C
- M. Cristofanilli, D. F. Hayes, G. T. Budd, et al., “Circulating Tumor Cells: A Novel Prognostic Factor for Newly Diagnosed Metastatic Breast Cancer,” Journal of Clinical Oncology, Vol. 23, No. 7, 2005, pp. 1420-1430. http://dx.doi.org/10.1200/JCO.2005.08.140
- S. Riethdorf, H. Fritsche, V. Müller, et al., “Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System,” Clinical Cancer Research, Vol. 13, No. 3, 2007, pp. 920-928. http://dx.doi.org/10.1158/1078-0432.CCR-06-1695
- V. Müller, N. Stahmann, S. Riethdorf, et al., “Circulating Tumor Cells in Breast Cancer: Correlation to Bone Marrow Micrometastases, Heterogeneous Response to Systemic Therapy and Low Proliferative Activity,” Clinical Cancer Research, Vol. 11, No. 10, 2005, pp. 3678-3685. http://dx.doi.org/10.1158/1078-0432.CCR-04-2469
- M. G. Krebs, R. Sloane, L. Priest, et al., “Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients with Non-Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 29, No. 12, 2011, pp. 1556-1563. http://dx.doi.org/10.1200/JCO.2010.28.7045
- F. Tanaka, K. Yoneda, N. Kondo, et al., “Circulating Tumor Cell as a Diagnostic Marker in Primary Lung Cancer,” Clinical Cancer Research, Vol. 15, No. 22, 2009, pp. 6980-6986. http://dx.doi.org/10.1158/1078-0432.CCR-09-1095
- O. Szolar, S. Stranner, I. Zinoecker, et al., “Qualification and Application of a Surface Plasmon Resonance-Based Assay for Monitoring Potential HAHA Responses Induced after Passive Administration of a Humanized Anti Lewis-Y Antibody,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 41, No. 4, 2006, pp. 1347-1353. http://dx.doi.org/10.1016/j.jpba.2006.03.026
- M. Schuster, A. Nechansky and R. Kircheis, “Cancer Immunotherapy,” Biotechnology Journal, Vol. 1, No. 2, 2006, pp. 138-147. http://dx.doi.org/10.1002/biot.200500044
- M. M. Heiss, P. Murawa, P. Koralewski, et al., “The Trifunctional Antibody Catumaxomab for the Treatment of Malignant Ascites Due to Epithelial Cancer: Results of a Prospective Randomized Phase II/III Trial,” International Journal of Cancer, Vol. 127, No. 9, 2010, pp. 2209-2221. http://dx.doi.org/10.1002/ijc.25423
- M. M. Heiss, M. A. Ströhlein, M. Jäger, R. Kimmig, A. Burges, A. Schoberth, K. W. Jauch, F. W. Schildberg and H. Lindhofer, “Immunotherapy of Malignant Ascites with Trifunctional Antibodies,” International Journal of Cancer, Vol. 117, No. 3, 2005, pp. 435-443. http://dx.doi.org/10.1002/ijc.21165
- F. Li, B. Lin, Y. Hao, Y. Li, J. Liu, J. Cong, L. Zhu, Q. Liu and S. Zhang, “Lewis Y Promotes Growth and Adhesion of Ovarian Carcinoma-Derived RMG-I Cells by Upregulating Growth Factors,” International Journal of Molecular Sciences, Vol. 11, No. 10, 2010, pp. 3748-3759. http://dx.doi.org/10.3390/ijms11103748
- J. A. Westwood, W. K. Murray, M. Trivett, et al., “The Lewis-Y Carbohydrate Antigen Is Expressed by Many Human Tumors and Can Serve as a Target for Genetically Redirected T Cells Despite the Presence of Soluble Antigen in Serum,” Journal of Immunotherapy, Vol. 32, No. 3, 2009, pp. 292-301. http://dx.doi.org/10.1097/CJI.0b013e31819b7c8e
- X. F. Wen, G. Yang, W. Mao, A. Thornton, J. Liu, R. C. Bast Jr. and X. F. Le, “HER2 Signaling Modulates the Equilibrium between Proand Antiangiogenic Factors via Distinct Pathways: Implications for HER2-Targeted Antibody Therapy,” Oncogene, Vol. 25, No. 52, 2006, pp. 6986-6996. http://dx.doi.org/10.1038/sj.onc.1209685
- A. Nechansky, M. Schuster, W. Jost, P. Siegl, S. Wiederkum, G. Gorr and R. Kircheis, “Compensation of Endogenous IgG Mediated Inhibition of Antibody-Dependent Cellular Cytotoxicity by Glyco-Engineering of Therapeutic Antibodies,” Molecular Immunology, Vol. 44, No. 7, 2007, pp. 1826-1828. http://dx.doi.org/10.1016/j.molimm.2006.08.013
- R. Kircheis, N. Halanek, M. Mayer, K. Hajszan, W. Jost, F. Altmann, G. Gorr and A. Nechansky, “Correlation of ADCC with Cytokine Release Induced by the Stably Expressed Glyco-Engineered Humanized Lewis Y Specific Monoclonal Antibody MB314,” MAbs, Vol. 4, No. 4, 2012, pp. 532-541. http://dx.doi.org/10.4161/mabs.20577
Abbreviations
LeY: Lewis Y;
EpCAM: epithelial cell adhesion molecule;
mAbs: monoclonal antibodies;
CDC: complement dependent cytotoxicity;
ADCC: antibody dependent cellular cytotoxicity;
CT: computer tomography;
pts: patients;
HAHA: human anti human antibody;
SDS: sodium dodecyl sulphate;
CEA: carcinoembryogenic antigen;
FITC: fluorescein isothiocyanate;
OPD: o-phenylenediamine dihydrochloride.
NOTES
*Corresponding author.


